Abstract
Urbanization, industrialization, and natural earth processes have potentially increased the contamination of heavy metals (HMs) in water bodies. These HMs can accumulate in human beings through the consumption of contaminated water and food chains. Various clean-up technologies have been applied to sequester HMs, especially conventional methods including electrolytic technologies, ion exchange, precipitation, chemical extraction, hydrolysis, polymer micro-encapsulation, and leaching. However, most of these approaches are expensive for large-scale projects and require tedious control and constant monitoring, along with low efficiency for effective HMs removal. Algae offer an alternative, sustainable, and environmentally friendly HMs remediation approach. This review presents a state-of-the-art technology for potential use of algae as a low-cost biosorbent for the removal of HMs from wastewater. The mechanisms of HMs removal, including biosorption and bioaccumulation along with physical and chemical characterization of the algae are highlighted. The influence of abiotic factors on HMs removal and changes in algal biocomponents (including, carbohydrate, lipid, and protein) are discussed. Recent progresses made in the development of HMs-tolerant algal strains and the direction of future research toward the development of sustainable technology for advanced wastewater treatment and biomass production are covered.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surface and sub-surface water contamination caused by heavy metals (HMs) is of substantial global concern (Kobielska et al. 2018). HMs are released into the environment by natural processes including wind and floods, as well as through anthropogenic activities (Gupta et al. 2016). HMs present in the air and soil end up in water bodies due to precipitation and water run-off (Singare et al. 2010; Warmate et al. 2011). They are non-biodegradable and persistent, have a deleterious impact on both ecosystems and human health (Alqadami et al. 2018; Kwaansa-Ansah et al. 2019). Figure 1 schematically represents the toxic effects of HMs on different human organs. As the presence of HMs in aquatic environments may limit clean water availability for its intended usage (Dixit et al. 2015), therefore, stringent environmental regulations have been imposed to reduce HMs concentration in wastewater below permissible limits before discharging into natural water reservoirs. The maximum permissible limits of HMs reported by the United States Environmental Protection Agency (US-EPA) and the toxic effects of HMs on human health are presented in Table 1.
Schematic representation showing the organs and systems targeted in humans by HMs (de Namor et al. 2012)
Various conventional techniques for HMs removal from polluted sites includes electrolytic technologies, ion exchange, precipitation, chemical extraction, hydrolysis, polymer micro-encapsulation, and leaching (Jais et al. 2017). However, the major concern is that most of these methods are ineffective, expensive when applied to large-scale projects, and require tedious control and constant monitoring. Table 2 covers the merits and demerits of conventional treatment processes. Therefore, biological treatment (bioremediation) is recommended as an alternative and eco-friendly approach for efficient removal of HMs from contaminated sites.
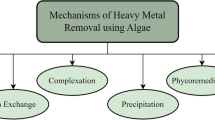
Bioremediation by algal species (Fig. 2), termed as “phycoremediation”, has recently emerged as an appealing technique for HMs removal from wastewater (Ahmad 2016; Babu et al. 2013; Oyetibo et al. 2016; Poo et al. 2018). Phycoremediation has numerous advantages over other bioremediation processes including: (1) algal biomass can be applied in wastewater with higher metal concentration than for membrane processes (Brinza et al. 2007); (2) no need to synthesis algal biomass; (3) biomass can be regenerated and reused in several adsorption/desorption series; (4) high uptake capacity and efficiency of HMs removal (Ajayan et al. 2011); (5) no sludge or toxic chemical produced; (6) Macroalgal biomass does not essential to be immobilized; (7) algal biomass can be applied in discontinuous and continuous regimes; (8) by using dead biomass, no nutrient or oxygen supply needed; (9) appropriate for anaerobic and aerobic effluent treatment units; (10) algal biomass can be used all around year (Darda et al. 2019); and (11) cost effective (Kotrba 2011).
Therefore, considering the importance of algae as a promising agent for HMs removal, this review gives an overview on recent progresses made on HMs remediation by algae. The main mechanisms of HMs removal, including biosorption and bioaccumulation, are highlighted. The influence of several abiotic factors on HMs removal and changes in algal biocomponents are comprehensively discussed. Furthermore, recent progresses in the development of HMs-tolerant algal strains and directs future research toward the development of sustainable technology for wastewater treatment and biomass production are covered.
