Abstract
More has yet to be indicated on the adsorption and degradation processes, determining herbicides recycling in the environment. The sorption and degradation of 2, 4-D, affected by organic carbon (1.92–2.81%), soil clay (20–30%) and pH of the citrus orchards of Mazandaran province, Iran was investigated using HPLC equipped with UV detector for the identification and quantification of soil 2, 4-D. The adsorption (kd) and degradation (Kdeg) coefficients were determined using Freundlich and the first-degree kinetic equations. Gardens C (2.45 mL g−1), and B (0.3 mL g−1), with the highest (8.2 g day−1) and least (2.7 g day−1) degradation coefficients, had the highest and lowest Kd values. Kd variations with pH indicated higher adsorption of 2, 4-D in acidic pH. Due to the high presence of functional groups and soil biological activities, organic carbon affected the adsorption and degradation rates more effectively, which is of economic and environmental significance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Although herbicides are not environmentally recommendable, and they have become weed resistant with time, they are used as one of the most important components of integrated weed management, worldwide (Salehian and Mohammadzadeh 2018). Accordingly, herbicides have always been consumed at a higher rate compared with the other pesticides in the world. The herbicide 2, 4-D was the fifth most widely used pesticide in 2012, globally (Atwood and Paisley-Jones 2017). However, in Iran, among the 20 most important pesticides, 2, 4-D was ranked 11th in 2017 (Zand et al. 2019).
Investigating the important phenomenon of pesticide recycling in the soil, which is controlled by the complex processes of adsorption and degradation, is of economic and environmental significance. The adsorption process is a function of plant and soil particles, leaching, runoff, and evaporation, while the degradation processes is affected by hydrolysis, photo-decomposition and oxidation–reduction reactions (Islam et al. 2018; Liu et al. 2018a).
The degradation of pesticide generally reduces its toxicity but in some cases the metabolites are more toxic than the original compounds. The adsorption of herbicides on soil particles indicates the presence of biological activities. Organic matter and clay content can importantly control adsorption of most pesticides. Additionally, sugars and amino acids increase soil organic matter and microbial activity, affecting herbicides degradation (Wu et al. 2017; Mierzejewska et al. 2020). The processes of adsorption and degradation are often interrelated; degradation may be limited to soil solution and the adsorbed molecules may be resistant to microbial mineralization (Ren et al. 2018; Liu et al. 2018b).
The adsorption mechanism of herbicides is controlled by their molecular structure, soil acidity and soil solute concentration. The adsorption and degradation of ionizing pesticides is often pH dependent as pH fluctuations of soil solution can alter the adsorption and degradation amount (Tulp et al. 2009). Due to higher bioactivity, the rate of pesticide degradation by microorganisms is usually faster in alkaline pHs (Kah and Brown 2006; 2007). The herbicides with acidic or weak basic properties are affected by soil acidity (Gámiz et al. 2019). For example, 2,4 -D is non-ionic at pH < 6 but ionic at pH > 6, and accordingly, due to negative charges of soil particles, its adsorption at pH < 6 is higher (Qisse et al. 2020).
Research has indicated acidic herbicides are absorbed by soil colloids with less intensity than alkaline and non-ionic herbicides (Weber et al. 2007). The dominant forms of acidic herbicides are often anionic in pHs higher than acid-ionization constant, and hence are not adsorbed by soil colloids with negative charge (Werner et al. 2013). With increasing acidity, the solubility of acidic herbicides increases resulting in the increased adsorption of acidic herbicides, due to protonation, neutralization of the acidic group or their reduced solubility. The anionic form of an herbicide is more soluble in water, increasing its adsorption by plant roots (Islam et al. 2018).
It is an important research topic to investigate the key role of adsorption and degradation processes in the recycling of phenoxy herbicides including their storage and leaching in the soil, affecting groundwater pollution. Accordingly, since there is little data on the adsorption and degradation of 2, 4-D in the orchards, the objective was to investigate the effects of adsorption and degradation processes on the recycling of 2, 4-D in the orange orchards of Mazandaran province, Iran.
Material and Methods
For the present research, four citrus orchards (Citrus sinensis L. Qsbeck) were selected in four areas of Sari county (the capital of Mazandaran province in the north of Iran).
The soil samples of the orchards, highly variable in terms of organic carbon content (with amplitude of 41%), were taken from a depth of 0–15 cm in May 2020. The amount of clay was relatively small and its range was less compared to organic carbon (with a range of 33%) (Table 1). The ratio of clay percentage to the amount of organic carbon varied from 7.8 to 10.4.
