Abstract
Soils consist of various components that can influence significantly heavy metal control in the environment. Understanding the adsorption characteristic of soil is important in combating pollution problems around farming areas. This work explored the sorption characteristics and retention of Pb and Cd by soils from Isu Aniocha farming area in Anambra state, Nigeria. The influence of temperature, metal concentration, pH and time on the sequestration of Pb2+ and Cd2+ was evaluated by batch sorption technique. Physicochemical properties of the soil were determined by standard techniques. Isotherm evaluation was performed by the Langmuir, Tempkin and Freundlich models. Pb (II) ion showed higher adsorption characteristics on the soil than Cd (II) from the maximum uptake capacity obtained. The maximum adsorption values for Pb range from 38.46 to 47.62 mg/g, while that for Cd range from 30.30 to 41.46 mg/g. Kinetic evaluation was conducted by the application of the pseudo first order, pseudo second order and intraparticle diffusion rate equations. The best fit on metal removal on the soils was achieved with the pseudo-second order model. The results showed that soils from a farming area can be effective in decreasing heavy metals pollution, especially Pb and Cd ions from solution phase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Soil is a complex heterogeneous medium which consist of soil matrix for heavy metal accumulation due to its tendency to attract and bind various chemicals [1]. The chemicals exist in soils in various forms bounded to soil particles by different forces [2]. It is necessary to evaluate the interaction as chemical toxicity is strongly dependent on the form they occur in the environment. Also, environmental properties and the soil variability may change equilibrium found in soil resulting in leaching of soil bound toxic elements [3]. Heavy metals chemicals once introduced to one part of the environment by any means may spread to other components of the environment, depending on the natural systems nature of interaction. Heavy metals interact physically or chemically with the compounds present in the natural environment, thus changing the nature they occur in the environment. They may change their oxidation state when in contact with oxidizing or reducing agents. Also their solubility changes with variation in temperature and pH [4]. Agricultural soils uptake of heavy metals is of increasing concern as a result of potential health risk, food safety issues and the detrimental effects on soil ecosystems. Volcanic eruptions, anthropogenic activities, forest fires, marine aerosols and natural occurrences are the main sources of elements in soils [5]. Traffic emissions (tire wear particles, particles of brake lining wear, vehicle exhaust particles and particles of weathered street surfaces), weathering of pavement and building surfaces, domestic emissions and industrial emissions (auto repair shops, power plants, metallurgical industry, chemical plants, coal combustion etc) are the anthropogenic heavy metal sources in soils [6].
Heavy metal contamination of agricultural soil usually leads to decrease in quality and crop output which affects through the food chain human health and also results in deterioration of water and air environmental quality [7]. Several works on the uptake of heavy metal by plants showed accumulated toxic levels harmful to human health [8]. Generally, there is a correlation between the heavy metal uptake by the plant and the soil contamination level [9]. Heavy metal level in the plant tends to increase with increasing soil contamination. However, since agricultural soils offer a possible sink for pollutants, there is a need to investigate how these pollutants are sorbed by soils and the fate of these pollutants in the environment, precisely plants, as this will aid in proffering solutions towards the remediation of soils polluted with heavy metals [10]. Usually, several metal ions are present at the same time in the soils, hence competitive adsorption and selective retention by the soil is essential to evaluate their mobility through the soils and the availability to plants [11]. In this study, cadmium and lead were considered because among various heavy metals, they are readily absorbed by plants and fairly mobile. Also, lead and cadmium ions are particularly of concern due to their toxicity even at low concentrations and can result in asthma, fertility issues, renal abnormalities, actute poisioning, cancer as well as liver, kidney and lungs infections [12].
Literature report revealed that agricultural soil contamination with heavy metals in Awka, Nigeria is largely associated with the use of poorly treated or untreated waste water from solid wastes and water bodies [13]. Also, the application of organic and inorganic fertilizers and pesticides has posed as a major ecological challenge in this area [14].
Heavy metals persistence in soils and mobility reduction involves some physicochemical processes such as dissolution, oxidation/reduction, complexation, precipitation, desorption and sorption. Although these phenomena usually take place simultaneously, sorption process is known to control significantly metal solubility and availability in soils. Adsorption phenomenon in soils influences the complexes in soil solution and metal ion concentration hence affects translocation of metal ions in plants [15]. Soils and clays have been reported by several researchers as potential adsorbents for heavy metals as well as other pollutants [16,17,18,19,20,21,22].
