Abstract
The constant spread of heavy metal contamination creates an increasing global environmental issue that results in considerable deterioration of land and water ecosystems leading to a decline in the health of plants, animals and humans. Novel, algal-based filtration technologies have been gaining a great deal of attention given their eco-friendly, effective and easy to implement processes. This review focuses on the potential roles that phytohormones can play in heavy metal stress response in microalgae. It emphasizes phytohormone efficiency and proposes the use of these signaling molecules for enhanced metal stress alleviation in microalgae. Furthermore, future implications for algal-based filtration technologies involving modifications of phytohormone metabolism towards improved heavy metal biodegradation rates are presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heavy metals: pollution, toxicity, and bio-removal
Heavy metal (HM) pollution is a worldwide environmental concern. Various industrial processes and the use of chemicals in many areas deliberately or accidentally release toxic HMs into the environment. Effluent wastes are released to rivers and wetlands causing water pollution, seriously damaging ecosystems and threatening human health (Afonne and Ifediba 2020). Among many HMs that originate mainly from agro-industrial wastewaters, nickel (Ni), copper (Cu), cadmium (Cd), arsenic (As), lead (Pb), and chromium (Cr) are considered as priority pollutants, owing to their high toxicity and non-biochemically degradable properties (Zeraatkar et al. 2016; Azimi et al. 2017). Exposure to HM contaminants leads to cytotoxicity, carcinogenicity, teratogenicity, and mutagenicity, thus increasing the risk of cancer and cancer-related diseases (Zeraatkar et al. 2016; Afonne and Ifediba 2020). Several physical and chemical methods can be used for HM removal from the environment, including replacement or washing of soil, metal precipitation, oxidation, ion exchange, or adsorption in water. However, these methods are costly and time- and labor-consuming and, in natural ecosystems, their success is often limited due to the large areas of contaminated land or water. These methods can also create a form of secondary pollution (Khalid et al. 2017).
Bio-removal of HMs has therefore been gaining a great deal of attention given its eco-friendly, effective, and easy to operate processes. Algal cells present diverse adaptive responses and physiochemical mechanisms to clean up metal contamination from water, which make them interesting material to explore for their bioremediation potential (Zeraatkar et al. 2016; Qin et al. 2020).
Microalgae: powerful organisms for bioremediation
The use of microalgae as bioremediation agents has become a promising solution for cleaning up HMs from the environment (Qin et al. 2020). Algae acclimate to, accumulate, and remove HMs from the polluted habitats using diverse defense systems, including tolerance against HM toxicity through ROS detoxification (Moenne et al. 2016) and bio-removal of HMs via bioadsorption, bioaccumulation, and chelation (Urrutia et al. 2019; Khatiwada et al. 2020). Algae-based bioremediation has been a focus of attention owing to the associated removal capacity, low cost, time and energy saving, and ease of handling operations (Urrutia et al. 2019). As a downstream bioremediation process, the remaining algal biomass could be disposed of with a much lower environmental footprint or used in industrial processing to produce biofuel or algal-derived, beneficial compounds. These advantages could be intensified through the application of phytohormones (Piotrowska-Niczyporuk et al. 2012; Sytar et al. 2019; Zhao et al. 2019a).
Phytohormones: elegant and effective signal molecules to enhance bioremediation
Phytohormones are the signaling molecules that are responsible for many important plant physiological activities, and they also play a pivotal role in HM stress tolerance in higher plants (Bücker-Neto et al. 2017). In microalgae, phytohormones help to increase cell growth, protein, and metabolite accumulation and they can improve abiotic stress tolerance (Zhao et al. 2019a). Enhanced algal biomass production can be achieved through the direct supply of phytohormones, such as the exogenous treatment of CKs (induction of growth rate, pigment and monosaccharides content) (Piotrowska and Czerpak 2009; de Jesus Raposo and de Morais 2013), auxin (boosted biomass, lipid and fatty acid (FA) accumulation) (Liu et al. 2017b; Salama et al. 2017), abscisic acid (ABA) (enhanced cell concentration, lipid and triacylglycerol content) (Contreras-Pool et al. 2016), gibberellins (GAs) (high value polyunsaturated FA production) (Udayan et al. 2018), or ethylene (ET) (saturated FA accumulation) (Kim et al. 2016).
Many findings linking phytohormones with various abiotic stress responses in algae have been reported in examples such as ABA (improved drought stress tolerance) (Kobayashi et al. 1997), nitrogen depletion (Sulochana and Arumugam 2016), brassinosteroids (BRs) (involved in short-term heat stress response) (Bajguz 2009) and auxin (improved salt stress tolerance) (El Arroussi et al. 2015). Phytohormones such as GAs, auxin, and CKs enhance HM stress tolerance and yield of Chlorella vulgaris (Falkowska et al. 2011; Piotrowska-Niczyporuk et al. 2012). Phytohormones are also helpful in reducing HM toxicity by increasing antioxidant enzyme activities (Piotrowska-Niczyporuk et al. 2012). Additionally, phytohormones are possibly beneficial in alleviating the negative effects of HMs via the inducement of non-enzymatic antioxidant components, such as proline and astaxanthin (Gao et al. 2012a, b; Lee et al. 2016). Algal cells with optimized phytohormone profiles would present improved growth and stress tolerance and open new windows for future water waste treatment practices (Fig. 1).
Timeline identifying important scientific milestones in phytohormone research in algae. CK cytokinin, ET ethylene, GA gibberellin, JA jasmonic acid, ROS reactive oxygen species, SA salicylic acid. Based on Pratt (1938); Mowat (1965); Stewart et al. (1968); Jacobs et al. (1985); Zhang et al. (1989); Maillard et al. (1993); Kobayashi et al. (1997); Yoshida et al. (2004); Lau et al. (2009); Piotrowska and Czerpak (2009); Le Bail et al. (2010); Falkowska et al. (2011); Piotrowska-Niczyporuk et al. (2012); Piotrowska-Niczyporuk and Bajguz (2014); Lu and Xu (2015); Lee et al. (2016); Liu et al. (2017a); Tiwari et al. (2018)
The above evidence shows that phytohormones can provide a new approach for developing effective and environmentally friendly bioremediation methods and resilient algal bio-filtration formulations. Thus, this review focuses on the current knowledge of the functional aspects and proposes phytohormone signaling pathways involved in algal HM stress response. Furthermore, novel concepts are emphasized using modern integrated -omic techniques to realize algal-based bioremediation technologies.
Exogenous phytohormone application can advance algae-based bioremediation
Phytohormones are critical for a wide range of HM stress tolerance mechanisms in higher plants (Shukla et al. 2017; Table 1). Exogenous treatment with phytohormones have been utilized to impact a broad spectrum of physiological and biochemical processes in algae, including abiotic stress tolerance, biomass enhancement, and accumulation of FAs, oil, and other valuable metabolites. However, regulatory functions of phytohormones in HM stress alleviation in algae remain relatively unexplored as compared with higher plants. To date, the model green alga C. vulgaris is the most successful algal system with significant influences of phytohormones for HM stress tolerance. Among the HMs, cadmium (Cd), lead (Pb), and copper (Cu) are highly phytotoxic metals that negatively impact general cellular metabolism and disturb ROS balance. Metal bio-adsorption and reduction of oxidative-induced damage under HM exposure can be directly controlled by CKs, GAs and auxin (Table 1).