Organic carbon of soil samples was measured by oxidation using potassium bichromate and concentrated sulfuric acid (Hesse 1971). Soil pH was also determined in saturated soil extract by pH meter (WTW-V30) (Rhoades 1982). Additionally, cation exchange capacity (Bower et al. 1952), bulk density (Black 1965) and soil texture (Bouyoucos 1962) were measured in the laboratory.
In this experiment 2, 4-D (Fig. 1) was used with water-soluble (SL) formulation and 72% purity. It is a selective and post emergence herbicide used to control annual and perennial broadleaf weeds in cereals, sugarcane and orchards. It has pKa = 2.97 and a solubility of 0.6 g L−1 (Villaverde et al. 2008).
The molecular structure and pH-dependent speciation of 2, 4-D. By Monolemma—Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=19239250
Freundlich equation was used to measure the adsorption coefficient (Eq. 1) (Muller et al. 2007):
S is the amount of herbicide adsorbed per unit weight of adsorbent (mg kg−1) and C is the equilibrium concentration of herbicide in solution phase (mol L−1). Kd or adsorption coefficient (dispersion or distribution coefficient), expressed in L Kg−1 (or mL g−1), is an important and practical indicator for the description and adsorption behavior of herbicides. To determine Kd, the numerator and denominator of Eq. 1 must be measured. S is the difference between the two initial concentrations of herbicide in the soil (E1) and the amount of herbicide drained from soil (E2).
The experiment was done by adding 550 g of soil (collected from each garden) to each PVC cylindrical column (32 cm high). With respect to the number of gardens and three replications for each treatment, 12 cylinders were prepared. Each column of soil (E1) was treated with 2, 4-D (SL, 72%) to achieve a dose of 1.5 L ha−1. Accordingly, in order to ensure the effect of the herbicide on the soil, after 72 h, the drained solution was extracted and transferred to the laboratory.
High performance liquid chromatography (HPLC) system equipped with UV detector was used for the identification and quantification of 2, 4-D in the soil samples. The wavelength of the UV detector was set at 230 nm, and using the buffer solution of 20 mM ammonium acetate adjusted to pH 4.0 by formic acid, the soil samples were measured. The mobile phase was a combination of 30% acetonitrile and 70% buffer solution. All separations were carried out on a reversed-phase C18 column (250 mm × 4 mm, 5 µm) at 20°C with a flow rate of 1 mL min−1.
Prior to the HPLC analyses, the samples were passed through 0.45 µm cellulose acetate syringe filters and were manually injected (50 µL) into the HPLC system. The calibration curves prepared by the injection of 50 μL from each of the standard solutions were used to determine the corresponding line equation (indicating the relationship between the peak area in the chromatogram and the concentration of the injected sample) and subsequent calculation of the residual 2, 4-D concentrations in the samples (Kashyap et al. 2005).
The following method was used to determine the equilibrium concentration of herbicide in solution phase (C) (m mol L−1) (Hiller et al. 2012); following the removal of the first extract from the tested pot soils, a thick solution was left in the soil, which was placed in a shaker for 24 h, and then washed with 550 mL distilled water. The solution was left for 72 h and its extract (second extract) was taken to the laboratory and its 2, 4-D amount was measured like the first extract (Morillo et al. 2001). According to the measurement of S and C values, the amount of herbicide adsorption coefficient (Kd) was obtained using Eq. 1.
In each garden, an area of 4 m2, with no history of herbicide spraying, was sprayed with 2, 4-D at the rate of 1.5 L ha−1 using a manual sprayer. Using an auger with a drill diameter of 50 mm, soil samples were taken from a depth of 0–5 cm, 10 (T1), 20 (T2), 30 (T3), 40 (T4) and 50 (T5) days after herbicide application (Noshadi et al. 2014). The samples were immediately transferred to the laboratory, stored in dark environment, dried, weighed and sieved. Using glass containers, the samples were stored in the freezer at − 20°C until analysis. Prior to chemical extraction, the samples were taken out of the freezer and placed in the room for some time to equilibrate with room temperature.
Degradation of herbicides in the soil generally follows a first order kinetic equation (Villaverde et al. 2008). In this study, the first-order kinetic Eq. (2) was used to interpret the alteration of herbicide in the soil with time:
c: herbicide concentration (µg cm−3 soil), t: time (day) and k: herbicide degradation coefficient.