However, since soil properties vary from one location to another due to the interaction with the environment, it is necessary to evaluate the adsorption behavior of soil from different locations for metals. A thorough literature search revealed paucity of information on the use of tropical soil from Awka Anambra Nigeria for heavy metal adsorption despite the risk of pollution of heavy metals in this area. The present study thus investigated the physicochemical parameters and the Cd and Pb adsorption capacities of classified soils in a farming area at Isu Aniocha town, Awka, Nigeria by assessing the operational variables for the adsorption process. The mechanistic kinetics and isotherm of the abstraction process were analyzed by suitable empirical models, which help to provide insight on baseline data for Pb (II) and Cd (II) uptake capacity by the tropical soils investigated.
2 Materials and Methods
2.1 Soil sample Collection and Preparation as Adsorbents
The tropical soil were obtained from Nodu, Ifite-isu, Ekeagba, Umudunu and Umuodu in Isu Aniocha town, Awka North, Anambra State, Nigeria with the aid of a stainless trowel at a depth of 0–15 cm (top soil) from sample sites in a quadrant. The soil was bulked and homogenized to form composite sample. The tropical soils were air dried for 72–96 h until the loss in mass of the soil samples were not greater than 5% per 24 h. The air dried samples were then crushed gently with a pestle and mortar and sieved through a 2 mm mesh size sieve and stored in closed glass sterile bottles.
2.2 Physicochemical Evaluation
Characterization of the adsorbents was carried out by quantitative spectroscopic analysis. The lead and cadmium concentration in the soil samples was evaluated by the atomic absorption spectrophotometer (AAS) after wet digestion of the samples using concentrated nitric and hydrochloric acid [23]. The Bouyoucos hydrometer method was used to determine the particle size distribution [24]. Organic carbon (OC) and organic matter were determined using wet oxidation method as described in literature [25]. Soil pH in water and KCl was determined potentiometrically by reported method [26]. Cation exchange capacity was determined using literature procedure [27]. Sodium and potassium was estimated colorimetrically using Flame photometer. Determination of Exchangeable Acidity (Al3+, H+) was based on titration using 0.05 M of NaOH [26] whereas the determination of Exchangeable Bases (Ca2+ and Mg2+) was based on titration using 0.01 M EDTA [28].
2.3 Preparation of Adsorbate Solutions
Analytical grade chemicals were used in the current study without further purification and obtained from Sigma–Aldrich. The adsorbate solutions of 1000 mg/L of Pb2+ and Cd2+ were prepared by dissolving an appropriate weight of Cd(NO3)2 and Pb(NO3)2 in distilled water in 1000 mL standard volumetric flasks. The solution was diluted several times to obtain solutions from 50 to 250 mg/L.
2.4 Adsorption Procedure
Batch sorption process was used to evaluate the influence of metal concentration, pH, and temperature and contact time on the sequestration of metal ions on the tropical soils. 0.10 g air-dried and sieved samples were placed in plastic vials and 20 mL of the working concentrations of Cd(NO3)2 and Pb(NO3)2 were added and tightly sealed with Teflon lined screw caps and mechanically agitated on a horizontal shaker, for 1 h at 120 rpm at room temperature (25 ± 1 °C). After equilibration, the vials were centrifuged at 3000 rpm for 30 min, allowed to settle and filtered via Whatmann110 mm filter paper into plastic vials, the filtrate were analyzed for Pb, and Cd equilibrium concentration. The influence of initial adsorbate concentration (50–250 mg/L), pH (3.0–11.0), temperature (303, 313, 323, 333, 343 K) and contact time (20, 30, 40, 50, 60 min) were studied. Samples were obtained from the vials at regular time intervals and analyzed for the amount of cadmium and lead remaining in solution by the AAS. The quantity of lead and cadmium ions removed (mg/g) by the tropical soils was determined by the equation described [29, 30]:
where qe (mg/g) is the uptake capacity of the tropical soils for cadmium and lead ions, Co (mg/L) is the cadmium and lead concentration present initially in solution, Ce (mg/L) is the residual amount after the adsorption process was carried out, m (g) is the mass of tropical soil adsorbents used and V (L) signifies the volume of solution used for the sorption process. The percentage sequestration of the heavy metals from solution on the tropical soils was evaluated from the given equation:
3 Results and Discussion
3.1 Characterization of Soil Samples
Table 1 gives the mean and standard deviation of concentrations of cadmium (Cd) and lead (Pb) in tropical soil samples. The amount of cadmium in soil was present in the range of 0.01–6.68 mg/kg, which is above WHO permissible limit of 1.0 mg/kg for some of the tropical soils. The mean concentration of Cd in soil samples is high because fertilizers are added regularly to soils to replenish N, K, P for crop growth especially phosphatic fertilizer which contains (2–200 mg/kg) of Cd. The concentration of Cd can be immobilized by increasing soil pH through addition of liming materials. The concentration of Pb in the samples ranged between 8.55 and 16.15 mg/kg. The permissible limit for lead (Pb) according to WHO standard is 10 mg/kg. The high level of Pb was traced from application of agrochemicals, fungicides and pesticides like lead arsenate that are used at various stages of crop production.