CKs, GAs, and auxin regulate Cu, Pb, and Cd tolerance through the ROS detoxification
Multiple roles of CKs in HM stress tolerance
CKs are responsible for many important physiological activities, and they also play a pivotal role in HM stress tolerance (Mohan et al. 2016; Jalmi et al. 2018). The manipulations of CKs during HM stress have targeted either CK biosynthesis/degradation or enhanced levels via exogenous CK treatment, and these have revealed promising outcomes in higher plants (Table 1). For example, exogenous CK treatment mitigated Cd-induced damage in pea seedlings (Al-Hakimi 2007). Increased endogenous CK levels in tobacco resulting from overexpression of a CK biosynthetic gene (IPT) enhanced plant tolerance to Cu stress, and this was explained by an increased transcriptional pattern of a metallothionein-like gene (Thomas et al. 2005). In Arabidopsis (A. thaliana), CK depletion caused by mutations in the ipt1, ipt3, ipt5, and ipt7 genes activated selenium (Se) tolerance; induced activities of catalase (CAT), ascorbate peroxidase (APX), and glutathione peroxidase (GPX); and increased glutathione (GSH) content (Jiang et al. 2019). CK-deficient Arabidopsis and tobacco plants overexpressing a CK degradation enzyme (CKX1) presented higher accumulations of thiol compounds like phytochelatins (PCs), leading to improved As tolerance (Mohan et al. 2016).
CKs regulate a wide range of important cellular processes in both micro- and macroalgal species including photorespiration in Chlamydomonas reinhardtii (Tian et al. 2006), carbon metabolism in C. vulgaris (Piotrowska and Czerpak 2009), or photosynthesis in Gracilaria caudata (Souza and Yokoya 2016). Exogenous application of CKs and its combined treatment with ABA induced 1.4-fold increase in cell yield in Euglena gracilis after 144 h of cultivation (Noble et al. 2014). CK supply improved salt stress tolerance in Haematococcus pluvialis and Dunaliella salina grown under 10% salinity (3.2-fold and 4.1-fold increase in cell number, respectively) (de Jesus Raposo and de Morais 2013). When exposed to nitrogen depletion, a time-series transcriptional analysis showed an activation of the ABA biosynthetic pathway and antagonistic transcription of CK biosynthesis genes, indicating there are antagonistic roles between CKs and ABA (Lu et al. 2014). In the context of hormonal cross-talk, CKs (10 nM tZ) interacted synergistically with BR (10 nM brassinolide (BL)) in C. vulgaris, leading to improved cell growth (almost 4 time increase in cell density) and accumulation of proteins (2.5-fold increase), chlorophyll (at least 3-fold increase), and monosaccharides (3-fold increase) (Bajguz and Piotrowska-Niczyporuk 2014). Regarding HM stress tolerance, CKs alleviated toxic effects of Cd, Pb, and Cu on growth of C. vulgaris (order of CKs stimulating properties: DPU (N,N′-diphenylurea) > CPPU (forchlorfenuron) > TDZ (thidiazuron) > tZ (trans-zeatin) > BA (N6-benzyladenine) > Kin (kinetin) > 2iP (2-isopentenyladenine)) (Piotrowska-Niczyporuk et al. 2012).
Apart from regulatory functions in multiple physiochemical processes, CKs have a capacity to control scavenger ROS and protect cellular proteins, pigments, and sugars. This makes CKs outstanding molecules for algal HM tolerance. Exogenous application of CKs stimulated antioxidant enzymes (superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT)) and caused ascorbate and GSH accumulation (over 2-fold higher with various HM treatment), reducing oxidative stress expressed by lipid peroxidation and hydrogen peroxide level in C. vulgaris. With respect to metabolite levels under metal exposure (Cd/Cu/Pb), CKs improved protein content (11–85% recovery of protein), carotenoids (60–71%), and monosaccharide accumulation (order of stimulating properties: DPU > CPPU > TDZ > Z > BA > Kin > 2iP) (Piotrowska-Niczyporuk et al. 2012). Exogenous CK (tZ) application reduced Pb toxicity through the manipulation of the endogenous phytohormones (auxins and CKs) and phytochelatin (PC) precursor levels in the green algae, Acutodesmus obliquus (Piotrowska-Niczyporuk et al. 2020). The above studies show that CKs help control metal stress tolerance in algae through the modification of the ROS detoxification system and regulation of main cellular pathways.
GAs associate with ROS systems during HM stress
In higher plants, exogenous GA treatment increased antioxidant potential and reduced oxidative damage under Cr, Cd, and Ca stresses (Meng et al. 2009; Gangwar et al. 2011; Siddiqui et al. 2011; Zhu et al. 2012). At a molecular level, GAs reduce Cd toxicity via suppression of expression of a Cd uptake transporter gene. The functions of GAs in providing protection to HM stress through ROS systems are presented in Table 1. Many deleterious effects of HM toxicities (Cu, Cd, and Pb) on the growth of C. vulgaris were reduced by supplementing cultures with gibberellin (GA3) (Piotrowska-Niczyporuk et al. 2012). Under the concentration of 100 μM of Cu, Cd, and Pb, GA3 had a positive effect on protein (6–14% increase), chlorophyll a (20–29% increase), chlorophyll b (28–52% increase), monosaccharides (~ 30% increase), ascorbate (52–76% increase), and GSH (58–79% increase) content (Piotrowska-Niczyporuk et al. 2012). GA3 significantly improved carotenoid content, and SOD and CAT activities under Cu exposure, surpassing its effect when exposure involved similar concentrations of Cd and Pb (Piotrowska-Niczyporuk et al. 2012). Hence, GAs appear to be involved in HM stress tolerance in algae and to be associated with photosynthesis pathways and ROS networks (Fig. 2). Moreover, GA3 (10−5 M) induced the biosorption capacity of C. vulgaris, thus enhancing cell capacity to bioaccumulate toxic metals (Pb/Cd) (Falkowska et al. 2011). GAs also induce FAs and lipid biosynthesis in three different algae (Chlorella pyrenoidosa, Nannochloropsis oceanica and Aurantiochytrium sp. (Yu et al. 2016; Du et al. 2017; Udayan et al. 2018), suggesting that FA and lipid pathways are regulated by GAs under HM stress.