Equation 3 is the integral of Eq. 2, if t = 0, and c = C0,
C0 is the concentration of herbicide at the time of zero (µg cm−3 soil). The values of C0 and k parameters are obtained from the fit of the equation on the data of herbicide concentration in the soil at different times after the application of the herbicide. In order to determine the concentration of 2, 4-D in the soil (C), the method by Hiller et al. (2012) was used again.
The use of half-life (DT50) is the most applicable method to express the persistence of herbicides and compare their degradation potential in the soil (Bowman 1991). The value of DT50 was calculated using Eq. 4, by the calculation of the k parameter in Eq. 3:
Results and Discussion
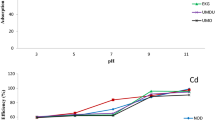
Table 1 presents the adsorption coefficients for the soils of different gardens. The highest and the lowest Kd values were obtained for gardens C, and B, respectively. The variations of Kd with pH (Fig. 2a) indicated that the adsorption of 2, 4-D in acidic pH was higher. The adsorption coefficient was positively correlated with soil properties except pH (Table 2).
The effect of pH on the adsorption of 10 ionic pesticides by nine soils of southern England has been shown (Kah and Brown 2007). It has also been indicated at high pH, the adsorption of 2, 4-D is reduced by up to 10 times (Tulp et al. 2009; Werner et al. 2013). Organic matter at acidic pHs (4.5–7.5) highly adsorb anionic herbicides (Tulp et al. 2009). Celis et al. (1999) obtained the adsorption coefficient for 2, 4-D in humic soil with a pH 2.9 equal to 60 L Kg−1 indicating the adsorption of 2, 4-D on soil organic matter is completely dependent on soil pH.
Faria et al. (2018) investigated the effects of soil pH on tebuthiuron leaching. The higher rate of organic matter and clay decreased the rate of herbicide leaching. The rate of leaching was noticeable (up to 50 cm depth) even at the higher rates of organic matter and clay. The authors indicated the leaching of nonionic herbicides is affected by soil pH, and liming increased the herbicide leaching by increasing soil pH.
The relatively high rate of adsorption by soil C cannot be related only to pH as it has the highest amount of organic carbon (Table 1). Different research has indicated the quantity of organic matter determines the adsorption process. Werner et al. (2013) in a review of 53 researches on 46 soils of North America and Europe examined the adsorption of several phenoxy acid herbicides, and found that the adsorption coefficient had a positive correlation with the amount of organic matter and a negative correlation with soil pH.
Tulp et al. (2009) obtained the 2, 4-D adsorption coefficient of 35.7 L Kg−1 in one type of peat soil. Figure 2b shows a direct and positive correlation between Kd and organic carbon, which was higher and more significant than clay (Table 2). A strong correlation between soil organic matter and the adsorption of phenoxy herbicides has also been indicated by other researchers (Hiller et al. 2012; Jiang et al. 2018). Accordingly, 2, 4-D adheres less to the soil particles and its adsorption has a positive correlation with the amount of soil organic matter and a negative correlation with soil pH (Fig. 2c) (Werner et al. 2013; Di Prima et al. 2018).
The ability of a particle to absorb molecules mainly depends on its surface area. Due to the association between inorganic and organic components of soil, it is difficult to separate their relative role in adsorption. According to the theory of blockage of clay particles by organic matter, when the ratio of clay to organic carbon is less than 30, the role of clay particles in adsorption is low (Morillo et al. 2004; James et al. 2019). In this experiment, the average ratio of clay to organic carbon in all soils was about 9.5. Research has also shown that when the share of soil organic carbon is more than 0.5%, sorption by the mineral part of the soil is reversed (Rebhun et al. 1992).
The minimum amount of organic carbon, was 1.92% (Table 1) indicating the importance of organic matter in the adsorption of 2, 4-D. However, determining the effect of a single component on sorption is difficult, as soil properties are often interrelated. Although research has indicated the contribution of clay to 2, 4-D adsorption might be significant, the sorption of herbicide by clay particles in most soils is low, because clay particles, especially in highly fertile soils are covered with a relatively thick layer of organic matter (Haberhauer et al. 2000; Marin-Benito et al. 2018).