The results of characterization of the tropical soil samples are given in Table 2. They are classified as sandy loam and loamy sand soils. The organic matter content of the soil samples observed in five agricultural soils vary from 0.825 to 4.402% and adsorption occurs in soil with higher organic matter content. The clay particle content and cation exchange capacity are necessary to be evaluated as it provides better insights of sorption process. At high clay content, the surface of the soils provides active sites for cation exchange.
3.2 Effect of Solution pH and Metal Concentration on Metal Ions Removal
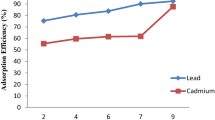
Figure 1 showed the influence of solution pH on the percentage of Pb2+ and Cd2+ removed by Isu Aniocha soil samples. At low pH of solution, the sequestration percentage was low and increased with pH due to the electrostatic repulsion between Pb2+ and Cd2+ ion and the edge groups having positive charges (Si–OH2+) on the adsorbents surface [31].With solution acidity decrease, the adsorbent functional group become de-protonated leading to increase in the negative charge density on the adsorbent surface facilitating the binding of metal ions [32]. A sharp increase in adsorption was observed from pH 5 to 7 while maximum adsorption occurred from pH 9 to 11 for both Pb2+ and Cd2+. The order of affinity of the metal ions on the soils is Pb2+ > Cd2+. Similar observation has been reported [33].
Initial concentration of solution is capable of affecting the mass transfer resistance between metal ions and the soil adsorbents by providing a driving force to overcome this effect. The influence of initial metal ion concentration on the adsorption of Pb2+ and Cd2+ onto Isu Aniocha soil samples are shown in Fig. 2. As the initial Pb2+ and Cd2+ concentration increased from 50 to 250 mg/L, adsorption efficiency of the soil samples for the metal ions decreased on all soil samples studied. The decrease in the percentage uptake is attributed to the saturation of the limited active sites of the tropical soil adsorbent with concentration increase [34]. On the other hand, an increase in the uptake capacity of the tropical soils with increase in cadmium and lead concentration was recorded. This is mainly attributed to the increase in driving force from increasing concentration which overcame the mass transfer resistance of lead and cadmium ions from solution to the surface of the adsorbents [22]. The affinity of the heavy metals for our utilized soils was in the order Pb2+ > Cd2+ for all the five samples. Similar trend was documented [35].
3.3 Effect of Contact Time and Temperature
The uptake of lead (II) and Cadmium (II) ions on the tropical soils under the influence of contact time is shown in Fig. 3. The sequestration rate was initially fast on all the soils and then became slower as the adsorption progressed until equilibrium was reached for both lead and cadmium ions. At this equilibrium point on the soil adsorbents there was no further removal of both heavy metals with increase in time. The equilibrium was attained after 60 min for the five tropical soil samples. The fast removal of both metal ions on the five adsorbents initially was due to the presence of abundant active sites on the soils. The abundant active sites were occupied as time progressed attaining saturation leading to no further sequestration [36]. The sorption was initially controlled by the rapid diffusion of lead and cadmium from the solution to the surface of the tropical soils. The variation in the rate of sequestration by the tropical agricultural soils could be attributed to the adsorption characteristics of the soils’ constituents which can affect the active available sorption sites on the soils and consequently affect the mass transfer of the heavy metals from solution to the soil surfaces. Similar trend have also been reported [37, 38].