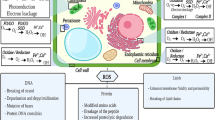
The proposed model of action of phytohormones in HM stress tolerance in algae. a ABA, CKs, and GAs’ function as regulators of Cu, Pb, and Cd biosorption and toxicity. b Proposed synergistic functions of BRs with other hormones. c Regulatory action of ABA, ET, JA, and SA under HM stress through antioxidant systems. d Key ROS processing pathways under regulation of phytohormones during HM stress in algae (adapted from Mhamdi and Van Breusegem (2018). e Non-enzymatic oxidant system associated with phytohormones under HM treatment. ABA abscisic acid, APX ascorbate peroxidase, BRs brassinosteroids, CAT catalase, Cd cadmium, CK cytokinin, Cu copper, ET ethylene, FA fatty acid, GA gibberellin, HM heavy metal, JA jasmonic acid, Pb lead, ROS reactive oxygen species, SA salicylic acid, SOD: superoxide dismutase
ROS and their detoxification are auxin-dependent components in algae
Auxin is directly involved in HM stress in higher plants ((Wang et al. 2015; Jalmi et al. 2018; Table 1). Although manipulation of auxin polar transport genes (aux1-7 and pin2-1) had no effect on lateral root formation under Cd stress (Hu et al. 2013), exogenously applied auxin interacted with miRNA to regulate HMs (Srivastava et al. 2013). In algae, the auxin role is mainly associated with the ROS detoxification system under HM stress. Even without HM stress, auxin stimulates enzymatic (APX, CAT, SOD) and non-enzymatic (ascorbate, GSH) antioxidant systems and reduces peroxidation and hydrogen peroxide levels (Piotrowska-Niczyporuk and Bajguz 2014). These components of cellular redox systems have a prominent role under the regulation of auxin-dependent processes. HM treatment in the combination with auxin-related ROS detoxification forms the auxin-dependent processes in C. vulgaris (Zhao et al. 2019a). In this context, auxin reduces hydrogen peroxide and malondialdehyde (MDA) content, which is attributed to the improved activities of ROS detoxification components (SOD, CAT, and APX) and enhanced ascorbate (43–57%) and GSH (24–40%) levels, ultimately leading to the improved cell growth under Cu, Pb, and Cd stress (order of auxin stimulating properties: IAA (indole-3-acetic acid) > NAA (1-naphthaleneacetic acid) > PAA (phenylacetic acid) > IBA (indole-3-butyric acid) (Piotrowska-Niczyporuk et al. 2012). In algae A. obliquus, exogenous application of auxins (0.01 μM IAA, 0.1 μM IBA, 0.1 μM PAA) stimulated the coordinated activation of metal tolerance mechanisms, resulting in an increased in PC synthase activity and accumulation of PCs and their precursors, enhancing Pb sequestration (Piotrowska-Niczyporuk et al. 2020).
Do ABA and BRs have synergistic function with other phytohormones in HM stress responses?
In consideration of in planta studies and potential cross-talk with other phytohormones under stress, ABA is a key player in plant HM stress responses. An elevated level of ABA under Zn, Pb, and As exposure occurs in higher plants (Atici et al. 2005; Srivastava et al. 2013). Exogenous supply of ABA increased antioxidant activities and reduced Pb-induced oxidative damage in the Chinese herb, Atractylodes macrocephala (Wang et al. 2013), whereas in poplar tree, ABA regulated Zn uptake and detoxification, reducing Zn concentration (Shi et al. 2015). In black mustard, ABA induced accumulation of miR159, which regulates GA signaling and ET biosynthesis, thus playing an important role in the As stress response (Srivastava et al. 2013). Thus, ABA can reverse the toxic effects of HMs and function as a central cross-talking agent among other plant hormone groups in higher plants. Metal-induced toxicity on higher plant and algal growth is concomitant with an increase in cellular ABA levels (Piotrowska-Niczyporuk et al. 2017). In A. obliquus cells, exposure to Pb stress increased ABA level by 111% as compared with control. The ameliorative effects of exogenous application of CKs (tZ, Kin, DPU), auxins (IAA, IBA, PAA), and BR (EBL- 24-epibrassinolide) on A. obliquus growth and tolerance upon Pb stress have been associated with a reduction in endogenous ABA level (Talarek-Karwel et al. 2020). Cellular events such as modification of carbohydrate, lipid, and amino acid fluxes are fundamental features in cell adjustment to effectively adapt to unfavorable conditions; thus, there is a redirection away from growth pathways and towards stress response investments (Signorelli et al. 2019). Moreover, modulation of lipid components plays a critical function in cell-adaptive responses during stress (Hou et al. 2016). In Scenedesmus quadricauda, ABA (2 μM) improved cell growth (2.1-fold increase in dry biomass yield after 24 h) while 5 μM concentration of ABA induced accumulation of saturated FA (12%) and decrease of unsaturated FA content (11%) under nitrogen (N) starvation (Sulochana and Arumugam 2016). A possible role of ABA in HM stress response in algae was further supported using ABA for inducing cell growth in Chlorella saccharophila (Contreras-Pool et al. 2016) and E. gracilis (Noble et al. 2014) and increasing lipid accumulation in C. saccharophila (Contreras-Pool et al. 2016). In hormonal cross-talk, ABA interacted synergistically with BRs (BL enhanced the ABA content) during short-term heat stress in C. vulgaris (Bajguz 2009). Exogenously applied tZ (10−7 M) combined with ABA (10−9 M) increased cell growth by 140% after 144 h in E. gracilis (Noble et al. 2014). Hence, ABA could be a promising candidate for manipulating HM stress response regulation in algae.
In higher plants, BRs upregulated gene expression of SOD, CAT, APX, and GR and thereby enhanced antioxidant systems and strengthen Cr stress tolerance ((Sharma et al. 2016; Table 1). Exogenous application of BRs also reduced Zn and Cd toxicity (Sharma and Bhardwaj 2007; Li et al. 2016). In algae, BRs have a synergistic effect with auxin and CKs on enhancing growth, metabolite content (proteins, chlorophylls and monosaccharides), and antioxidant responses in C. vulgaris (Bajguz and Piotrowska-Niczyporuk 2013, 2014). These data indicate that combinations of treatments with BRs and two other well-known HM stress alleviators (CKs and auxin) enhance algae HM tolerance. The study confirmed that EBL treatment increased BR, GA3, auxin, and CK content under Pb stress and reduced unfavorable consequences of HM on A. obliquus growth (Talarek-Karwel et al. 2020).
ET and SA potentially regulate HM stress through the enzymatic and non-enzymatic antioxidant systems
ET, SA, and JA have long been recognized for their roles in mediating the response to HM stress in plants mainly through the ROS detoxification system (Table 1). However, no reports have yet uncovered ET and SA roles in HM tolerance in algae. The decrease in cell number, chlorophyll, carotenoid, monosaccharide, soluble protein, ascorbate, and GSH content as well as antioxidant enzyme activity was a response to JA and HMs (Cu, Pb and Cd) in C. vulgaris (Piotrowska-Niczyporuk et al. 2012) suggesting that JA play a negative role in HM stress response in algae.
ET (200 μM ethephon) significantly improves metabolite profiles in C. vulgaris, especially that of proline content after 7-days treatment (Kim et al. 2016). Proline acts as an effective hydroxyl radical scavenger of ROS species under HM exposure (Smirnoff and Cumbes 1989). Similar to the case of sugars, proline has a critical role as a direct scavenger of ROS species in the protection of membranes, photosynthesis activities, and cellular homeostasis (Rejeb et al. 2014). 1-Aminocyclopropane-1-carboxylic acid (ACC), an ethylene precursor, also promoted astaxanthin accumulation (11 and 22% increase in 0.1 and 1 mmol L−1 ACC-treated cultures, respectively) and upregulated transcriptional patterns of SOD, and CAT genes, which have an important function in HM stress response in H. pluvialis (Vo et al. 2016). Astaxanthin is a carotenoid which showed the greatest increase in concentration and antioxidant capacity under abiotic stress (Zhao et al. 2019b), and this process has been associated with metal cation chelating capacity (Kim et al. 2016). A similar effect to ET was observed for SA. The effect of SA has been well documented in H. pluvialis, where SA improved astaxanthin content, APX, and CAT enzyme activities and transcriptional expression patterns of eight carotenoid genes (Raman and Ravi 2011). Overall, the role of three hormones, ET, JA and SA, in algal HM tolerance ranges from forming ROS antioxidant enzymes and role in non-enzymatic antioxidant mechanisms (astaxanthin and proline) to minimizing HM stress-oxidative damage (Fig. 2).