The correlation between the amount of sorption and degradation rate of 2, 4-D (Kdeg), in the present research, was positive (Table 2) (Fig. 3). Park et al. (2001) showed in their proposed model, when 2, 4-D is adsorbed on soil particles, its degradation increases. The adsorption coefficient, organic carbon and biological activity are highly related. The herbicide adsorbed on the organic matter increases the activity of microorganisms (Zhang et al. 2020) as the predominant mechanism of 2,4-D degradation in the soil is through decomposition by bacteria (Shi et al. 2021). Accordingly, it seems reasonable that soil C with higher rate of organic carbon resulted in higher 2, 4-D degradation related to the other soil types (Table 1) (Zhu and Guo 2020). Our results are confirmed by Ismail and Azlizan (2002) indicating soil sterilization severely reduced the degradation of metsulfuron‐methyl.
The results of the present experiment indicated a significant positive correlation between degradation rate with the amounts of clay, organic carbon and a negative correlation with soil pH (Table 2). The negative correlation of soil pH with degradation rate can be explained according to the following: as the pH increases, the sorption of 2, 4-D decreases and this compound is less exposed to biodegradation due to leaching (Nowak et al. 2011; Zhu and Guo 2020).
The activity of microorganisms including biodegradation is affected by environmental conditions such as acidity and soil type (Liang et al. 2020; Yang et al. 2020). Due to the non-uniformity of soil conditions, it is not logical to extend the herbicide half-life from one soil to another. Soil C had a 2, 4-D half-life of 8.4 days, which was less than other soils (Table 1). Such results can be attributed to the higher amount of organic carbon and clay.
Ghafoor et al. (2011) investigated pesticide half-life and its correlation with soil properties and found that half-life has a negative correlation with soil organic matter. Other studies have estimated the half-life of 2, 4-D in the soil to be around 10 days (IUPAC 2011). Experiments by Juhler et al. (2008) on herbicide MCPA in 23 samples indicated that the average half-life of this herbicide is 9 days. However, soil B, had the longest 2,4-D half-life (25.6 days). The long-term presence of 2, 4-D in the soil may be due to a lack of degradability or a small population of microorganisms (Germaine et al. 2006).
Conclusion
Although the factors of organic carbon and soil clay had an effect on the adsorption of 2, 4-D., organic carbon content was the more effective one. Accordingly, organic carbon played a more decisive role in the adsorption of 2, 4-D. The results indicated pH was negatively correlated with herbicide sorption. Similar to the process of adsorption, the amount of 2, 4-D degradation was positively correlated with organic carbon and soil clay and negatively correlated with soil pH. With respect to the important processes of adsorption and degradation affecting the recycling and leaching potential of an herbicide, the use of 2, 4-D is recommendable in the regions with organic carbon of about 2%, clay of about 27%, and pH of less than 6.8.
References
Atwood D, Paisley-Jones C (2017) Pesticides industry sales and usage. US Environmental Protection Agency, Washington, p 24
Black CA (1965) Methods of soil analysis (V.I). American Society of Agronomy, Madison, p 1572
Bouyoucos GJ (1962) Hydrometer method improved for making particle size analyses of soils. Agron J 54:464–465
Bower CA, Reitemeier RF, Fireman M (1952) Exchangeable cation analysis of saline and alkali soils. Soil Sci 73:251–262
Bowman BT (1991) Mobility and dissipation studies of metribuzin, atrazine and their metabolities in plainfield sand using field lysimeters. Environ Toxicol Chem 10:573–579
Celis R, Hermosin MC, Cox L, Comejo J (1999) Sorption of 2,4-dichlorophenoxyacetic acid by model particles simulating naturally occurring soil colloids. Environ Sci Technol 33:200–1206
Di Prima S, Rodrigo-Comino J, Novara A, Iovino M, Pirastru M, Keesstra S, Cerdà A (2018) Soil physical quality of citrus orchards under tillage, herbicide, and organic managements. Pedosphere 28:463–477