Phycoremediation of HMs
Phycoremediation is defined as an application of algae in the treatment process of wastewater pollution (Jais et al. 2017). Algae are classified on the basis of their morphology, pigments, cell walls, stored food materials, reproductive structures, and life history patterns into seven major groups: Rhodophyta, Chlorophyta, Charophyta, Chrysophyta, Euglenophyta, Pyrrhophyta, and Phaeophyta (Hallmann 2015; Namdeti and Pulipati 2014; Wang and Chen 2009). Various HMs, such as Mn2+, Ni2+, Cu2+, Mo2+, Fe2+, and Zn2+, are essential to algal growth and are known as ‘trace elements’ that are desirable as micronutrients. In contrast, other HMs, including Sn2+, Au3+, Cd2+, Pb2+, Sr2+, Ti3+, and Hg2+, have no essential biological function and are toxic to algae (Jais et al. 2017). Detailed studies of the physiochemical composition of algal cells have helped in revealing the usefulness of algae in environmental pollution control, especially in the area of HMs removal from domestic and industrial wastewaters. Some algae have shown exceptional tolerance and survival in water polluted with relatively high HMs concentration (Kotrba 2011).
Besides living cells (Fig. 3a, b), the dead algal cells can also remove HMs from contaminated water as both can perform biosorption of HMs present in their surrounding environment (Fig. 3c). However, the efficiency of living algae cells during wastewater treatment is higher than that of dead biomass, as they can remove and retain a greater quantity of metals using both biosorption and bioaccumulation mechanisms for a longer time period. The HMs removal efficiencies of various algal species in various wastewater sources (e.g., municipal, petrochemical, electroplating, and dairy) are shown in Table 3. For example, Spirulina sp. removed 91 and 98% of Cu2+ and Ca2+ after cultivation in municipal wastewater, respectively (Al-Homaidan et al. 2015; Anastopoulos and Kyzas 2015). When grown in municipal wastewater, the removal efficiency of Chlorella minutissima was 62, 84, 74, and 84% for Zn2+, Mn2+, Cd2+, and Cu2+, respectively (Yang et al. 2015). After cultivation in oil sands tailings ponds, Cladophora fracta removed 99% of Cu2+ and 85% of Zn2+ (Mahdavi et al. 2012). After grown in acid mine drainages, the removal efficiency of Oedogonium sp. was 46, 34, 48, and 50% for Cu2+, Ni2+, Zn2+, and Co2+, respectively (Bakatula et al. 2014). In Vasant Kunj, New Delhi, India, arsenic (As) was completely removed from drinking water by the filamentous green alga Cladophora (Jasrotia et al. 2014). Chlorella sp., Scenedesmus sp., and Chlamydomonas sp. have shown to be effective in removing HMs, some toxic organic compounds, and secondary pollutants from wastewaters with a wide range of initial pollutant concentrations (Gao et al. 2016; Matamoros et al. 2015; Yang et al. 2016).
Various binding groups (COO−, OH−, PO43−, NO3−, SH−, RNH2−, RO−, and RS−) stimulate metal ion biosorption (a). A schematic representation of surface binding, uptake, and intracellular accumulation of metal ions by a living algal cell. A variety of transporters is involved in uptake of metal ions, and the cell has numerous intracellular sites for binding and sequestration of metal ions (b). A schematic representation of some mechanisms of HMs sequestration, translocation, and uptake in living (left) and non-living (right, brown-shaded) algae (c) (Kumar et al. 2015, 2016; Zeraatkar et al. 2016)
Algal biomass could be considered as an alternate to conventional adsorbent materials (including microbial, agricultural waste or other type of biomasses) for the treatment of HMs due to: (1) algae can be grown in a wide range of environmental conditions (Abou-Shanab et al. 2011); (2) they show high growth rates because of short cell cycle time; (3) they require low nutrient concentrations compared to other biomass organisms; (4) they do not need agricultural land for cultivation; (5) due to lower water requirements, algae cultivation can be achieved in wastewater (Salama et al. 2017); and (6) they can be further used for other applications such as biofuel generation (Mantzorou et al. 2018).