Phytohormone biosynthesis and signaling in algal HM stress response: a look beyond higher plants
The current evidence shows there are significant impacts of phytohormones on HM stress response in microalgae. Although the response of endogenous phytohormones under HM stress remains largely unmeasured, the physiochemical effects of exogenous application and genome based-metabolic/signaling constructions suggest that phytohormones are functional and their signal transduction networks work in a manner similar to that of higher plants. The interactions between phytohormones and ROS detoxification system under HM stress occur in algae, where ROS detoxification seems to be a key player interacting with phytohormones as a part of HM tolerance (Fig. 2). The transcriptional activities of SOD and CAT genes were also regulated by ET precursor in Haematococcus (Vo et al. 2016). Thus, ET controls ROS transcriptional components, leading to the antioxidative defense. Less clear are the metabolic and signaling components of phytohormone metabolism in algae and their response to HM stress. Figure 3 integrates the available information on the current metabolomic and de novo transcriptomic studies involving the role of phytohormones. Based on the available information, phytohormone signaling pathways are proposed to be among the main mechanisms that control algae response to HMs. This summary will help direct our understanding of hormonal signal transduction in algae and how it can be manipulated to enhance HM tolerance.
A schematic model of phytohormone signaling pathways involved in HM stress responses of algae. Dash-outlined oval shapes indicate proposed components that have not yet been identified in algae genomes. ABA abscisic acid, ABP1 auxin binding protein 1, ALC ALCATRAZ factors, APX ascorbate peroxidase, ARF AUXIN RESPONSE FACTOR, AUA/IAA auxin transcriptional repressors, CAT catalase, CK cytokinin, DELLA/SLEEPY DELLA-domain protein and the F-box protein SLEEPY1, EIN4 ETHYLENE INSENSITIVE4, ERF1 ETHYLENE RESPONSE FACTOR1, ET ethylene, ETR1/ERS ETHYLENE RESPONSE 1/ETHYLENE RESPONSE SENSOR, GA GIBBERELLIN, GID1 GIBBERELLIN INSENSITIVE DWARF1, HK HISTIDINE KINASE, HM heavy metal, HP HISTIDINE-CONTAINING PHOSPHOTRANSMITTER, PP2C PYRABACTIN RESISTANCE 1-like (PYL)–PROTEIN PHOSPHATASE 2C, SnRK2: SNF1-RELATED PROTEIN KINASE 2, SOD superoxide dismutase, TF ABA dependent/independent transcriptional factor, type B RR type B ARABIDOPSIS RESPONSE REGULATOR (Ju et al. 2015; Lu and Xu 2015; Sun et al. 2019)
Genes in biosynthesis pathways in some of the classic phytohormones (auxin, ABA, CKs, and ET) are structurally highly conserved in microalgae (Ju et al. 2015; Lu and Xu 2015). IPT genes, which are responsible for CK biosynthesis, form two functional classes in Arabidopsis: t-RNA degradation (AtIPT02 and AtIPT09) and ATP/ADP pathway (AtIPT1, AtIPT3–8). The green microalgae Micromonas sp. RCC299 and Ostreococcus tauri have two IPT homologs, one of which is more closely related to ATP/ADP IPTs and the remaining putative algal IPT exhibits higher level of similarity to tRNA IPTs of Arabidopsis (Lu and Xu 2015). The unicellular red alga, Porphyridium purpureum, has homologs of the ET biosynthesis–related genes, 1-aminocyclopropane-1-carboxylic acid (ACC) synthase enzyme and ACC oxidase (ACO) homologs (Ju et al. 2015). ET synthesis occurs in unicellular green algae H. pluvialis (Maillard et al. 1993), although the enzymes responsible for this synthesis are largely unknown.
Although known homologs of plant genes responsible for biosynthesis of phytohormones are highly conserved between higher plants and microalgae, this is not the case for signaling pathways. In the GA signaling pathway, orthologs encoding the GA receptor GIBBERELLIN INSENSITIVE DWARF1 (GID1) have been identified in microalgae. The remaining signaling components have been found in plants but not in microalgae. This includes the ALCATRAZ (ALC) response regulator and others which are involved in mediating the GA signaling such as the DELLA-domain proteins and the F-box protein SLEEPY1 (Lu and Xu 2015). Likewise, orthologs of the higher plant ET receptors, ETHYLENE RESPONSE 1/ETHYLENE RESPONSE SENSOR/ETHYLENE INSENSITIVE 4 (ETR1/ERS/EIN4), are widely present in microalgal lineages. However, there are no known orthologs for the remaining signaling components, such as ETHYLENE-INSENSITIVE 3 (EIN3) and the ERF1 response regulator (Ju et al. 2015). ABA signaling module PYRABACTIN RESISTANCE 1-like (PYL)–PROTEIN PHOSPHATASE 2C (PP2C)–SNF1-RELATED PROTEIN KINASE 2 (SnRK2) exists in the charophyte macroalgae (Sun et al. 2019). In microalgae, no ABA receptor has been identified, even though the downstream phosphatases from ABA signaling (SNF1-RELATED PROTEIN KINASE 2) are conserved (Lu and Xu 2015).
More composed than ET, ABA and GA signaling, CK signaling generally work as a two-component system that consists of sensor kinases (HKs), histidine phosphotransfer proteins (HPs), and response regulators (RRs) (To and Kieber 2008). Homologs of the CK receptor, ARABIDOPSIS HISTIDINE KINASE 1 (AHK1), are common in algal genomes. Homologs of the downstream CK signaling cascade (type B ARABIDOPSIS RESPONSE REGULATORS and HISTIDINE-CONTAINING PHOSPHOTRANSMITTER 1) also occur in green microalgae (Lu and Xu 2015). In auxin signaling, C. variabilis NC64A, C. pyrenoidosa, and C. reinhardtii genomes have putative orthologs of the auxin receptor AUXIN-BINDING-PROTEIN1 (ABP1) which are well characterized in higher plants (Lu and Xu 2015). Algae also share the conserved motif of Auxin Binding Protein 1 (ABP1) with land plants to form an auxin receptor.
Evidence has emerged that some phytohormone signaling components (likely not full sets except for CKs) of higher plants are present in microalgae. The current view is that phytohormones have a clear beneficial effect in HM stress alleviation in algae and their interplay with the ROS system is similar to the mechanisms known in higher plants. There is a possible existence of coordination signaling cascades between hormone signaling components and ROS detoxification genetic frameworks in microalgae under HM stress as proposed in this paper. However, these transcriptional trajectories need to be further explored (Fig. 3).