Faria AT, Souza MF, de Jesus Passos ABR, da Silva AA, Silva DV, Zanuncio JC, Rocha PRR (2018) Tebuthiuron leaching in three Brazilian soils as affected by soil pH. Environ Earth Sci 77:214
Gámiz B, Velarde P, Spokas KA, Celis R, Cox L (2019) Changes in sorption and bioavailability of herbicides in soil amended with fresh and aged biochar. Geoderma 337:341–349
Germaine KJ, Liu X, Cabellos GG, Hogan JP, Ryan D, Dowling DN (2006) Bacterial endophyte-enhanced phytoremediation of the organochlorine herbicide 2,4-D. FEMS Microbiol Ecol 57:302–310
Ghafoor A, Moeys J, Stenstrom J, Tranter G, Jarvis NJ (2011) Modeling spatial variation in microbial degradation of pesticides in soil. Environ Sci Technol 45:6411–6419
Haberhauer G, Pfeiffer L, Gerzabek MH (2000) Influence of molecular structure on sorption of phenoxyalkanoic herbicides on soil and its particle size fractions. J Agric Food Chem 48:3722–3727
Hesse PR (1971) A text book of soil chemical analysis. John Murray, London
Hiller E, Tatarkova V, Simonovicova A, Bartal M (2012) Sorption, desorption and degradation of 4-chloro-2-methylphenoxy acetic acid in representative soils of the Danubian lowland. Slovakia Chemosphere 87:437–444
Ismail BS, Azlizan BA (2002) Persistence and bioactivity of metsulfuron-methyl in three soils. J Environ Sci Health B 37:345–353
Islam F, Wang J, Farooq MA, Khan MSS, Xu L, Zhu J, Zhao M, Munos S, Li QX, Zhou W (2018) Potential impact of the herbicide 2,4-dichlorophenoxyacetic acid on human and ecosystems. Environ Int 111:332–351
IUPAC (2011) IUPAC FOOTPRINT pesticides properties database. Available at http://sitem.herts.ac.uk/aeru/iupac/index.htm. Accessed 02 April 2011.
James TK, Ghanizadeh H, Harrington KC, Bolan NS (2019) Effect on herbicide adsorption of organic forestry waste products used for soil remediation. J Environ Sci Health B 54:407–415
Jiang R, Wang M, Chen W, Li X (2018) Ecological risk evaluation of combined pollution of herbicide siduron and heavy metals in soils. Sci Total Environ 626:1047–1056
Juhler RK, Henriksen TH, Ernstsen V (2008) Impact of basic soil parameters on pesticide disappearance investigated by multivariate partial least square regression and statistics. J Environ Qual 37:1719–1732
Kah M, Brown CD (2006) Adsorption of ionisable pesticides in soils. Rev Environ Con Toxicol 188:149–217
Kah M, Brown CD (2007) Prediction of the adsorption of ionizable pesticides in soils. J Agric Food Chem 55:2312–2322
Kashyap SM, Pandya GH, Kondawar VK, Gabhane SS (2005) Rapid analysis of 2,4-D in soil samples by modified Soxhlet apparatus using HPLC with UV detection. J Chromatog Sci 43:81–86
Liang Q, Yan Z, Li X (2020) Influence of the herbicide haloxyfop-R-methyl on bacterial diversity in rhizosphere soil of Spartina alterniflora. Ecotoxicol Environ Saf 194:110366
Liu X, Wu H, Hu T, Chen X, Ding X (2018) Adsorption and leaching of novel fungicide pyraoxystrobin on soils by 14 C tracing method. Environ Mon Assess 190:86
Liu Y, Lonappan L, Brar SK, Yang S (2018) Impact of biochar amendment in agricultural soils on the sorption, desorption, and degradation of pesticides: a review. Sci Total Environ 645:60–70
Marín-Benito JM, Sánchez-Martín MJ, Ordax JM, Draoui K, Azejjel H, Rodríguez-Cruz MS (2018) Organic sorbents as barriers to decrease the mobility of herbicides in soils. Modelling Leaching Process Geoderma 313:205–216
Mierzejewska E, Baran A, Urbaniak M (2020) Biodegradation potential and ecotoxicity assessment in soil extracts amended with phenoxy acid herbicide (2, 4-D) and a structurally-similar plant secondary metabolite (ferulic acid). Bull Environ Con Toxicol 104:200–205
Morillo E, Perez-Martinez JI, Gines JM (2001) Leaching of 2,4-D from a soil in the presence of β-cyclodextrin: laboratory columns experiments. Chemosphere 44:1065–1069
Morillo E, Undabeytia T, Cabrera A, Villaverde J, Maqueda C (2004) Effect of soil type on adsorption-desorption, mobility, and activity of the herbicide norflurazon. J Agric Food Chem 52:884–890