Mechanisms of HMs phycoremediation
Several studies have reported the potential of phytoplankton to sequester HMs from aqueous media (Jan and Parray 2016; Lahiri et al. 2017). Microalgae remove HM ions from wastewater through two mechanisms: biosorption and bioaccumulation (Table 4). Biosorption is an independent metabolic process that occurs in both live and dead cells (Fig. 3). In this process, HM ions attached to functional groups on the cell surface as a result of ion exchange, complexation, chelation, and microprecipitation (Kumar et al. 2015; Park et al. 2016). Studies suggest that the components of algal cell walls, such as alginate and fucoidan, which have key functional groups, are chiefly responsible for biosorption of HM ions (Anastopoulos and Kyzas 2015; Zeraatkar et al. 2016). Through ion exchange, the HM ions in wastewater surrounding the algae are exchanged with elemental ions held on the cell surface, such as Ca2+, Na+, and K+. The viability of this process depends on important factors such as metal selectivity and regeneration potential. Selectivity in biosorption is generally low because HM ions bind to the cell surface through physicochemical interactions. However, selectivity can be increased through chemical modification of the biomass, such as cross-linking with epichlorohydrin, or oxidation by potassium permanganate (Luo et al. 2006). Figures 3 and 4 present the biosorption and bioaccumulation processes for HM ions removal.
Biosorption
Biosorption is a physiochemical property of biological material that results in the removal of pollutants, mostly HMs, from wastewater by either ionic or covalent bonding (He and Chen 2014; Zeraatkar et al. 2016). Various binding groups, such as COO−, SH−, OH−, RNH2−, RS−, and RO−, promote metal ion biosorption (Fig. 3a, b). These binding groups are present at the cell surface and in the cytoplasm, especially inside vacuoles. Studies have shown that algal cell walls carry a net negative charge due to the presence of COO−, PO43−, and other groups used for bonding metals through ion exchange. Some algal species, including Ditylum brightwellii, secrete a special substance called Cu ligands (Rijstenbil and Gerringa 2002). The carboxyl functional group (COO−) is the most abundant acidic functional group in the cell walls of brown algae. Excretion and exclusion of metal from the cell, as well as the production of proteins like proline and other binding compounds like metallothioneins (MTs) and glutathione (GSH), are among the mechanisms employed by algae to prevent metal-induced damage (Aude-Garcia et al. 2016). Differences in cell wall components among various algal species result in different functional groups. The metal uptake of biosorbent and the matrix system was quantitatively evaluated using Pb, Cd, Ni, and Zn and corresponded well with the Langmuir isotherm model (Aziz et al. 2016). The selectivity of HMs uptake depends on the encapsulation of microalgae and its cellulose derivatives (Wang et al. 2016a). Desorption of the adsorbed HMs can be achieved through a reduction in the suspension pH. Therefore, a reversible loading/unloading of the adsorbed HMs, using HCl or citric acid for the desorption process, is possible.
Metal biosorption experiments have been carried out with freshwater green microalgae (e.g., Chlorella sp., Scenedesmus sp., and Chlamydomonas sp.), brown algae (e.g., Fucus vesiculosus and Laminaria japonica), and blue-green algae (e.g., Microcystis aeruginosa and Oscillatoria sp.) (Khan et al. 2017). Several HMs removal technologies, for example, high rate algal ponds (HRAP) and algal turf scrubbers (ATS), have been supported for practical applications around the globe. However, these technologies are still insufficient for large-scale application. As an innovative clean-up technology, phycoremediation depends mainly on the biosorption and bioaccumulation abilities of algae, with biosorption dominating the bioremediation process (Furey et al. 2016).
Algae are efficient and cost-effective biosorbents due to their low nutrient requirements. Based on statistical analysis of the potentiality of algae for biosorption, the biosorption efficiency of algae has been reported as approximately 15.3–84.6% higher than other microbial biosorbents (e.g., bacteria and fungi) (Anastopoulos and Kyzas 2015; Kanchana et al. 2014; Sweetly 2014).
Bioaccumulation and detoxification of heavy metals in algae
Through bioaccumulation, HM ions are transported across living cell membranes in various ways (e.g., active and passive transport systems) and accumulated within cells (Figs. 3 and 4a). HMs accumulation inside the cell causes inhibition of photosynthesis activity and thus reduce the algal growth, irreversible increase in plasma-lemma permeability leading to the loss of cell solutes, disruption of membrane integrity owing to deterioration of protein structure, enzyme inhibition due to displacement of essential metal ions, abnormal morphological development, and loss of flagella in certain algae (Fig. 4b). Intracellular and extracellular metal binding approaches (such as ion exchanges, chelation, physical adsorption, and complexation) have been implemented by algae to overcome HMs toxicity (Priyadarshini and Priyadarshini 2019). These mechanisms are effective as they alter the toxic metal into non-toxic forms (Mantzorou et al. 2018).