A plot showing the effect of varying temperature on adsorption of Pb (II) and Cd (II) by Isu Aniocha soils is presented in Fig. 4. As temperature increased, a gradual increase in percentage of cadmium and lead sequestrated by the tropical soil samples was recorded. The increase in removal of both metal ions on the soils may be due to increase in collision of the metal ions with the soil adsorbents as temperature increases [39]. Temperature increase may result to increase in active adsorption sites on the tropical soil surfaces due to dissociation of some surface components and greater possession of kinetic energy by the adsorbate for better interaction with the tropical soil surfaces [40]. This increase in adsorption with temperature suggest feasible utilization of the soils for lead and cadmium sequestration in tropical regions where the temperatures could be high at times.
3.4 Adsorption Isotherms Application on Soil Adsorption Potential
Sorption isotherms help to provide insight on the relationship between adsorbent and adsorbate i.e the soils and metal ions. Equilibrium isotherm parameters obtained from the interaction of soils with Pb (II) and Cd (II) ions are given in Tables 3 and 4.
The Langmuir sorption was used in the adsorption process and expressed linearly as [41]:
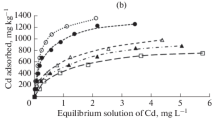
where KL is related to the adsorption energy known as the Langmuir constant (L/mg), qe is the monolayer uptake capacity of the tropical soils (mg/g), qL is the maximum uptake monolayer capacity of the soils estimated from the model (mg/g). The linear plots of Ce/qe versus Ce showed that Langmuir model gave a very good fit to the adsorption data of Cd2+ with correlation coefficient (R2) values varying from 0.928 to 0.992 while for Pb2+ only three soils (EKG, UMD, UMO) fitted to the linearised Langmuir isotherm with R2 varying from 0.960 to 0.983 thus suggesting the occurrence of monolayer coverage of the soils for Pb2+ and Cd2+ ions. The tropical soils showed higher maximum uptake capacity for Pb than Cd, as seen in Tables 3 and 4. This is due to the hard–soft acid–base principle in which adsorption on the soils increases with increasing electronegativity and decreasing ionic radii and polarizability thus decreasing hardness. Similar trend have been documented [42]. The removal process is said to be linear (RL = 1), favorable (0 < RL < 1), irreversible (RL = 0) or unfavorable (RL > 1) [43]. Values of initial cadmium and lead concentration from 50 to 250 mg/L on the five tropical soils used, showed RL ranged from 0.03 to 0.34 showing good affinity of our topical soil adsorbents for lead and cadmium ions from aqueous media.
The Freundlich isotherm model was used in its linear form as [44, 45]:
where n and KF (mg/g) (mg/L), are Freundlich model constants. The obtained parameter for regression coefficient (R2) for Pb2+ ion is 0.987 for Nodu soil and 0.962 for Ifite-isu soil sample which shows that the two soil samples gave a good fit for Freundlich isotherm than Langmuir isotherm thus indicates that the surface of the adsorbents are heterogeneous and adsorption is non uniform distribution of heat of sorption.
The linear form of the isotherm Temkin model is represented as [46, 47]:
where B and A are the Tempkin model adsorption constants for removal on the soils, T and R are the absolute temperature and ideal gas constants respectively. The values of the regression coefficient (R2) in all the five studied soil samples vary from (0.917–0.950) for Pb2+ ion and (0.783–0.982) for Cd2+ ion which are lower than the Langmuir model and better than Freundlich model in some soil samples. Therefore this isotherm did not give best fit to the sorption of both metals on the five tropical samples studied and so was not further analyzed or discussed.
3.5 Adsorption Kinetics Modeling
Kinetic modeling was performed by pseudo-first, intraparticle diffusion and pseudo-second order model [48]. The constant parameters are given in Tables 5 and 6. Pseudo-first order equation was applied as given [49]:
KI is the adsorption rate constant (min−1). Good fits were not provided by the pseudo-first order model for the sequestration of cadmium and lead on the five tropical soils evaluated due to the low regression (R2) values presented for the sorption process.