Muller K, Magesan GN, Bolan NS (2007) A critical review of the influence of effluent irrigation on the fate of pesticides in soil. Agric Ecosys Environ 120:93–116
Noshadi E, Homaee M, Mahmoudian Shooshtari M (2014) Transport and degradation of herbicides in soil under different herbigation systems. Iranian J Soil Water Res 45:255–266
Nowak KM, Miltner A, Gehre M, Schaffer A, Kastner M (2011) Formation and fate of bound residues from microbial biomass during 2,4-D degradation in soil. Environ Sci Technol 45:999–1006
Park JH, Kay D, Zhaoa X, Boyd SA, Voice TC (2001) Kinetic modeling of bioavailability for sorbed-phase 2,4-dichlorophenoxyacetic acid. J Environ Qual 30:1523–1527
Qisse N, Alouani ME, Azzouzi LE, Fadil IE, Saufi H, Belghiti MAE, Zrineh A, Azzouzi ME (2020) Adsorption of Imazalil herbicide onto Moroccan agricultural soils: kinetic and isotherm adsorption studies. Groundwater Sustain Develop 11:100468
Rebhun M, Kalabo R, Grossman L, Manka J, Rav-Acha CH (1992) Sorptions of organics on clay and synthetic humic-clay complexes simulating aquifer processes. Water Res 26:79–84
Ren X, Zeng G, Tang L, Wang J, Wan J, Liu Y, Yu J, Yi H, Ye S, Deng R (2018) Sorption, transport and biodegradation–an insight into bioavailability of persistent organic pollutants in soil. Sci Total Environ 610:1154–1163
Rhoades JD (1982) Soluble salts. Methods of soil analysis, Part 2. Chemical and microbiological Properties. American Society of Agronomy, Madison, pp 167–179
Salehian H, Mohammadzadeh M (2018) Weed ecology is affected by succession in differently aged gardens of Citrus sinensis and C. reticulata. Rend Lincei-Sci Fis 29:35–41
Shi A, Chakrawal A, Manzoni S, Fischer BM, Nunan N, Herrmann AM (2021) Substrate spatial heterogeneity reduces soil microbial activity. Soil Biol Biochem 152:108068
Tulp HC, Fenner K, Schwarzenbach RP, Goss KU (2009) pH-Dependent sorption of acidic organic chemicals to soil organic matter. Environ Sci Technol 43:9189–9195
Villaverde J, Kah M, Brown CD (2008) Adsorption and degradation of four acidic herbicides in soils from southern Spain. Pest Manage Sci 64:703–710
Weber JB, Taylor KA, Wilkerson GG (2007) Soil and herbicide properties influenced mobility of atrazine, metolachlor, and primisulfuron-methyl in field lysimeters. Agron J 98:8–15
Werner D, Garratt JA, Pigott G (2013) Sorption of 2, 4-D and other phenoxy herbicides to soil, organic matter and minerals. J Soils Sed 13:129–139
Wu X, Wang W, Liu J, Pan D, Tu X, Lv P, Wang Y, Cao H, Wang Y, Hua R (2017) Rapid biodegradation of the herbicide 2, 4-dichlorophenoxyacetic acid by Cupriavidus gilardii T-1. J Agric Food Chem 65:3711–3720
Yang Y, Singh RP, Song D, Chen Q, Zheng X, Zhang C, Zhang M, Li Y (2020) Synergistic effect of Pseudomonas putida II-2 and Achromobacter sp. QC36 for the effective biodegradation of the herbicide quinclorac. Ecotoxicol Environ Saf 30:109826
Zand E, Nezamabadi N, Baghestani MA, Shimi P, Mousavi SK (2019) A guide to chemical control of weeds in Iran. Jahad Daneshgahi Publication, Tehran, p 216
Zhang Y, Li W, Zhou W, Jia H, Li B (2020) Adsorption-desorption characteristics of pyraclonil in eight agricultural soils. J Soils Sed 20:1404–1412
Zhu Y, Guo J (2020) Impact of dichlorprop on soil microbial community structure and diversity during its enantioselective biodegradation in agricultural soils. J Environ Sci Health B 55:974–982
Acknowledgements
The authors would like to thank very much, the international publisher, AbtinBerkeh Scientific Ltd. Company (https://AbtinBerkeh.com), Isfahan, Iran, for editing the manuscript and revising it according to the journal format
Funding
There was not any funding for the present research.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they do not have any conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jamshidi, M.H., Salehian, H., Babanezhad, E. et al. The Adsorption and Degradation of 2, 4-D Affected by Soil Organic Carbon and Clay. Bull Environ Contam Toxicol 108, 151–157 (2022). https://doi.org/10.1007/s00128-021-03362-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-021-03362-w