Metal detoxification by algae is achieved through several approaches including binding to specific intracellular organelle or transport to specific cellular components (such as polyphosphate bodies/vacuoles), flushing out into the solution by efflux pump, and synthesis of phytochelatins or class III metallothioneins (Perales-Vela et al. 2006). A detoxification process can reduce the toxicity of HM ions on living cells through precipitation in a carbonate, phosphate, or sulfide forms (Juang and Chang 2016). Cladophora glomerata, a green alga, was able to remove Pb, Cd, Ni, Cr, and V at 7.9, 0.1, 15.6, 1.7, and 37.7 mg kg−1, respectively, from a refinery sewage lagoon (Chmielewská and Medved 2001). Fucus vesiculosus, a macroalga, showed high capacity for HMs accumulation from contaminated saltwater, removing 65, 95, and 76% of Pb, Hg, and Cd, respectively. Bioconcentration factors for Pb, Hg, and Cd ranged from 600 to 2300, with all metal removed from the solution accumulated into the biomass (Henriques et al. 2017).
Abiotic factors influencing HM remediation by algae
Media pH
Availability of the metal-binding groups on algae invariably depends on pH of the media. These groups can maintain negatively charged surface under acidic conditions. However, extreme pH (< 2) was reported for lowering the metal biosorption by microalgae. High concentrations of H+ ions decrease metal biosorption by preventing them from binding to ligands on the cell surface (Volesky 2007; Zeraatkar et al. 2016). Various binding groups and ligand atoms in algae biomolecules are listed in Table 5. According to the pKa of functional groups, carboxyl groups, sulfonate, phosphate, and phosphodiester are the largest contributors in metals biosorption. Different algae exhibit different capacity for metal ions biosorption because of the relative abundance of each functional groups for different algal strains (Priyadarshini and Priyadarshini 2019).
Optimization of the suspension pH is vital for maximum biosorption capacity and efficiency. Therefore, efforts have been made to determine the optimum pH values for enhancement in metal ions removal by algae (Sheng et al. 2005). Biosorption of Cs+ by Padina australis was optimal at pH 4 (Jalali-Rad et al. 2004). Cu2+ biosorption was strongly governed by solution pH. Lower Cu2+ biosorption was observed at acidic pH (~ 2), gradual increased at higher suspension pH. The sharpest increase was observed between pH 3 and 4 (Yu and Kaewsarn 1999). Biosorption of Pb2+ by Durvillaea potatorum was optimal at pH 5 (Jalali-Rad et al. 2004). The biosorption of some metal ions such as Cu2+ and Pb2+ might increase using living algal cells, because of consequent increase in suspension pH due to photosynthetic activity (Raeesossadati et al. 2014). Thus, injection of CO2 can be used to control the acidity of the culture medium (Zeraatkar et al. 2016).
For efficient HMs removal by an algal biosorbent, the ratio of free metal ions [Mn+] to total metal concentration [M]T should remain high (Babarinde and Onyiaocha 2016). The ratio of [Mn+] to [M]T in a solution can be determined by the free ligand concentration and stability constant (β). [Mn+]/[M]T is often low for Fe3+, Co2+, Cu2+, Zn2+, Pb2+, and Hg2+ at circumneutral pH due to relatively low solubility and frequent surface precipitation on microalgae has been also observed (Abou-Shanab et al. 2013; Babarinde and Onyiaocha 2016). Most of the relevant studies have disregarded this important aspect during screening of algal species for biosorption of metals from metal solutions or industrial effluents.