Integration of omics approaches to study the function of phytohormones in HM stress response in algae
Hormonal application has potential to be a game-changing solution for HM bioremediation as well as global HM pollution challenges. Phytohormones can program algal cells to express specific metal binding proteins and strengthen antioxidant systems for enhanced HM tolerance (Table 1).
From unpredictable occurrences in HM contamination by global industries and agriculture, to ever-increasing health problems caused by the HM pollution, a thorough understanding of promising advances of algae and phytohormones provides an excellent remedy and proposes new forms of algae-based filtration methods for this scenario. Unraveling thus-far overlooked mechanisms of phytohormonal regulation of metal stress response will improve our knowledge of algae stress physiology, allowing for identification of new pathways for HM stress tolerance. Realizing these goals will require enormous efforts of researchers and industries to extend the utility and economic feasibility of phytohormone applications for HM stress alleviation in algae. These efforts should be directed towards studying (i) comprehensive inventories of exogenous application of phytohormones under HM stress, (ii) systematic characterization of hormone metabolism and signaling components under HM stress, and (iii) in algal characterization of potential molecular components of HM stress response.
The effect of phytohormones on HM stress response is dose dependent (Han et al. 2018). During stress, hormonal treatment generates economically valuable biomass associated with production of lipids, biofuels, and other value-added compounds (Zhao et al. 2019a). There is a need to apply a combination of suitable doses of phytohormones and omics-based approaches, such as metaproteomics and metabolomics, to illustrate mechanisms of bio-absorption/accumulation/chelation in HM stress controlled by phytohormones. More research on scale-up studies and technical aspects of phytohormone bioremediation systems are needed to effectively remove HMs and stimulate beneficial algae biomass production. While clear evidence exists to support the claim that phytohormones regulate HM stress responses via antioxidative systems and metal binders, an obvious question remains—can these aspects provide practical value for using microalgae in “phycoextraction” of HM-contaminated soils and water bodies on the industrial scale? Can algal phytohormone–based remediation be used for a wide range of HM bioremovals? Is it feasible to extract metal-binding compounds as well as algal bioactive compounds from HM algal filtration systems? Future work should provide further insight towards novel algae-based filtration techniques and methods to combat the global metal pollution.
Phytohormones work interactively through the complex signaling networks and they often cross-talk with each other. However, compared with higher plants, hormonal signaling networks and their response to HMs in algae is still in its infancy and there is a need to identify hormone-related HM stress–responsive algal genes using transcriptomic approaches. The next important step in this context will be in algal characterization, via overexpression, of potential phytohormone signaling components under HM exposure, to produce transgenic algae. In the view of successful results achieved previously using transgenic approaches for bioremediation, such as in C. reinhardtii (Cai et al. 1999; Siripornadulsil et al. 2002; Ibuot et al. 2017), algae bioremediation could benefit from genetic modification of the phytohormone biosynthesis components as well as the molecular signaling cascades. Hence, several issues need to be considered before the transgenic algae can be utilized in industrial application (Cheng et al. 2019). CRISPR/Cas technology for algal genome editing to induce knockout hormonal signaling components is also an option. Based on the successful results achieved using CK-deficient Arabidopsis for HM bioremediation (Mohan et al. 2016; Jiang et al. 2019), CK-deficient algae obtained through the editing of CK biosynthesis genes is a promising option. Furthermore, a new pathway of HM-binding compounds and hormone-associated components can be discovered based on these functional studies, thus opening new exciting areas in algal HM stress physiology and phytohormone studies.
Conclusions and future needs
Over the last decade, significant efforts have been made to understand the biological functions of phytohormones in HM stress tolerance in higher plants. As discussed in this review, the available studies indicate that HM stress tolerance in microalgae can also be improved by phytohormones (CKs, Auxin, GAs, BRs, and ABA). Although the physiological roles of ET and SA remain largely unknown in HM stress response in microalgae, the essential and bioactive forms of these phytohormones have been detected in a wide range of algal lineages (Table 1). The biological insights derived from these data should act as significant drivers of translational innovation and lead to broadening the algal industry applications towards remediation strategies for HM pollution. For a more complete understanding of the potential of phytohormones in algal bioremediation systems, there is a need for a sustained collaborative endeavors on several fronts to generate a comprehensive picture from transcriptomic and metabolomic trajectory maps of the bioremediation mechanisms and to design scale-up studies that can be translated into algal biotechnology.
References
Afonne OJ, Ifediba EC (2020) Heavy metals risks in plant foods – need to step up precautionary measures. Curr Opin Toxicol 22:1–6
Al-Hakimi AMA (2007) Modification of cadmium toxicity in pea seedlings by kinetin. Plant Soil Environ 53:129–135
Atici Ö, Aǧar G, Battal P (2005) Changes in phytohormone contents in chickpea seeds germinating under lead or zinc stress. Biol Plant 49:215–222
Azimi A, Azari A, Rezakazemi M, Ansarpour M (2017) Removal of heavy metals from industrial wastewaters: a review. Chem Bio Eng Rev 4:37–59
Bajguz A (2009) Brassinosteroid enhanced the level of abscisic acid in Chlorella vulgaris subjected to short-term heat stress. J Plant Physiol 166:882–886
Bajguz A, Piotrowska-Niczyporuk A (2013) Synergistic effect of auxins and brassinosteroids on the growth and regulation of metabolite content in the green alga Chlorella vulgaris (Trebouxiophyceae). Plant Physiol Biochem 71:290–297