The kinetics of pseudo-second order rate equation was given by Ho and Mckay [50]:
K2 represents the pseudo second order equation rate constant (g/mg min) and h (mg/g min) signifies the initial adsorption rate calculated from the given equation:
From the table a good fit of the pseudo second order model for adsorption of lead and cadmium on the tropical soils was indicated by the high R2 values obtained. The qe calculated values although higher were closer to the experimental values than the pseudo second order model which indicates further the suitability of the kinetic model for metal sorption on the tropical soils. This suggests that removal of both metal ions on the soils was through chemisorptions process [22].
The intraparticle diffusion can be estimated by using the model equation to elaborate the diffusion mechanism of lead and cadmium on the tropical soils expressed as [36, 51]:
where I represent the intercept and Kd denotes the rate constant of abstraction (mg/gmin1/2). From the diffusion analysis it was observed from the R2 that linear plots were achieved but deviated from the origin due to the occurrence of the intercept. This showed the involvement of intraparticle diffusion mechanism on lead and cadmium removal on the tropical soil. However the occureence of intercepts showed that surface mechanism (liquid film diffusion) was also involved in the overall sequestration process of the metal ions on the five tropical soils used.
4 Conclusion
This study investigated the feasibility of selected tropical soils of Isu Aniocha farming area used as eco-friendly adsorbents for cadmium and lead sequestration from aqua solution by batch sorption technique. Various impart factors such as solution initial heavy metal concentration, pH, temperature and contact time were evaluated for metal ions removal. The adsorption capacity of tropical soils for Pb2+ and Cd2+ as pH, contact time, concentrations and temperature increased. The order of sorption on the tropical soils was Pb (II) > Cd (II). The adsorption of Cd (II) and Pb (II) in all the soil samples gave good fit for the Langmuir model, showing the monolayer uptake capacity onto Isu Aniocha tropical soils. Although, NOD and IFTU soils gave better fit with the Freundlich model for Pb (II) depicting a heterogeneous multilayer removal on the soil surfaces. Pseudo-second order model was found to provide the best fit in the abstraction of both metal ions on the tropical soils studied. In addition, the intraparticle diffusion of the metal ions into micropores was not the sole rate controlling step. It is therefore suffices to conclude that Isu Aniocha tropical agricultural soils could serve as readily available, cheap, efficient adsorbent for the abstraction of Pb2+ and Cd2+ from contaminated waters. Also, the soils could serve as lining material under landfills for heavy metals adsorption in order to maintain safer ground water resources. The significance of the study is that the readily obtainable soil in Awka Nigeria can be utilized as potential adsorbent to treat heavy metal release from wastewaters into the environment thereby reducing the health effect of these metals to humans and ecosystems.
References
Heike BB (2004) Adsorption of heavy metal ions on soils and soils constituents. J Colloid Interface Sci 277:1
Dube A, Zbytniewski R, Kowalkowski T, Cukrowska E, Buszewski B (2011) Adsorption and migration of heavy metal in soil. Pol J Environ Stud 20:1
Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. Int Sch Res Net 40:26
Emenike PC, Omole DO, Ngene BU, Tenebe IT (2016) Potentiality of agricultural adsorbent for the sequestering of metal ions from wastewater. Glob J Environ Sci Manag 2:411
Hegazi HA (2013) Removal of heavy metal from wastewater using agriculture and industrial wastes as adsorbents. HBRC J 9:276
Tang X, Zhenze L, Yunmin C (2009) Adsorption behavior of Zn(II) on calcinated Chinese loss. J Hazard Mater 161:824
Raymond AW, Felix EO, Rebecca NV (2014) Mixed contaminants interaction in soils: implications for bioavailability, risk assessment and remediation. Afr J Environ Sci Technol 8:691