Ionic strength
The ionic strength influence is caused by the competition between HMs and Na+ for electrostatic binding to the algal biomass, which carries a negative charge. Most of the negative charges in the algal biomass balanced at the high ionic strength. However, at lower ionic strength, the electrostatic attraction leads to higher intraparticle protons concentration than the bulk proton concentration (Andrade et al. 2005; Schiewer 1999). Characterizations of Ulva fascia (green alga), Sargassum hemiphyllum, Petalonia fascia, and Colpomenia sinuosa (brown seaweeds) were performed in terms of their charge density, binding sites, and intrinsic proton binding constant (pKa). The number of identified binding sites were highest on Petalonia and Sargassum and lowest on Colpomenia and Ulva (Schiewer and Wong 2000). Due to the large number of binding sites, Sargassum and Petalonia were most effective for biosorption applications. A decrease in proton binding with increased ionic strength and pH was described using the Donnan model, in conjunction with an ion exchange biosorption isotherm. Electrostatic attraction between protons and negatively charged carboxyl sites results in intraparticle proton concentrations that are higher than the bulk proton concentration, resulting in proton release from intraparticle space into the bulk solution (Ungureanu et al. 2016). A pKa value of 3.0 was used for all algae, and it was assumed that the cation binding volume was proportional to the number of binding sites. The Cu2+ binding constants decreased in the following order: Sargassum > Petalonia > Colpomenia > Ulva. The intrinsic binding constant for Cu2+ was 30-90 times higher than that for Ni2+. Covalent binding was more important for Cu2+ than for Ni2+, which was bound predominantly by electrostatic attraction (Kleinübing et al. 2013). Virtually no covalent metal binding took place in Ulva, possibly, because green algae, which lack alginate, do not offer carboxyl groups spaced at suitable distances for metal ions to bridge between two binding sites. Brown algae are more suited for biosorption applications than green algae because of their higher metal binding capacity and affinity (Davis et al. 2003).
Temperature
The biosorption efficiency of algal species for each metal ions is effected by temperature (Chairat and Bremner 2016; Gupta et al. 2010). Although the constants for metal–ligand complex formation are primarily a function of temperature, some studies have claimed that a potential increase in metal ions biosorption is due to an increases in algal culture temperatures, without considering changes to formation constants (Khan et al. 2012; Yi et al. 2016). The possible reasons for an increase in biosorption with an increase in temperature are: (a) increase in the number of active sites involved in metal ions uptake; (b) increase in the tendency of active sites to absorb metal ions; (c) a reduction in mass transfer resistance in the diffusion layer due to a reduction of the diffusion boundary layer thickness around the biosorbent groups; or (d) a change to the complex formation constant with temperature (Bayes et al. 2012; Zhu and Wachs 2016). However, other studies have suggested that for some algae, the metal ions uptake was exothermic, so by lowering the temperature, uptake capacity increases. Several studies reported temperature-linked changes in metal ions uptake by living algal cells, while others also showed that temperature has no significant influence on metal ions uptake by dead algal cells (Balarak et al. 2016). These seemingly incompatible results may be resolved by noting that optimum temperatures are usually a narrow range for active biological reactions in living cells. A biomass of Chlorella vulgaris achieved maximum biosorption of Cd2+ and Ni2+ at 20 and 45 °C, respectively (Aksu 2001). Temperature also influences metal ions biosorption on non-living algal biomass, as the biosorption equilibrium is determined by the exothermic or endothermic nature of the process (Al-Homaidan et al. 2014). A number of studies have examined the effects of temperature on biosorption isotherms, metal uptake, and biosorption thermodynamics parameters (Pokethitiyook and Poolpak 2016). Due to biosorption and the involvement of enzymes in ion transfer, increased temperature might have a greater impact on the biosorption capacity of living algae compared to non-living algae (Goher et al. 2016). In the available literature reported on temperature effect, it is difficult to develop a relationship between temperature and metal ions uptake. However, different algal strains behave differently to uptake metals ions at varied temperatures (Chang 2019; Furuhashi et al. 2019; Mantzorou et al. 2018; Vilar et al. 2005).
Effect of counter ions
The presence or absence of other ions in the medium along with nutrient level, growth rate, and illumination greatly influence metal ions biosorption by living algae. The uptake of Cd2+ by Aphanocapsa increased with increased NO3− concentration in the culture medium (Quan et al. 2016). The growth phase of the algal culture also influences metal ions biosorption. Biosorption of Ni2+ on the surface of C. vulgaris was higher for cultures in the stationary and decline phases than in the exponential phase, this might be a result of higher exposure of the metal binding sites or from creation of additional sites on the cell surface during these phases. Metal ions biosorption characteristics of the biomass may be influenced by growth conditions as it effects the cell surface composition which is a key player in metal ions biosorption (Wu et al. 2016).
