Abstract
Heavy metal (HM) contamination of the environment is a major issue worldwide, creating an ever-increasing demand for remediation techniques. Remediation with algae offers an ecologically safe, cost-effective, and efficient option for HM removal. Similar to plants, growth and development of algae are controlled by the hormonal system, where phytohormones are involved in HM tolerance and thus can regulate remediation ability; however, the underlying mechanisms of phytohormone function remain elusive. This review aims to draw a comprehensive model of phytohormone contributions to algal performance under HM stress. We focus on the mechanisms of HM biosorption, uptake and intracellular storage, and on how phytohormones interact with algal defence systems under HM exposure. We provide examples of successful utilization of algae in remediation, and of post-processing of algal materials. Finally, we discuss the advantages and risks of using algae for remediation. An in-depth understanding of these processes can inform effective HM remediation techniques.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal (HM) pollution of the biosphere is a major environmental issue worldwide. While HMs occur as natural constituents of the earth’s crust, they are also released due to anthropogenic activities, which can lead to excess levels in the environment. Unlike organic chemicals, most metals cannot be degraded into less toxic compounds through natural processes, contributing to their persistence and wide distribution in water and soil (Selvi et al. 2019). At high concentrations, HMs represent a serious threat to environmental and human health (Cheng et al. 2019; Sen Gupta et al. 2020). Thus, the need to remove excess HMs from the environment is internationally recognized (Mansour 2014).
Several techniques have been developed to remove HMs from environmental media, such as oxidation, precipitation, and ion exchange; but, these practices can be costly, time-consuming, and associated with secondary pollution (Cheng et al. 2019). Remediation with plants or algae offers an ecologically safer, less expensive, and more efficient method to remove HMs (Cheng et al. 2019; Selvi et al. 2019). The use of algae is preferable in many situations, as plants can take a long time to extract HMs and their application is limited by climatic and geological conditions. Therefore, algae can play a crucial role in the restoration of degraded environments, especially where plant use is less facilitated (El-Sheekh and Mahmoud 2017).
Algae are a diverse group of photosynthetic organisms built of simple vegetative structures without a vascular system, and possessing chlorophyll a as their primary photosynthetic pigment. They have a range of morphologies: unicellular, colonial, constructed of filaments, or composed of simple tissues (Sheath and Wehr 2015). Hormone regulatory networks control the growth and development of algae, and so-called “phytohormones” (which we can also consider as “phycohormones”) are synthesized to regulate critical physiological processes (Tarakhovskaya et al. 2007). Major classes of phytohormones found in algae include abscisic acid (ABA), auxins, brassinosteroids (BRs), cytokinins (CKs), and gibberellins (GAs) (Lu and Xu 2015; Tarakhovskaya et al. 2007). Understanding how these hormones influence molecular and physiological responses of algae to HM exposure is critical for developing successful remediation technologies.
Algae used for remediation of HMs are often essential components of the aquatic ecosystems they live in. Several reviews have identified algae as effective remediators for different metals (e.g., Ahmad et al. 2020; Sen Gupta et al. 2020), and others have characterized the role of phytohormones for algal growth and development (e.g., Noble et al. 2014; Lu and Xu 2015; Romanenko et al. 2016). Case studies have linked both aspects and reported that phytohormones could affect the response to HM exposure in the green algal species Chlorella vulgaris (e.g.,Bajguz 2011; Falkowska et al. 2011; Piotrowska-Niczyporuk et al. 2012), and Acutodesmus obliquus (Piotrowska-Niczyporuk et al. 2017, 2018a). However, the molecular mechanisms by which microalgae cope with HM stress remain unclear; moreover, the knowledge of algal responses to HMs is largely based on observations in higher plants (Bajguz 2011). Nevertheless, because of the differences between plant and algae morphology and physiology, the same mechanisms obviously are not always relevant to both groups.
In this review, we aim to draw a comprehensive model of phytohormone involvement in algal responses under HM stress. We present an overview of how algae respond to HM stress and the potential roles of phytohormones in the alleviation of the HM toxicity. We focus on the mechanisms of HM biosorption, uptake and intracellular storage in algae, and on how phytohormones interact with algal defence systems under HM exposure. An in-depth understanding of these processes will be valuable to inform effective HM remediation techniques in the future.
Algae and Metal Remediation
The Toxicity of an Excess Heavy Metal Exposure
Metals, both essential and non-essential, are toxic when present at a level that induces a deleterious response in an organism (Ahmad et al. 2019; Sen Gupta et al. 2020). Non-essential HMs can disrupt metabolic functioning by competing with the essential metals, facilitated by their similar size, charge and oxidation state (Sen Gupta et al. 2020). General metal toxicity mechanisms are: interactions with essential functional groups of biomolecules, disruption of the integrity of biomembranes, and production of reactive oxygen species (ROS). Metals bind to many functional groups of cellular macromolecules that form essential cell structures, such as lipids, polysaccharides, and proteins. HMs can damage DNA, denaturate proteins, and inhibit enzyme activity (Ahmad et al. 2019).