Bajguz A, Piotrowska-Niczyporuk A (2014) Interactive effect of brassinosteroids and cytokinins on growth, chlorophyll, monosaccharide and protein content in the green alga Chlorella vulgaris (Trebouxiophyceae). Plant Physiol Biochem 80:176–183
Belkadhi A, Djebali W, Hédiji H, Chaïbi W (2016) Cellular and signaling mechanisms supporting cadmium tolerance in salicylic acid treated seedlings. Plant Sci Today 3:41–47
Bücker-Neto L, Paiva ALS, Machado RD, Arenhart RA, Margis-Pinheiro M (2017) Interactions between plant hormones and heavy metals responses. Genet Mol Biol 40:373–386
Bueso E, Alejandro S, Carbonell P, Perez-Amador MA, Fayos J, Bellés JM, Rodriguez PL, Serrano R (2007) The lithium tolerance of the Arabidopsis cat2 mutant reveals a cross-talk between oxidative stress and ethylene. Plant J 52:1052–1065
Cai XH, Brown C, Adhiya J, Traina SJ, Sayre RT (1999) Growth and heavy metal binding properties of transgenic Chlamydomonas expressing a foreign metallothionein gene. Int J Phytoremediation 1:53–65
Cao S, Chen Z, Liu G, Jiang L, Yuan H, Ren G, Bian X, Jian H, Ma X (2009) The Arabidopsis ethylene-Insensitive 2 gene is required for lead resistance. Plant Physiol Biochem 47:308–312
Cheng SY, Show PL, Lau BF, Chang JS, Ling TC (2019) New prospects for modified algae in heavy metal adsorption. Trends Biotechnol 37:1255–1268
Contreras-Pool PY, Peraza-Echeverria S, Ku-González ÁF, Herrera-Valencia VA (2016) The phytohormone abscisic acid increases triacylglycerol content in the green microalga Chlorella saccharophila (Chlorophyta). Algae 31:267–276
de Jesus Raposo MF, de Morais RMSC (2013) Influence of the growth regulators kinetin and 2,4-D on the growth of two chlorophyte microalgae, Haematococcus pluvialis and Dunaliella salina. J Basic Appl Sci 9:302–308
Du H, Ahmed F, Lin B, Li Z, Huang Y, Sun G, Ding H, Wang C, Meng C, Gao Z (2017) The effects of plant growth regulators on cell growth, protein, carotenoid, PUFAs and lipid production of Chlorella pyrenoidosa ZF strain. Energies 10:1696
El Arroussi H, Benhima R, Bennis I, El Mernissi N, Wahby I (2015) Improvement of the potential of Dunaliella tertiolecta as a source of biodiesel by auxin treatment coupled to salt stress. Renew Energy 77:15–19
Falkowska M, Pietryczuk A, Piotrowska A, Bajguz A, Grygoruk A, Czerpak R (2011) The effect of gibberellic acid (GA3) on growth, metal biosorption and metabolism of the green algae Chlorella vulgaris (Chlorophyceae) Beijerinck exposed to cadmium and lead stress. Pol J Environ Stud 20:53–59
Gangwar S, Singh VP, Srivastava PK, Maurya JN (2011) Modification of chromium (VI) phytotoxicity by exogenous gibberellic acid application in Pisum sativum (L.) seedlings. Acta Physiol Plant 33:1385–1397
Gao Z, Meng C, Zhang X, Xu D, Zhao Y, Wang Y, Lv H, Yang L, Chen L, Ye N (2012a) Differential expression of carotenogenic genes, associated changes on astaxanthin production and photosynthesis features induced by JA in H. pluvialis. PLoS One 7:e42243
Gao Z, Meng C, Zhang X, Xu D, Miao X, Wang Y, Yang L, Lv H, Chen L, Ye N (2012b) Induction of salicylic acid (SA) on transcriptional expression of eight carotenoid genes and astaxanthin accumulation in Haematococcus pluvialis. Enzym Microb Technol 51:225–230
Hadi F, Bano A, Fuller MP (2010) The improved phytoextraction of lead (Pb) and the growth of maize (Zea mays L.): the role of plant growth regulators (GA3 and IAA) and EDTA alone and in combinations. Chemosphere 80:457–462
Han X, Zeng H, Bartocci P, Fantozzi F, Yan Y (2018) Phytohormones and effects on growth and metabolites of microalgae: a review. Fermentation 4:25
Hanaka A, Wójcik M, Dresler S, Mroczek-Zdyrska M, Maksymiec W (2016) Does methyl jasmonate modify the oxidative stress response in Phaseolus coccineus treated with Cu? Ecotoxicol Environ Saf 124:480–488
Hou Q, Ufer G, Bartels D (2016) Lipid signalling in plant responses to abiotic stress. Plant Cell Environ 39:1029–1048
Hu YF, Zhou G, Na XF, Yang L, Nan WB, Liu X, Zhang YQ, Li JL, Bi YR (2013) Cadmium interferes with maintenance of auxin homeostasis in Arabidopsis seedlings. J Plant Physiol 170:965–975
Ibuot A, Dean AP, McIntosh OA, Pittman JK (2017) Metal bioremediation by CrMTP4 over-expressing Chlamydomonas reinhardtii in comparison to natural wastewater-tolerant microalgae strains. Algal Res 24:89–96
Jacobs W, Falkenstein K, Hamilton R (1985) Nature and amount of auxin in algae: IAA from extracts of Caulerpa paspaloides (Siphonales). Plant Physiol 78:844–848
Jalmi SK, Bhagat PK, Verma D, Noryang S, Tayyeba S, Singh K, Sharma D, Sinha AK (2018) Traversing the links between heavy metal stress and plant signaling. Front Plant Sci 9:12
Jiang L, Liu C, Cao H, Chen Z, Yang J, Cao S, Wei Z (2019) The role of cytokinin in selenium stress response in Arabidopsis. Plant Sci 281:122–132
Ju C, Van de Poel B, Cooper ED, Thierer JH, Gibbons TR, Delwiche CF, Chang C (2015) Conservation of ethylene as a plant hormone over 450 million years of evolution. Nat Plants 1:14004
Jusoh M, Loh SH, Chuah TS, Aziz A, San Cha T (2015) Elucidating the role of jasmonic acid in oil accumulation, fatty acid composition and gene expression in Chlorella vulgaris (Trebouxiophyceae) during early stationary growth phase. Algal Res 9:14–20
Khalid S, Shahid M, Niazi NK, Murtaza B, Bibi I, Dumat C (2017) A comparison of technologies for remediation of heavy metal contaminated soils. J Geochem Explor 182:247–268
Khatiwada B, Hasan MT, Sun A, Kamath KS, Mirzaei M, Sunna A, Nevalainen H (2020) Proteomic response of Euglena gracilis to heavy metal exposure – identification of key proteins involved in heavy metal tolerance and accumulation. Algal Res 45:101764
Kim SH, Lim SR, Hong SJ, Cho BK, Lee H, Lee CG, Choi HK (2016) Effect of ethephon as an ethylene-releasing compound on the metabolic profile of Chlorella vulgaris. J Agric Food Chem 64:4807–4816
Kobayashi M, Hirai N, Kurimura Y, Ohigashi H, Tsuji Y (1997) Abscisic acid-dependent algal morphogenesis in the unicellular green alga Haematococcus pluvialis. Plant Growth Regul 22:79–85
Lau S, Shao N, Bock R, Jürgens G, De Smet I (2009) Auxin signaling in algal lineages: fact or myth? Trends Plant Sci 14:182–188
Le Bail A, Billoud B, Kowalczyk N, Kowalczyk M, Gicquel M, Le Panse S, Stewart S, Scornet D, Cock JM, Ljung K (2010) Auxin metabolism and function in the multicellular brown alga Ectocarpus siliculosus. Plant Physiol 153:128–144
Lee C, Choi YE, Yun YS (2016) A strategy for promoting astaxanthin accumulation in Haematococcus pluvialis by 1-aminocyclopropane-1-carboxylic acid application. J Biotechnol 236:120–127