Impact of contact time
HM ions biosorption is highly dependent on contact time. The kinetics of HM ions biosorption on algae cell surfaces in previous studies report that the biosorption mechanism is specific to various algal strains (Sooksawat et al. 2016; Zhang et al. 2016). Biosorption occurs in two stages (Chang 2019; Gupta et al. 2017). First, for algal biomass, metal ions were passively adsorbed to cell membranes, and biosorption of metal ions occurs rapidly within the first minute. Second, for live algae, active biosorption occurs as the algal cell slowly uptakes the HM ions. The uptake of uranium (U) by biomass of non-living C. vulgaris during the first 5 min was more than 90% (Sooksawat et al. 2016; Vogel et al. 2010). Biomass of Chlamydomunas reinhardtii microalgae rapidly adsorbed free ions of Hg2+, Cd2+, and Pb2+, with biosorption equilibrium achieved in 60 min (Nowicka et al. 2016; Tüzün et al. 2005). This demonstrates that biosorption of HM ions is a passive process that occurs relatively rapidly even when algal cells are non-living. In living algae, contact time has a greater effect on biosorption capacity.
Phytohormones
Earlier studies have shown that exogenous application of phytohormones can improve protection against HMs toxicity. Acting as chemical messengers with highly complex regulation, these molecules allow algae to retain growth plasticity during the development. Additionally, phytohormones are collectively the main means by which plants respond to abiotic and biotic stresses (Asgher et al. 2016; Krantev et al. 2008; Masood et al. 2016). Phytohormones (i.e., auxins, cytokinins, gibberellin, and polyamine) alleviate the effects of HMs stress on growth and prevent degradation of photosynthetic pigments, monosaccharides, and proteins. These compounds prompt a mechanism of plant stress tolerance, which is associated with the blockage of HMs entry into the cell and the activation of antioxidant defense responses that reduce oxidative damage stimulated by HMs. Piotrowska-Niczyporuk et al. (2012) clearly indicated the ameliorative influence of auxins, cytokinins, gibberellin, and polyamine on algal resistance to HMs and growth improvement. Jasmonic acid acted as a stressor that stimulated metal biosorption, which led to inhibition of algal growth and metabolite oxidative degradation. These results suggest that phytohormones plays a vital role in the ability of C. vulgaris to grow and develop adaptively in aquatic ecosystems contaminated with HMs (Piotrowska-Niczyporuk et al. 2012). The interactions among HMs, phytohormones, and polyamine are unclear and require further study. Figure 5 shows a schematic representation of phytohormones reaction, including abscisic acid, auxin, brassinosteroids, and ethylene, under HMs exposure.
A schematic illustration showing reactions of some phytohormones under HMs exposure: abscisic acid (a), auxin (b), brassinosteroids (c), and ethylene (d) (Bücker-Neto et al. 2017)
Effect of HMs on the bio-components of algae
Lipid production combined with HMs removal is a cost-effective and environmentally friendly approach for algae biofuel production and waste management (Gupta et al. 2017; Singh et al. 2017). Chlorella minutissima UTEX 2341 had strong resistance to Cd2+, Cu2+, Mn2+, and Zn2+ ions under heterotrophic culture conditions and could efficiently eliminate them through intracellular accumulation and extracellular immobilization (Yang et al. 2015). Lipid accumulation in algal cells was not inhibited by HMs. The algal lipid content was significantly increasing by 21 and 94% with the addition of Cd2+ and Cu2+, respectively (Yang et al. 2015). At low concentrations, HMs such as Pb2+, Al3+, and Co2+ exhibited stimulatory effects on the growth of Dunaliella tertiolecta and Monoraphidium minutum. Arsenate was found to support the growth of cyanobacterium (Nostoc minutum) and microalgae Chlorella salina and Chlorella sp. (Miazek et al. 2015). Table 6 presents the influence of HMs on the lipid contents of algae.
Higher HMs (namely, Cu2+ and Cd2+) concentrations affect Amphora coffearformis by reducing its growth and biochemical compositions (Anantharaj et al. 2011). Table 7 summarizes the impacts of HMs on the carbohydrate contents of algae. Metals in small concentrations are vital for algae cells to achieve cellular functions. They act as components for photosynthetic electron transport proteins (Fe3+ and Cu2+) and photosynthetic water oxidizing centers (Mn2+) and are elements in vitamins (Co2+). They also serve as co-factors for enzymes participating in CO2 fixation (Zn2+ in carbonic anhydrase) (Moroney et al. 2001), DNA transcription (Zn2+ in RNA polymerase), and phosphorus acquisition (Zn2+ in alkaline phosphatase) (Sunda 2012). Table 8 summarizes the effects of HMs on algal protein contents.