Algae possess molecular mechanisms that allow to discriminate between essential and non-essential HMs, and to maintain nontoxic concentrations of HMs inside their cells (Perales-Vela et al. 2006). In algae, HMs can adversely affect growth, cell division, photosynthesis, and can cause destruction of primary metabolites (Pokora and Tukaj 2010). The formation of ROS is a distinctive sign of metal toxicity owing to an imbalance between their generation and removal (Kováčik et al. 2017). Algal HM tolerance is based on two principal mechanisms: preventing HMs to enter into the cell through e.g. adsorbing them on the cell surface, and preventing bioavailability of toxic HMs inside the cell through e.g. complexation (Perales-Vela et al. 2006). Numerous protective mechanisms are induced in response to HMs in algal cells, allowing them to cope with the ROS excess, including the involvement of enzymatic (superoxide dismutase, SOD; ascorbate peroxidase, APX; catalase, CAT; glutathione reductase, GR) and non-enzymatic (ascorbic acid, glutathione, proline, cysteine or carotenoids) antioxidants (Perales-Vela et al. 2006; Simmons et al. 2009; Bajguz 2010).
Mechanisms of Uptake and Accumulation of Heavy Metals by Algae
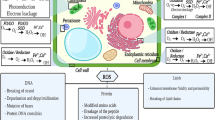
Mechanisms through which algae can remediate HMs include biosorption (a metabolism-independent passive binding process), and accumulation (a metabolism-dependent active uptake process) followed by intracellular localization and deposition (Ahmad et al. 2020; Salama et al. 2019) (Fig. 1). Any tolerance of algae towards HMs results from a combination of blocking HM entry into the cell and the activation of defence mechanisms that reduce HM toxicity (Salama et al. 2019; Ahmad et al. 2020; Sen Gupta et al. 2020).
Algae can absorb HMs to their extracellular matrix through several mechanisms, including electrostatic interactions among HM ions and ligands of the algal cell wall (e.g., ion exchange), complexation on the surface, micro-precipitation, and bonding to proteins and other polymers (Cheng et al. 2019; Salama et al. 2019; Ahmad et al. 2020). Algal cell walls consist mainly of polysaccharides, proteins, and lipids, all of which offer several functional groups with a high binding affinity for HM cations (Ahmad et al. 2020). For example, extracellular ligands such as organic acids and polypeptides can act as cation exchangers and complexing agents for HMs (Salama et al. 2019). However, components of the cell wall differ among algal species (Salama et al. 2019), and different sorption/binding pathways will therefore not be equally efficient for each HM (Andrade et al. 2005). Moreover, various environmental factors can influence the biosorption ability of algae, including pH, contact time, temperature, ionic strength, particle size, and coexistence of other contaminants (Park et al. 2010; Ahmad et al. 2020).
HMs can be uptaken into algal cells through transport systems on the cell membranes (Salama et al. 2019). Active transport systems have been described for various HMs in algae (e.g., Fortin and Campbell 2001; Kiran Marella et al. 2020). One transport system can take up multiple ions; thus, uptake can be competitive among nutrient and HM ions. After uptake into the cells, HMs may be compartmentalized and/or converted to more innocuous forms by their binding or precipitation (Kiran Marella et al. 2020). Metals can be transported intracellularly (Kiran Marella et al. 2020) and localized at intraprotoplast sites such as vacuoles or chloroplasts (Perales-Vela et al. 2006). Internal deposition involves the synthesis of metal-binding components or proteins (e.g., PCs, metallothioneins), which may function in detoxification (Perales-Vela et al. 2006).
The Role of Phytohormones in Algae Response to Heavy Metal Stress
In higher plants, phytohormones are chemical signaling molecules involved in a broad spectrum of physiological and biochemical processes at very low concentrations. Phytohormones, including auxins, GAs, CKs, BRs, ABA, jasmonic acid (JA), salicylic acid (SA), and ethylene (ET) have also been found in a variety of algae (Tarakhovskaya et al. 2007; Piotrowska et al. 2009; Stirk et al. 2013a, b; Tran and Pal 2014; Lu and Xu 2015) (Fig. 2). The most detailed data available on the algal phytohormone (phycohormone) system refer to marine macrophytes (Tarakhovskaya et al. 2007). Hormones of microscopic algae are poorly understood, which can be attributed to the extreme diversity of this group of organisms and the methodological difficulties of working with microscopic objects. However, principally the biological roles of phytohormones in microalgae and higher plants are comparable, as the former evolved from an algae lineage over 450 million years ago (Sanderson et al. 2004). In general, regarding five main phytohormone groups (ABA, auxins, CKs, GAs and ET), microalgae largely share phytohormone biosynthetic pathways with higher plants while much of the downstream regulation genes responsible for phytohormone signaling in response to the environmental factors need to be further investigated.