Asagi N, Veno H, Ebid A (2007) Application of soil nutrients replenishing additives. Int J Soil Sci 2:171
Ojiegbe RU (2005) Study of a waste disposal site and its ground water contamination potential. Int J Natl Appl Sci 1:21
Siti NAA, Mohd HSI, Md LK, Shamsul I (2013) Adsorption process of heavy metals by low-cost adsorbent: a review. World Appl Sci J 28:1518
Asadu CLA, Ukadike B, Agada C (2008) Assessment of sewage application in south eastern Nigeria and Impact of soil chemical properties, trace and heavy metal accumulation in soil and underground water. Outlook Agric 37:63
Ali I, Peng C, Naz I (2019) Removal of lead and cadmium ions by single and binary systems using photogenic magnetic nanoparticles functionalized by 3-marcaptopropanic acid. Chin J Chem Eng 27:949
Bessie ES, Edidiong AE (2014) Heavy metal concentration of cocoyam and pawpaw crops grown around anaekie Obiakor illegal dumpsite, Awka, Anambra State, Nigeria. Int J Innov Sci Res 8:210
Ojiako EN, Aduaka PC (2015) Effects of heavy metals in agricultural soils of Dunukofia local government area of Anambra state, Nigeria. Int J Res Stud Sci Eng Technol 2:28
Salman T, Temel FA, Turan NG, Ardali Y (2005) Adsorption of lead (II) ions onto diatomite from aqueous solutions: mechanism, isotherm and kinetic studies. Glob NEST J 17:15
Abukhadra MR, Adlii A, Bakry BM (2019) Green fabrication of bentonite/chitosan@cobalt oxide composite of enhanced adsorption and advanced oxidation removal of congo red dye and Cr (VI) from water. Int J Biol Macromol 126:402
Dawodu MO, Akpomie KG (2016) Evaluating the potential of a Nigerian soil as an adsorbent for tartrazine dye: isotherm kinetic and thermodynamic studies. Alex Eng J 55:3211
Peng L, Liu P, Feng X, Wang Z, Cheng T, Liang Y, Lin Z, Shi Z (2018) Kinetics of heavy metal adsorption and desorption in soils: developing a unified model based on chemical speciation. Geochim Cosmochim Acta 224:282
Uddin MK (2017) A review on the adsorption of heavy metals by clay minerals with special focus on the past decade. Chem Eng J 308:438
Elbana TA, Selim HM, Akrami N, Newnan A, Shaheen SM, Rinklebe J (2018) Freundlich sorption parameters for cadmium, copper, nickel, lead and zinc for different soils: influence of kinetics. Geoderma 324:80
Jock AA, Zaini MAA, Abdulsalam S, El-Nafaty UA, Aroke UO (2019) Isotherm studies of lead (II), manganese (II) and cadmium (II) adsorption by Nigerian bentonite clay in single and multi-metal solution. Part Sci Technol 37:399
Akpomie KG, Dawodu FA, Adebowale KO (2015) Mechanism on the sorption of heavy metals from binary-solution by a low cost montmorillonite and its desorption potential. Alex Eng J 54:757
Ure AM, Quevauviller PH, Muntau H, Griepink B (2003) Speciation of heavy metals in soils and sediments. An account of improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the Commission of the European Communities. Int J Environ Anal Chem 51:135
Bouyoucos GH (1964) A recalibration of the hydrometer for making mechanical analysis of soils. J Agron 43:434
Nelson DW, Sommers LE (1996) Total carbon, organic carbon and organic matter. In: Page AL (ed) Methods of soil analysis, part 2. Chemical and microbiological properties. 2nd edn. American Society of Agronomy, Madison
Mclean EO (1982) Soil pH and lime requirement. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. Part 2: chemical and microbiological properties, vol 1982, 2nd edn. American Society of Agronomy, Madison, pp 199–224
Chapman HD (1965) Cation exchange capacity. In: Black CA (ed) Methods of soil analysis, part 2. Agronomy No. 9, vol 1965. American Society of Agronomy. Inc., Madison. pp 891–901
Thomas GW (1982) Historical developments in soil chemistry. Ion exchange. Soil Sci Soc Am J 41:230
Shaban M, Abukhadra MR, Rabia M, Abd-Elkader Y, AbdEl-Halim MR (2018) Investigation the adsorption properties of graphene oxide and polyaniline nano/micro structures for efficient removal of toxic Cr (VI) contaminants from aqeous solutions: kinetic and equilibrium studies. Rend Lincei Sci Fisiche e Naturali 29:141