Biotechnological improvements of phycoremediation process for HM depilation
Phycoremediation is a part of environmental biotechnology that uses algae to treat contaminants (Amit et al. 2017; Apandi et al. 2019). One emerging research area is the design and development of new algal strains with increased affinity, capacity, and selectivity for biosorption of HM ions (Apandi et al. 2019). Biological mechanisms have been manipulated at the molecular level to develop new biosorbents and to produce genetically modified algae with higher biosorption capacity and selectivity for specific metal ions (Fig. 6). The high cost of conventional approaches using wild algae to decrease toxic metal ions concentrations in water to acceptable regulatory standards has stimulated exploration of genetic and protein-engineering methods to produce cost-effective ‘green’ biosorbents (Abedi 2019; Ansari et al. 2019; Rajamani et al. 2007). Many genes are involved in metal-uptake, detoxification, and tolerance of HMs toxicity (Mrudula et al. 2016). Manipulation of cysteine-rich peptides such as glutathione (GSH), lipopolysaccharides (LPSs), phytochelatins (PCs), and metallothioneins (MTs) that bind metal ions (e.g., Cd, Cu, and Hg) has been suggested for improvement of metal ions bioaccumulation (Godlewska-Zylkiewicz 2001). Tripeptide GSH, a low-molecular-weight thiol, plays a major role in metal ions detoxification by acting as storage for endogenous S and N (Gharieb and Gadd 2004). The genetic manipulation strategy has recently been adopted to increase cell surface MTs or PCs in order to increase the metal ions accumulation capacity of algal cells.
Capacities of microorganisms for bioremediation and biodegradation constitute forms of natural attenuation. However, these capacities may be improved by engineering techniques, either by addition of selected microorganisms (bioaugmentation) or by addition of nutrients (biostimulation). Genetic engineering is also used to develop the biodegradation abilities of microorganisms (Joutey et al. 2013)
The algal biomass that is commercially available is not produced for phycoremediation applications, and thus may not exhibits optimal performance. The use of dead biomass compromises the phycoremediation capacities of living cultures, particularly when dealing with low concentrations of HMs. The currently available approaches of immobilization have not proven to be satisfactory for large-scale applications, due to insufficient biomass production. There are many variables and parameters need to be considered for design and operation of phycoremediation, such as algal selection, containment types with contacting time, biomass recovery, disposal of spent biomass, and economic considerations for overall process.
Conclusion
HMs contamination of aquatic eco-systems is a matter of great concern because of its toxicity towards plants, animals, and human health. Various algal species have been recognized as promising candidates for HMs removal and/or detoxification, and potential low-cost alternatives to physicochemical remediation techniques. HMs removal can be achieved by biosorption and bioaccumulation. The efficiency of HMs removal by algae is influenced by several parameters including pH, temperature, ionic strength, contact time, and presence of counter ions. The supplementation of phytohormones improves algal resistance to HMs toxicity. The genetic manipulation of algae has developed HM-tolerant mutant strains with high specificity and metal removal efficiency. This review directs future research toward the development of a sustainable technology through algal bioremediation for simultaneous treatment of HM-rich wastewaters and massive production for producing biofuel.