Phytohormone signalling in the response to heavy metal stress involves early growth inhibition by abscisic acid (ABA), and ethylene, alleviating reactive oxygen species (ROS) by promoting antioxidant production, and heavy metal detoxification and accumulation by stimulating thiols, glutathione (GSH) and phytochelatins (PCs) biosynthesis. The arrow demonstrates the promoting and/or increasing effects, whereas the flat end indicates the negative effects. Abbreviations: BR, brassinosteroid; CK, cytokinin; GA3, gibberellic acid; JA, jasmonic acid
Abscisic acid
ABA is an essential hormone involved in plant adaptation under adverse conditions (Nambara and Marion-Poll 2005; Wilkinson and Davies 2010). Plants respond to biotic and abiotic stress factors by increasing the amount of active (free) form of the hormone, which takes place primarily by hydrolysis of bound ABA (Xu et al. 2014). ABA can also alleviate detrimental stress effects in microalgae (Khasin et al. 2017). ABA content in Acutodesmus obliquus and C. vulgaris increased with the increasing intracellular Pb level, ultimately resulting in growth reduction (Bajguz 2010, 2011; Piotrowska-Niczyporuk et al. 2012, 2018a). A similar effect of the increased content of ABA as a protection against metal exposure was observed in various plant species (Hsu and Kao 2005; Ozfidan et al. 2012; Wang et al. 2013). Molecular mechanisms of ABA involvement in abiotic stress response in algae is less understood than the ABA signal transduction pathway in plants, with just some signaling components known and no ABA receptor genes identified (Lu and Xu 2015).
Auxins and Cytokinins
Auxins and CKs play a vital role in the growth and development of microalgae, as they are involved in the induction of cellular growth and division, and augmentation of photosynthetic activity (Bajguz 2010, 2011; Piotrowska-Niczyporuk et al. 2012). Under HM stress, reductions in endogenous levels of auxin (indole-3-acetic acid, IAA) and CKs (trans-zeatin, tZ) were reported in green alga, C. vulgaris (Bajguz 2011) and A. obliquus (Piotrowska-Niczyporuk et al. 2017) subjected to Pb applied at a high concentration (100 µM). The mechanisms of endogenous auxin and CK-mediated regulation of HM stress were mainly characterized using their exogenous counterparts. The introduction of exogenous auxins and CKs mitigated toxicity, promoted growth and development, and regulated HM sorption in C. vulgaris (Piotrowska-Niczyporuk et al. 2012), and A. obliquus (Piotrowska-Niczyporuk et al. 2018a).
Exogenous CKs were found to protect proteins and components of photosynthetic apparatus (chlorophylls, carotenoids, xanthophylls), and significantly reduce damaging effects of HMs on green algae, C. vulgaris (Piotrowska-Niczyporuk et al. 2012), and A. obliquus (Piotrowska-Niczyporuk et al. 2018b). CKs also alleviated HM toxicity by inhibiting ROS formation in C. vulgaris when it was challenged by Cd, Cu, or Pb (Piotrowska-Niczyporuk et al. 2012). The impact of CKs on ROS homeostasis is also crucial in A. obliquus responding to Pb stress (Piotrowska-Niczyporuk et al. 2018a).
By contrast, auxins were less effective in reducing ROS content in A. obliquus cells subjected to Pb stress (Piotrowska-Niczyporuk et al. 2018a). Previous observations also suggest that auxins alone without abiotic stress factors stimulate SOD activity and provoke H2O2 generation (Piotrowska-Niczyporuk et al. 2018b). The higher ROS level in auxin-treated cells under Pb stress may be correlated with auxin stimulation of cell enlargement and promotion of HM biosorption by A. obliquus cells (Piotrowska-Niczyporuk et al. 2018a). In C. vulgaris the exogenous auxins, IAA and IBA (indole-3-butyric acid), reduced Cu biosorption. IAA did not affect Pb and Cd accumulation in the algal cells, while exogenous IBA significantly stimulated biosorption of these HMs by C. vulgaris (Piotrowska-Niczyporuk et al. 2012). Similarly, the application of auxins can also increase Pb accumulation in plants, resulting in a higher metal-removal yield, which is the primary objective of phytoextraction techniques (Liu et al. 2007; Fässler et al. 2010).