Li M, Ahammed GJ, Li C, Bao X, Yu J, Huang C, Yin H, Zhou J (2016) Brassinosteroid ameliorates zinc oxide nanoparticles-induced oxidative stress by improving antioxidant potential and redox homeostasis in tomato seedling. Front Plant Sci 7:615
Liu J, Qiu W, Song Y, Peng H, Zhao Y (2017a) The growth and lipid productivity of Chlorella pyrenoidosa enhanced by plant hormones under ammonium stress. Environ Prog Sustain Energy 36:1187–1193
Liu T, Liu F, Wang C, Wang Z, Li Y (2017b) The boosted biomass and lipid accumulation in Chlorella vulgaris by supplementation of synthetic phytohormone analogs. Bioresour Technol 232:44–52
Lu Y, Xu J (2015) Phytohormones in microalgae: a new opportunity for microalgal biotechnology? Trends Plant Sci 20:273–282
Lu Y, Jiang P, Liu S, Gan Q, Cui H, Qin S (2010) Methyl jasmonate- or gibberellins A3-induced astaxanthin accumulation is associated with up-regulation of transcription of β-carotene ketolase genes (bkts) in microalga Haematococcus pluvialis. Bioresour Technol 101:6468–6474
Lu Y, Tarkowská D, Turečková V, Luo T, Xin Y, Li J, Wang Q, Jiao N, Strnad M, Xu J (2014) Antagonistic roles of abscisic acid and cytokinin during response to nitrogen depletion in oleaginous microalga Nannochloropsis oceanica expand the evolutionary breadth of phytohormone function. Plant J 80:52–68
Luo ZB, He J, Polle A, Rennenberg H (2016) Heavy metal accumulation and signal transduction in herbaceous and woody plants: paving the way for enhancing phytoremediation efficiency. Biotechnol Adv 34:1131–1148
Maillard P, Thepenier C, Gudin C (1993) Determination of an ethylene biosynthesis pathway in the unicellular green alga, Haematococcus pluvialis Relationship between growth and ethylene production. J Appl Phycol 5:93–98
Maksymiec W, Wójcik M, Krupa Z (2007) Variation in oxidative stress and photochemical activity in Arabidopsis thaliana leaves subjected to cadmium and excess copper in the presence or absence of jasmonate and ascorbate. Chemosphere 66:421–427
Meng H, Hua S, Shamsi IH, Jilani G, Li Y, Jiang L (2009) Cadmium-induced stress on the seed germination and seedling growth of Brassica napus L., and its alleviation through exogenous plant growth regulators. Plant Growth Regul 58:47–59
Mhamdi A, Van Breusegem F (2018) Reactive oxygen species in plant development. Development 145:dev164376
Moenne A, González A, Sáez CA (2016) Mechanisms of metal tolerance in marine macroalgae, with emphasis on copper tolerance in Chlorophyta and Rhodophyta. Aquat Toxicol 176:30–37
Mohan TC, Castrillo G, Navarro C, Zarco-Fernández S, Ramireddy E, Mateo C, Zamarreño AM, Paz-Ares J, Muñoz R, García-Mina JM, Hernández LE (2016) Cytokinin determines thiol-mediated arsenic tolerance and accumulation. Plant Physiol 171:1418–1426
Mowat JA (1965) A survey of results on the occurrence of auxins and gibberellins in algae. Bot Mar 8:149–155
Noble A, Kisiala A, Galer A, Clysdale D, Emery RN (2014) Euglena gracilis (Euglenophyceae) produces abscisic acid and cytokinins and responds to their exogenous application singly and in combination with other growth regulators. Eur J Phycol 49:244–254
Pandey C, Gupta M (2015) Selenium and auxin mitigates arsenic stress in rice (Oryza sativa L.) by combining the role of stress indicators, modulators and genotoxicity assay. J Hazard Mater 287:384–391
Piotrowska A, Czerpak R (2009) Cellular response of light/dark-grown green alga Chlorella vulgaris Beijerinck (Chlorophyceae) to exogenous adenine- and phenylurea-type cytokinins. Acta Physiol Plant 31:573–585
Piotrowska-Niczyporuk A, Bajguz A (2014) The effect of natural and synthetic auxins on the growth, metabolite content and antioxidant response of green alga Chlorella vulgaris (Trebouxiophyceae). Plant Growth Regul 73:57–66
Piotrowska-Niczyporuk A, Bajguz A, Zambrzycka E, Godlewska-zyłkiewicz B (2012) Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Plant Physiol Biochem 52:52–65
Piotrowska-Niczyporuk A, Bajguz A, Zambrzycka-Szelewa E (2017) Response and the detoxification strategies of green alga Acutodesmus obliquus (Chlorophyceae) under lead stress. Environ Exp Bot 144:25–36
Piotrowska-Niczyporuk A, Bajguz A, Kotowska U, Zambrzycka-Szelewa E, Sienkiewicz A (2020) Auxins and cytokinins regulate phytohormone homeostasis and thiol-mediated detoxification in the green alga Acutodesmus obliquus exposed to lead stress. Sci Rep 10:10193
Pratt R (1938) Influence of auxins on the growth of Chlorella vulgaris. Am J Bot 25:498–501
Qin H, Hu T, Zhai Y, Lu N, Aliyeva J (2020) The improved methods of heavy metals removal by biosorbents: a review. Environ Pollut 258:113777
Ramakrishna B, Rao SSR (2015) Foliar application of brassinosteroids alleviates adverse effects of zinc toxicity in radish (Raphanus sativus L.) plants. Protoplasma 252:665–677
Raman V, Ravi S (2011) Effect of salicylic acid and methyl jasmonate on antioxidant systems of Haematococcus pluvialis. Acta Physiol Plant 33:1043–1049
Rejeb KB, Abdelly C, Savouré A (2014) How reactive oxygen species and proline face stress together. Plant Physiol Biochem 80:278–284
Salama ES, Jeon BH, Chang SW, Lee SH, Roh HS, Yang IS, Kurade MB, El-Dalatony MM, Kim DH, Kim KH, Kim S (2017) Interactive effect of indole-3-acetic acid and diethyl aminoethyl hexanoate on the growth and fatty acid content of some microalgae for biodiesel production. J Clean Prod 168:1017–1024
Schellingen K, Van Der Straeten D, Vandenbussche F, Prinsen E, Remans T, Vangronsveld J, Cuypers A (2014) Cadmium-induced ethylene production and responses in Arabidopsis thaliana rely on ACS2 and ACS6 gene expression. BMC Plant Biol 14:214
Sharma P, Bhardwaj R (2007) Effects of 24-epibrassinolide on growth and metal uptake in Brassica juncea L. under copper metal stress. Acta Physiol Plant 29:259–263
Sharma P, Kumar A, Bhardwaj R (2016) Plant steroidal hormone epibrassinolide regulate - heavy metal stress tolerance in Oryza sativa L. by modulating antioxidant defense expression. Environ Exp Bot 122:1–9
Shi GR, Cai QS, Liu QQ, Wu L (2009) Salicylic acid-mediated alleviation of cadmium toxicity in hemp plants in relation to cadmium uptake, photosynthesis, and antioxidant enzymes. Acta Physiol Plant 31:969–977
Shi WG, Li H, Liu TX, Polle A, Peng CH, Luo ZB (2015) Exogenous abscisic acid alleviates zinc uptake and accumulation in Populus×canescens exposed to excess zinc. Plant Cell Environ 38:207–223
Shukla A, Srivastava S, Suprasanna P (2017) Genomics of metal stress-mediated signalling and plant adaptive responses in reference to phytohormones. Curr Genomics 18:512–522
Siddiqui MH, Al-Whaibi MH, Basalah MO (2011) Interactive effect of calcium and gibberellin on nickel tolerance in relation to antioxidant systems in Triticum aestivum L. Protoplasma 248:503–511