Shaban M, Abukhadia MR, Mohamed AS, Shahien MG, Ibrahim SS (2018) Synthesis of mesoporous graphite functionalized by nitrogen for efficient removal of safranin dye utilizing risk husk ash: equilibrium studies and response surface optimization. J Inorg Organomet Polym Mater 28:279
Sheng PX, Ting YP, Chen JP, Hong L (2004) Sorption of lead, copper, cadmium, zinc and nickel by marine algal biomass: characterization of biosorptive capacity and investigation of mechanisms. J Colloid Inter Sci 275:131
Ofomaja AE, Unuabonah EI, Oladoja NA (2010) Competitive modeling for the biosorptive removal of copper and lead ions from aqueous solution by mansonia wood sawdust. Bioresour Technol 101:3844
Das B, Mondal NK (2011) Calcareous soil as a new adsorbent to remove lead from aqueous solution: equilibrium, kinetic and thermodynamic study. Univer J Environ Res Technol 1:515
Tsai WT, Chen HR (2010) Removal of malachite green from aqueous solution using low cost chlorella-based biomass. J Hazard Mater 175:844
Kalalagh SS, Babazadeh H, Nazemi AH, Manshouri N (2011) Isotherm and kinetic studies on adsorption of Pb, Zn and Cu by kaolinite. Casp J Environ Sci 9:243
Chukwuemeka-Okorie HO, Ekemezie PN, Akpomie KG, Olikagu CS (2018) Calcined concob-kaolinite combo as new sorbent for sequestration of toxic metal ions from polluted aqua media and desorption. Front Chem 6(1):2018
Eba F, Gueu S, Eya A, Ondo AJA, Yao BK, Ndong NJ, Kouya BR (2010) Evaluation of the absorption capacity of the natural clay from Bikougou (Gabon) to remove Mn (II) from aqueous solution. Int J Eng Sci Technol 2:501
Olaofe O, Olagboye SA, Akanji PS, Adamolugbe EY, Fowowe OT, Olaniyi AA (2015) Kinetic studies of adsorption of heavy metals on clays. Int J Chem 7:231
Jimoh TO, Yisa J, Ajai AI, Musa A (2013) Kinetics and thermodynamics studies of the biosorption of Pb(II), Cd(II) and Zn(II) ions from aqueous solution by sweet orange (Citrus sinensis) seeds. Int J Mod Chem 4:19
Ekop AS, Eddy NO (2010) Thermodynamic study on the adsorption of Pb2+ and Zn2+ from aqueous solution by human hair. E-J Chem 7:1296
Langmuir I (1918) The adsorption of gases in plane surfaces of glass, mica and Platinum. J Am Chem Soc 40:1361
Fonseca B, Maio H, Quintelas C, Teixeira A, Tavares T (2009) Retention of Cr (VI) and Pb (II) on a loamy sand soil kinetics, equilibria and breakthrough. Chem Eng J 152:212
Ani JU, Ochonogor AE, Akpomie KG, Olikagu CS, Igboanugo CC (2019) Abstraction of arsenic (III) on activated carbon prepared from Dialium guineense seed shell: kinetics, isotherm and thermodynamic studies. SN Appl Sci 1:1304
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385
Abukhadra MR, Mostafa M (2019) Effective decontamination of phosphate and ammonium utilizing novel muscovite/phillipsite composites: equilibrium investigation and realistic application. Sci Total Environ 667:101
Temkin MI, Pyzhev V (1940) Kinetics of ammonia synthesis on promoted ion catalyst”. Acta Physicochem 12:217
El-Nahas S, Salman HM, Seleeme WA (2019) Aluminium building scrap wire, take out food container, potato peels and bagasse as valueless waste materials for nitrate removal from water supplies. Chem Afr 2(143):2019
Akpomie KG, Onyeabor CF, Ezeofor CC, Ani JU, Eze SI (2019) Natural aluminosilicate clay obtained from South-Eastern Nigeria as potential sorbent for oil spill remediation. J Afr Earth Sci 155:118
Lagergren S (1898) About the theory of so called adsorption of soluble substances. Ksven Vetenskapsakad Handl 24:1
Ho YS, Mckay G (2000) The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Resour 34:735
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solutions. J Sanit Eng Div Am Soc Civ Eng 9:31
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Umeh, C., Asegbeloyin, J.N., Akpomie, K.G. et al. Adsorption Properties of Tropical Soils from Awka North Anambra Nigeria for Lead and Cadmium Ions from Aqueous Media. Chemistry Africa 3, 199–210 (2020). https://doi.org/10.1007/s42250-019-00109-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-019-00109-3