IAA and IBA are the predominant natural forms of auxin in microalgae (Romanenko2016). The evidence suggests that metal transport through algal cell membranes and its cellular translocation may be improved through the exogenous application of these auxins. Overall, biological roles of auxins and CKs are similar in algae to what is known in higher plants, with some gene homologs and signaling pathway conserved between two kingdoms (Lu and Xu 2015). This suggests that exogenous auxin and CK treatment improves algal tolerance to toxicants and complements the functions of these endogenous phytohormones, thereby being an effective method and potential strategy to counteract the adverse effects of HMs.
Gibberellins
Not many reports are available regarding the biological functions of GAs in algae; however, their presence has been confirmed in multiple microalgae strains (Stirk et al. 2013b). The available reports suggest significant roles of GAs in the alleviation of metal toxicity in algae. A positive effect of GA3 was revealed on growth, protein contents, chlorophylls а and b, carotenoids, and monosaccharides in C. vulgaris exposed to HMs (Falkowska et al. 2011; Piotrowska-Niczyporuk et al. 2012). It was observed that GA3 activated defence responses and decreased oxidative damages by promoting the production of thiol compounds which could bind to HM ions (Bajguz 2002) in cells of C. vulgaris (Falkowska et al. 2011; Piotrowska-Niczyporuk et al. 2012). The content of HMs in C. vulgaris gradually increased during the experimental period under exogenous GA3 application. These results indicate that GA3 can help algae to withstand the toxic concentrations of Cd and Pb based upon the efficiency of cellular division in C. vulgaris (Falkowska et al. 2011). Like other groups of phytohormones in algae, GAs share many genes in biosynthesis pathway with higher plants while the molecular regulation of GA signaling in algae remains highly elusive (Lu and Xu 2015).
Ethylene
Functions of ET in improving plant tolerance to HM stress have been studied extensively; however, the data of ET with respect to effects on algal responses to HMs is scarce and understanding physiological roles of ET as a phycohormone requires further work. In a green microalgae, Haematococcous pluvialis, ET activity increased at elevated concentrations of cobalt (Co), manganese (Mn), and silver (Ag), while ET production was inhibited by copper (Cu). Conversely, zinc (Zn), iron (Fe), and magnesium (Mg) had no effect on the activity of ET (Maillard et al. 1993). The adverse effects of exogenous ET on chlorophyll content was reported in marine macroalgae, Ulva intestinalis (Plettner et al. 2005). Based on the available evidence, algae at least partially share plant ET biosynthesis pathway, with aminocyclopropane-1-carboxylic acid (ACC) being the intermediate precursor for ET biosynthesis (Maillard et al. 1993) in both groups.
Brassinosteroids
Biological activities of BRs in algae correspond to their functions recognized in higher plants with an important role in phytohormone crosstalk. Studies on the endogenous BRs suggest that the activation of the early and late C6-oxidation pathways lead to brassinolide (BL) production in algae. Exogenous BL partially mitigated the inhibitory effect of HMs in C. vulgaris by decreasing the accumulation of HMs in the cells and by stimulation of ABA, IAA, and tZ production. Even though HM exposure is not known to affect endogenous BL content (Bajguz 2011), the application of BRs to C. vulgaris cultures reduced the impact of HMs stress on growth and enhanced chlorophyll, sugar, and protein levels (Bajguz 2002, 2011).
BL inhibited the degradation of lipids resulting from the overproduction of ROS and increased the activity of antioxidative enzymes (SOD, APX, GR, CAT) and content of antioxidants (glutathione, ascorbate) in C. vulgaris cells treated with HMs (Cd, Pb, Cu) (Bajguz 2010). Exogenous BRs also caused a rapid response in C. vulgaris by acceleration of phytochelatin (PC) synthesis (Bajguz 2010). These observations suggest that BRs can mitigate the effects of metal stress in microalgal cells through the precise regulation of the ROS level (Bajguz 2019).
Overall, the increase of resistance using exogenous BRs was reflected in the improvement of algal growth in the presence of HMs, indicating the ameliorative influence of BRs on the inhibitory effect of HMs. Moreover, lowering the pH in cell wall spaces stimulated the growth of C. vulgaris in presence of exogenous BRs (Bajguz 2000) while it is known that the optimum pH of metal ion sorption is between 4 and 6.