Signorelli S, Tarkowski ŁP, Van den Ende W, Bassham DC (2019) Linking autophagy to abiotic and biotic stress responses. Trends Plant Sci 24:413–430
Sirhindi G, Mir MA, Sharma P, Gill SS, Kaur H, Mushtaq R (2015) Modulatory role of jasmonic acid on photosynthetic pigments, antioxidants and stress markers of Glycine max L. under nickel stress. Physiol Mol Biol Plants 21:559–565
Siripornadulsil S, Traina S, Verma DPS, Sayre RT (2002) Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae. Plant Cell 14:2837–2847
Smirnoff N, Cumbes QJ (1989) Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 28:1057–1060
Souza JMC, Yokoya NS (2016) Effects of cytokinins on physiological and biochemical responses of the agar-producing red alga Gracilaria caudata (Gracilariales, Rhodophyta). J Appl Phycol 28:3491–3499
Srivastava S, Srivastava AK, Suprasanna P, D’Souza SF (2013) Identification and profiling of arsenic stress-induced microRNAs in Brassica juncea. J Exp Bot 64:303–315
Stewart WDP, Fitzgerald GP, Burris RH (1968) Acetylene reduction by nitrogen-fixing blue-green algae. Arch Mikrobiol 62:336–348
Sulochana SB, Arumugam M (2016) Influence of abscisic acid on growth, biomass and lipid yield of Scenedesmus quadricauda under nitrogen starved condition. Bioresour Technol 213:198–203
Sun Y, Harpazi B, Wijerathna-Yapa A, Merilo E, de Vries J, Michaeli D, Gal M, Cuming AC, Kollist H, Mosquna A (2019) A ligand-independent origin of abscisic acid perception. Proc Natl Acad Sci U S A 116:24892–24899
Sytar O, Kumari P, Yadav S, Brestic M, Rastogi A (2019) Phytohormone priming: regulator for heavy metal stress in plants. J Plant Growth Regul 38:739–752
Talarek-Karwel M, Bajguz A, Piotrowska-Niczyporuk A (2020) Hormonal response of Acutodesmus obliquus exposed to combined treatment with 24-epibrassinolide and lead. J Appl Phycol
Thomas JC, Perron M, LaRosa PC, Smigocki AC (2005) Cytokinin and the regulation of a tobacco metallothionein-like gene during copper stress. Physiol Plant 123:262–271
Tian BJ, Wang Y, Zhu YR, Lü XY, Huang K, Shao N, Beck CF (2006) Synthesis of the photorespiratory key enzyme serine: glyoxylate aminotransferase in C. reinhardtii is modulated by the light regime and cytokinin. Physiol Plant 127:571–582
Tiwari S, Patel A, Prasad SM (2018) Kinetin alleviates chromium toxicity on growth and PS II photochemistry in Nostoc muscorum by regulating antioxidant system. Ecotoxicol Environ Saf 161:296–304
To JPC, Kieber JJ (2008) Cytokinin signaling: two-components and more. Trends Plant Sci 13:85–92
Trinh NN, Huang TL, Chi WC, Fu SF, Chen CC, Huang HJ (2014) Chromium stress response effect on signal transduction and expression of signaling genes in rice. Physiol Plant 150:205–224
Udayan A, Kathiresan S, Arumugam M (2018) Kinetin and gibberellic acid (GA3) act synergistically to produce high value polyunsaturated fatty acids in Nannochloropsis oceanica CASA CC201. Algal Res 32:182–192
Urrutia C, Yañez-Mansilla E, Jeison D (2019) Bioremoval of heavy metals from metal mine tailings water using microalgae biomass. Algal Res 43:101659
Vo TT, Lee C, Han SI, Kim JY, Kim S, Choi YE (2016) Effect of the ethylene precursor, 1-aminocyclopropane-1-carboxylic acid on different growth stages of Haematococcus pluvialis. Bioresour Technol 220:85–93
Wang J, Chen J, Pan K (2013) Effect of exogenous abscisic acid on the level of antioxidants in Atractylodes macrocephala Koidz under lead stress. Environ Sci Pollut Res Int 20:1441–1449
Wang R, Wang J, Zhao L, Yang S, Song Y (2015) Impact of heavy metal stresses on the growth and auxin homeostasis of Arabidopsis seedlings. Biometals 28:123–132
Wu XX, Chen JL, Xu S, Zhu ZW, Zha DS (2016) Exogenous 24-epibrassinolide alleviates zinc-induced toxicity in eggplant (Solanum melongena L.) seedlings by regulating the glutathione-ascorbate-dependent detoxification pathway. J Hortic Sci Biotechnol 91:412–420
Yoshida K, Igarashi E, Wakatsuki E, Miyamoto K, Hirata K (2004) Mitigation of osmotic and salt stresses by abscisic acid through reduction of stress-derived oxidative damage in Chlamydomonas reinhardtii. Plant Sci 167:1335–1341
Yu XJ, Sun J, Sun YQ, Zheng JY, Wang Z (2016) Metabolomics analysis of phytohormone gibberellin improving lipid and DHA accumulation in Aurantiochytrium sp. Biochem Eng J 112:258–268
Zeraatkar AK, Ahmadzadeh H, Talebi AF, Moheimani NR, McHenry MP (2016) Potential use of algae for heavy metal bioremediation, a critical review. J Environ Manag 181:817–831
Zhang W, Yamane H, Takahashi N, Chapman DJ, Phinney BO (1989) Identification of a cytokinin in the green alga Chara globularis. Phytochemistry 28:337–338
Zhang F, Zhang H, Xia Y, Wang G, Xu L, Shen Z (2011) Exogenous application of salicylic acid alleviates cadmium toxicity and reduces hydrogen peroxide accumulation in root apoplasts of Phaseolus aureus and Vicia sativa. Plant Cell Rep 30:1475–1483
Zhao Y, Wang HP, Han B, Yu X (2019a) Coupling of abiotic stresses and phytohormones for the production of lipids and high-value by-products by microalgae: a review. Bioresour Technol 274:549–556
Zhao Y, Xing H, Li X, Geng S, Ning D, Ma T, Yu X (2019b) Physiological and metabolomics analyses reveal the roles of fulvic acid in enhancing the production of astaxanthin and lipids in Haematococcus pluvialis under abiotic stress conditions. J Agric Food Chem 67:12599–12609
Zhu XF, Jiang T, Wang ZW, Lei GJ, Shi YZ, Li GX, Zheng SJ (2012) Gibberellic acid alleviates cadmium toxicity by reducing nitric oxide accumulation and expression of IRT1 in Arabidopsis thaliana. J Hazard Mater 239:302–307
Funding
Financial support from the Natural Sciences and Engineering Council of Canada (RGPIN-05436) and NSERC Strategic Partnerships Grant Program (STPGP 521417) to RJNE is gratefully acknowledged. HNN was supported by NSERC Strategic Partnerships Grant Program (STPGP 521417) and International Graduate Scholarship (IGS) from Environmental Life Sciences Graduate (EnLS) program, Trent University.
Author information
Authors and Affiliations
Contributions
Hai Ngoc Nguyen: conceptualization; visualization; writing, original draft; writing, review and editing. Anna B. Kisiala: writing, review and editing. R.J. Neil Emery: funding acquisition, supervision, writing, review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nguyen, H.N., Kisiala, A.B. & Emery, R.J.N. The roles of phytohormones in metal stress regulation in microalgae. J Appl Phycol 32, 3817–3829 (2020). https://doi.org/10.1007/s10811-020-02271-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02271-5