Jasmonic Acid
The studies on the effect of the exogenous JA treatments suggest potentially significant roles of this phytohormone in elevating algae tolerance to HM stress. Presence of exogenous JA exacerbated Cd, Cu, or Pb toxicity accompanied by an increase in metal biosorption, lipid peroxidation and H2O2 level in C. vulgaris. Indicators of cell health and their ability to deal with HM like cell number, chlorophyll levels, carotenoids, monosaccharides, soluble proteins, ascorbate and glutathione content, as well as antioxidant enzyme activity, were considerably reduced in response to JA treatment under HM exposure (Piotrowska-Niczyporuk et al. 2012). These findings correspond well with other studies reporting that high amounts of JA are accumulated during HM stress in A. thaliana (Maksymiec 2007), accelerating the senescence program and algal cell death (Czerpak et al. 2006).
Phytohormonal Crosstalk
Phytohormones never act alone, but they rather work together or against each other in complex signalling networks. Under HM stress, the hormonal crosstalk in higher plants has been studied extensively, while the phycohormone interactions in microalgae are still poorly understood. The present evidence suggests that the endogenous auxins and CKs interact antagonistically with ABA in response to HM stress in algae (Piotrowska-Niczyporuk et al. 2018b) resulting in a highly controlled homeostasis between the different signaling molecules that allow optimal growth and survival response of algae under stress factors. Although the levels of endogenous BRs did not change under HM stress, their exogenous application promoted production of ABA, IAA, and tZ (Bajguz 2002, 2011). As most of the available results are derived from studies using exogenous phytohormones, it is worth to note that such treatments may alter the concentrations of the endogenous hormones and other signalling compounds, interacting with various signal transduction pathways. Given what little is known about it, phycohormone crosstalk research is clearly at its early stage and it is an avenue that should be more strongly developed in the coming years with the goal to optimize phytoremediation efficiency using algae. The promising examples show that simultaneous treatment of algae under stress conditions with multiple exogenous phytohormones can lead to the enhanced production of high-value compounds (Han et al. 2018).
Summary and Future Prospects
Alga species offer an appealing alternative for the novel and effective bioremediation techniques that can be applied for HM-contaminated wastewater. The unique ecology, morphological and physiological characteristics of algae create new options, not available for traditional methods that use plants for HM bioremoval. Some of the undeniable advantages of using algae as the cost-effective, biological agents for neutralizing excess HM presence in the environment, such as lower dependence on the climate conditions or location of the contaminated site, have been mentioned in the introduction of this review. Furthermore, algae often exhibit specificity toward different kinds of HMs combined with high removal efficacy at low concentrations (Sen Gupta et al. 2020).
Nevertheless, introducing bioremediation with algae on the broader, industrial scale requires careful consideration of any possible disadvantages of the proposed solution. One of the important issues is the release of algal phycotoxins that might diminish the efforts of successful algal bioremediation of HMs. Many biotoxins (e.g., microcystin, anatoxins, cylindrospermopsins) can be released as the effect of metabolic processing of the absorbed HMs as it is often observed during the bloom of toxic algae (Dorantes-Aranda et al. 2015). Therefore, the development of bioremediation technologies that involve modification of phytohormone metabolism should focus also on reducing the possible side effects of the increased concentration of HMs on the biochemical profile of algal biomass.
Chemical composition of algal biomass used for HM detoxification is important in the context of potential post-processing of algal materials that would additionally increase the sustainability of the developed technologies. Utilization of algal biomass in the biofuel, food and other industries have been already implemented (Khan et al. 2018) and growth enhancement by programmed phytohormone activities might facilitate post-processing of algae biomass after extraction of the absorbed HMs.
Considering the promising results of the here-reviewed literature that show algae’s great capabilities to reduce HM pollution, it is justifiable to continue work on the further improvement of the applicability of these techniques and their industrial scale implementation. Exploration of the roles phytohormones have in HM stress alleviation in algae could be the new and successful avenue leading to the development of the modern, more effective bioremediation technologies utilizing algae. Along with optimizing exogenous phytohormone treatment for improved HM tolerance, another important area of research that can accelerate our understanding of the phytohormones in algal metabolism is the genetic modification of algae aiming to enhance the functions of phytohormones for more effective detoxification of HMs. Examples of positive outcomes of the genetic engineering approach for improved bioremediation capacities are already known in the case of various transgenic algal strains (Ibuot et al. 2017; Chang et al. 2019).
References
Ahmad S, Pandey A, Pathak VV, Tyagi VV, Kothari R (2020) Phycoremediation: algae as eco-friendly tools for the removal of heavy metals from wastewaters. In: Bharagava RN, Saxena G (eds) Bioremediation of industrial waste for environmental safety. Springer, Singapore, pp 53–76
Andrade AD, Rollemberg MCE, Nóbrega JA (2005) Proton and metal binding capacity of the green freshwater alga Chaetophora elegans. Process Biochem 40:1931–1936. https://doi.org/10.1016/j.procbio.2004.07.007
Bajguz A (2000) Blockade of heavy metals accumulation in Chlorella vulgaris cells by 24-epibrassinolide. Plant Physiol Biochem 38:797–801. https://doi.org/10.1016/S0981-9428(00)01185-2
Bajguz A (2002) Brassinosteroids and lead as stimulators of phytochelatins synthesis in Chlorella vulgaris. J Plant Physiol 159:321–324. https://doi.org/10.1078/0176-1617-00654
Bajguz A (2010) An enhancing effect of exogenous brassinolide on the growth and antioxidant activity in Chlorella vulgaris cultures under heavy metals stress. Environ Exp Bot 68:175–179. https://doi.org/10.1016/j.envexpbot.2009.11.003
Bajguz A (2011) Suppression of Chlorella vulgaris growth by cadmium, lead, and copper stress and its restoration by endogenous brassinolide. Arch Environ Contam Toxicol 60:406–416. https://doi.org/10.1007/s00244-010-9551-0
Bajguz A (2019) Brassinosteroids in microalgae: application for growth improvement and protection against abiotic stresses. In: Hayat S, Yusuf M, Bhardwaj R, Bajguz A (eds) Brassinosteroids: Plant growth and development. Springer, Singapore, pp 45–58
Cheng SY, Show P-L, Lau BF, Chang J-S, Ling TC (2019) New prospects for modified algae in heavy metal adsorption. Trends Biotechnol 37:1255–1268. https://doi.org/10.1016/j.tibtech.2019.04.007
Czerpak R, Piotrowska A, Szulecka K (2006) Jasmonic acid affects changes in the growth and some components content in alga Chlorella vulgaris. Acta Physiol Plant 28:195–203. https://doi.org/10.1007/BF02706531
Dorantes-Aranda JJ, Seger A, Mardones JI, Nichols PD, Hallegraeff GM (2015) Progress in understanding algal bloom-mediated fish kills: the role of superoxide radicals, phycotoxins and fatty acids. PLoS ONE. https://doi.org/10.1371/journal.pone.0133549
El-Sheekh MM, Mahmoud YA-G (2017) Technological approach of bioremediation using microbial tools. In: Bhakta JN (ed) Handbook of research on inventive bioremediation techniques. IGI Global, Hershey, pp 134–154
Falkowska M, Pietryczuk A, Piotrowska A, Bajguz A, Grygoruk A, Czerpak R (2011) The effect of gibberellic acid (GA3) on growth, metal biosorption and metabolism of the green algae Chlorella vulgaris (Chlorophyceae) Beijerinck exposed to cadmium and lead stress. Pol J Environ Stud 20:53–59
Fässler E, Evangelou MW, Robinson BH, Schulin R (2010) Effects of indole-3-acetic acid (IAA) on sunflower growth and heavy metal uptake in combination with ethylene diamine disuccinic acid (EDDS). Chemosphere 80:901–907. https://doi.org/10.1016/j.chemosphere.2010.04.077
Fortin C, Campbell PG (2001) Thiosulfate enhances silver uptake by a green alga: role of anion transporters in metal uptake. Environ Sci Technol 35:2214–2218. https://doi.org/10.1021/es0017965
Han X, Zeng H, Bartocci P, Fantozzi F, Yan Y (2018) Phytohormones and effects on growth and metabolites of microalgae: a review. Fermentation 4(2):25. https://doi.org/10.3390/fermentation4020025
Hsu YT, Kao CH (2005) Abscisic acid accumulation and cadmium tolerance in rice seedlings. Physiol Plant 124:71–80. https://doi.org/10.1111/j.1399-3054.2005.00490.x
Ibuot A, Dean AP, McIntosh OA, Pittman JK (2017) Metal bioremediation by CrMTP4 over-expressing Chlamydomonas reinhardtii in comparison to natural wastewater-tolerant microalgae strains. Algal Res 24:89–96. https://doi.org/10.1016/j.algal.2017.03.002
Khan MI, Shin JH, Kim JD (2018) The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Fact 17(1):36. https://doi.org/10.1186/s12934-018-0879-x
Khasin M, Cahoon RE, Alvarez S, Beckeris R, Eyun S-i, Jia Q, Riethoven J-J, Nickerson KW, Riekhof WR (2017) Synthesis, secretion, and perception of abscisic acid regulates stress responses in Chlorella sorokiniana, bioRxiv. https://doi.org/10.1101/180547
Kiran Marella T, Saxena A, Tiwari A (2020) Diatom mediated heavy metal remediation: a review. Bioresour Technol 305:123068. https://doi.org/10.1016/j.biortech.2020.123068
Kováčik J, Klejdus B, Babula P, Hedbavny J (2017) Ascorbic acid affects short-term response of Scenedesmus quadricauda to cadmium excess. Algal Res 24:354–359. https://doi.org/10.1016/j.algal.2017.04.026
Liu D, Li T, Yang X, Islam E, Jin X, Mahmood Q (2007) Enhancement of lead uptake by hyperaccumulator plant species Sedum alfredii hance using EDTA and IAA. Bull Environ Contam Toxicol 78:280–283. https://doi.org/10.1007/s00128-007-9121-y
Lu Y, Xu J (2015) Phytohormones in microalgae: a new opportunity for microalgal biotechnology? Trends Plant Sci 20:273–282. https://doi.org/10.1016/j.tplants.2015.01.006
Maillard P, Thepenier C, Gudin C (1993) Determination of an ethylene biosynthesis pathway in the unicellular green alga, Haematococcus pluvialis. Relationship between growth and ethylene production. J Appl Phycol 5:93–98. https://doi.org/10.1007/BF02182426
Maksymiec W (2007) Signaling responses in plants to heavy metal stress. Acta Physiol Plant 29:177–187. https://doi.org/10.1007/s11738-007-0036-3
Mansour SA (2014) Heavy metal contamination as a global problem and the need for prevention/reduction measurements. In: Bhat R, Gómez-López VM (eds) Practical food safety: contemporary issues and future directions. Wiley Blackwell, Chichester, pp 257–280
Nambara E, Marion-Poll A (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56:165–185. https://doi.org/10.1146/annurev.arplant.56.032604.144046
Noble A, Kisiala A, Galer A, Clysdale D, Emery RN (2014) Euglena gracilis (Euglenophyceae) produces abscisic acid and cytokinins and responds to their exogenous application singly and in combination with other growth regulators. Eur J Phycol 49:244–254. https://doi.org/10.1080/09670262.2014.911353
Ozfidan C, Turkan I, Sekmen AH, Seckin B (2012) Abscisic acid-regulated responses of aba2-1 under osmotic stress: the abscisic acid-inducible antioxidant defence system and reactive oxygen species production. Plant Biol (Stuttg) 14:337–346. https://doi.org/10.1111/j.1438-8677.2011.00496.x
Park D, Yun Y-S, Park JM (2010) The past, present, and future trends of biosorption. Biotechnol Bioprocess E 15:86–102. https://doi.org/10.1007/s12257-009-0199-4
Perales-Vela HV, Peña-Castro JM, Cañizares-Villanueva RO (2006) Heavy metal detoxification in eukaryotic microalgae. Chemosphere 64:1–10. https://doi.org/10.1016/j.chemosphere.2005.11.024
Piotrowska A, Bajguz A, Godlewska-Żyłkiewicz B, Czerpak R, Kamińska M (2009) Jasmonic acid as modulator of lead toxicity in aquatic plant Wolffia arrhiza (Lemnaceae). Environ Exp Bot 66:507–513. https://doi.org/10.1016/j.envexpbot.2009.03.019
Piotrowska-Niczyporuk A, Bajguz A, Zambrzycka E, Godlewska-Żyłkiewicz B (2012) Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Plant Physiol Biochem 52:52–65. https://doi.org/10.1016/j.plaphy.2011.11.009
Piotrowska-Niczyporuk A, Bajguz A, Zambrzycka-Szelewa E (2017) Response and the detoxification strategies of green alga Acutodesmus obliquus (Chlorophyceae) under lead stress. Environ Exp Bot 144:25–36. https://doi.org/10.1016/j.envexpbot.2017.08.013
Piotrowska-Niczyporuk A, Bajguz A, Zambrzycka-Szelewa E, Bralska M (2018a) Exogenously applied auxins and cytokinins ameliorate lead toxicity by inducing antioxidant defence system in green alga Acutodesmus obliquus. Plant Physiol Biochem 132:535–546. https://doi.org/10.1016/j.plaphy.2018.09.038
Piotrowska-Niczyporuk A, Bajguz A, Kotowska U, Bralska M, Talarek-Karwel M (2018b) Growth, metabolite profile, oxidative status, and phytohormone levels in the green alga Acutodesmus obliquus exposed to exogenous auxins and cytokinins. J Plant Growth Regul 37:1159–1174. https://doi.org/10.1007/s00344-018-9816-9
Plettner I, Steinke M, Malin G (2005) Ethene (ethylene) production in the marine macroalga Ulva (Enteromorpha) intestinalis L. (Chlorophyta, Ulvophyceae): effect of light-stress and co-production with dimethyl sulphide. Plant Cell Environ 28:1136–1145. https://doi.org/10.1111/j.1365-3040.2005.01351.x
Pokora W, Tukaj Z (2010) The combined effect of anthracene and cadmium on photosynthetic activity of three Desmodesmus (Chlorophyta) species. Ecotoxicol Environ Saf 73:1207–1213. https://doi.org/10.1016/j.ecoenv.2010.06.013
Romanenko EA, Kosakovskaya IV, Romanenko PA (2016) Phytohormones of microalgae: biological role and involvement in the regulation of physiological processes. Pt II. Cytokinins and gibberellins. Algologia 26:203–229. https://doi.org/10.15407/alg26.02.203
Salama E-S, Roh H-S, Dev S, Khan MA, Abou-Shanab RAI, Chang SW, Jeon B-H (2019) Algae as a green technology for heavy metals removal from various wastewater. World J Microbiol Biotechnol 35:75. https://doi.org/10.1007/s11274-019-2648-3
Sanderson MJ, Thorne JL, Wikström N, Bremer K (2004) Molecular evidence on plant divergence times. Am J Bot 91:1656–1665. https://doi.org/10.3732/ajb.91.10.1656
Selvi A, Rajasekar A, Theerthagiri J, Ananthaselvam A, Sathishkumar K, Madhavan J, Rahman PKSM (2019) Integrated remediation processes toward heavy metal removal/recovery from various environments-a review. Front Environ Sci 7:171. https://doi.org/10.3389/fenvs.2019.00066
Sen Gupta G, Yadav G, Tiwari S (2020) Bioremediation of heavy metals: a new approach to sustainable agriculture. In: Upadhyay AK, Singh R, Singh DP (eds) Restoration of wetland ecosystem: a trajectory towards a sustainable environment. Springer, Singapore, pp 195–226
Sheath RG, Wehr JD (2015) Introduction to the freshwater algae. In: Wehr JD (ed) Freshwater algae of North America. Elsevier, New York, pp 1–11
Simmons DBD, Hayward AR, Hutchinson TC, Emery RJN (2009) Identification and quantification of glutathione and phytochelatins from Chlorella vulgaris by RP-HPLC ESI-MS/MS and oxygen-free extraction. Anal Bioanal Chem 395:809–817. https://doi.org/10.1007/s00216-009-3016-1
Stirk WA, Ördög V, Novák O, Rolčík J, Strnad M, Bálint P, van Staden J (2013a) Auxin and cytokinin relationships in 24 microalgal strains. J Phycol 49:459–467. https://doi.org/10.1111/jpy.12061
Stirk WA, Bálint P, Tarkowská D, Novák O, Strnad M, Ördög V, van Staden J (2013b) Hormone profiles in microalgae: gibberellins and brassinosteroids. Plant Physiol Biochem 70:348–353. https://doi.org/10.1016/j.plaphy.2013.05.037
Tarakhovskaya ER, Maslov YI, Shishova MF (2007) Phytohormones in algae. Russ J Plant Physiol 54:163–170. https://doi.org/10.1134/S1021443707020021
Tran L-SP, Pal S (2014) Phytohormones: a window to metabolism, signaling and biotechnological applications. Springer, New York
Wang J, Chen J, Pan K (2013) Effect of exogenous abscisic acid on the level of antioxidants in Atractylodes macrocephala Koidz under lead stress. Environ Sci Pollut Res Int 20:1441–1449. https://doi.org/10.1007/s11356-012-1048-0
Xu Z-Y, Yoo Y-J, Hwang I (2014) ABA conjugates and their physiological roles in plant cells. In: Zhang D-P (ed) Abscisic acid: metabolism, transport and signaling. Springer, Dordrecht, pp 77–87
Wilkinson S, Davies WJ (2010) Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant Cell Environ 33:510–525. https://doi.org/10.1111/j.1365-3040.2009.02052.x
Acknowledgements
This work was conceptualized with the help of Aaron Woodcock. Financial support came from the Ontario Trillium Scholarship for PhD Studies to VS, and from NSERC Discovery Grant No. RGPIN-05436 to RJNE.
Author information
Authors and Affiliations
Contributions
TQN and VS contributed to conceptualization and literature research. The first manuscript draft was written by TQN and VS. AK and RJNE commented on previous drafts and critically revised the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
VS was invited as Assistant Editor for the International Institute for Environmental Studies (IIES) Special Issue. All other authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nguyen, T.Q., Sesin, V., Kisiala, A. et al. The Role of Phytohormones in Enhancing Metal Remediation Capacity of Algae. Bull Environ Contam Toxicol 105, 671–678 (2020). https://doi.org/10.1007/s00128-020-02880-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-020-02880-3