References
Abedi S et al (2019) Decoupling a novel Trichormus variabilis-Synechocystis sp. interaction to boost phycoremediation. Sci Rep 9:1–10
Abou-Shanab RAI, Hwang J-H, Cho Y, Min B, Jeon B-H (2011) Characterization of microalgal species isolated from fresh water bodies as a potential source for biodiesel production. Appl Energy 88:3300–3306
Abou-Shanab RA, Ji MK, Kim HC, Paeng KJ, Jeon BH (2013) Microalgal species growing on piggery wastewater as a valuable candidate for nutrient removal and biodiesel production. J Environ Manage 115:257–264
Afkar E, Ababna H, Fathi A (2010) Toxicological response of the green alga Chlorella vulgaris, to some heavy metals. Am J Environ Sci 6:230–237
Ahmad P (2016) Plant metal interaction: emerging remediation techniques. Elsevier, Amsterdam
Ajayan KV, Selvaraju M, Thirugnanamoorthy K (2011) Growth and heavy metals accumulation potential of microalgae grown in sewage wastewater and petrochemical effluents. Pak J Biol Sci 14:805–811
Aksu Z (2001) Equilibrium and kinetic modelling of cadmium (II) biosorption C. vulgaris by in a batch system: effect of temperature. Sep Purif Technol 21:285–294
Alfarra RS, Ali NE, Yusoff MM (2014) Removal of heavy metals by natural adsorbent: review. Int J Biosci 4:130–139
Al-Homaidan AA, Al-Houri HJ, Al-Hazzani AA, Elgaaly G, Moubayed NMS (2014) Biosorption of copper ions from aqueous solutions by Spirulina platensis biomass Arab J Chem 7:57–62
Al-Homaidan AA, Alabdullatif JA, Al-Hazzani AA, Al-Ghanayem AA, Alabbad AF (2015) Adsorptive removal of cadmium ions by Spirulina platensis dry biomass. Saudi J Biol Sci 22:795–800
Alqadami AA, Khan MA, Otero M, Siddiqui MR, Jeon B-H, Batoo KM (2018) A magnetic nanocomposite produced from camel bones for an efficient adsorption of toxic metals from water. J Clean Prod 178:293–304
Amit, Chandra R, Ghosh UK, Nayak JK (2017) Phycoremediation potential of marine microalga Tetraselmis indica on secondary treated domestic sewage for nutrient removal and biodiesel production. Environ Sci Pollut Res Int 24:20868–20875
Anantharaj K, Govindasamy C, Natanamurugaraj G, Jeyachandran S (2011) Effect of heavy metals on marine diatom Amphora coffeaeformis (Agardh. Kutz). Glob J Environ Res 5:112–117
Anastopoulos I, Kyzas GZ (2015) Progress in batch biosorption of heavy metals onto algae. J Mol Liq 209:77–86
Andrade A, Rollemberg M, Nobrega J (2005) Proton and metal binding capacity of the green freshwater alga Chaetophora elegans. Process Biochem 40:1931–1936
Ansari FA, Ravindran B, Gupta SK, Nasr M, Rawat I, Bux F (2019) Techno-economic estimation of wastewater phycoremediation and environmental benefits using Scenedesmus obliquus microalgae. J Environ Manage 240:293–302
Apandi NM, Mohamed R, Al-Gheethi A, Kassim AHM (2019) Microalgal biomass production through phycoremediation of fresh market wastewater and potential applications as aquaculture feeds. Environ Sci Pollut Res Int 26:3226–3242
Asgher M, Per TS, Masood A, Fatma M, Freschi L, Corpas FJ, Khan NA (2016) Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environ Sci Pollut Res 24:1–13
Aude-Garcia C et al (2016) Different in vitro exposure regimens of murine primary macrophages to silver nanoparticles induce different fates of nanoparticles and different toxicological and functional consequences. Nanotoxicology 10:586–596
Aziz N, Jayasuriya N, Fan L (2016) Adsorption study on Moringa oleifera seeds and Musa cavendish as natural water purification agents for removal of lead, nickel and cadmium from drinking water. In: IOP Conference Series: Mater Sci Eng . vol 1. IOP Publishing, p 012044
Babarinde A, Onyiaocha GO (2016) Equilibrium sorption of divalent metal ions onto groundnut (Arachis hypogaea) shell: kinetics, isotherm and thermodynamics. Chem Int 2:37–46
Babu AG, Kim J-D, Oh B-T (2013) Enhancement of heavy metal phytoremediation by Alnus firma with endophytic Bacillus thuringiensis GDB-1. J Hazard Mater 250:477–483
Bakatula E, Cukrowska E, Weiersbye I, Mihaly-Cozmuta L, Peter A, Tutu H (2014) Biosorption of trace elements from aqueous systems in gold mining sites by the filamentous green algae (Oedogonium sp.). J Geochem Explorat 144:492–503
Balarak D, Azarpira H, Mostafapour FK (2016) Thermodynamics of removal of cadmium by adsorption on Barley husk biomass. Der Pharma Chemica 8:243–247
Bayes GS, Raut SS, Patil VR, Lokhande RS (2012) Formation of diazepam-lanthanides (III) complexes in the 50–50 volume% ethanol-water solvent system and study of the effect of temperature on the complex formation constants. J Solut Chem 41:241–248
Birungi Z, Chirwa E (2014) The kinetics of uptake and recovery of lanthanum using freshwater algae as biosorbents: comparative analysis. Bioresour Technol 160:43–51
Brinza L, Dring MJ, Gavrilescu MJEE, Journal M (2007) Marine micro and macro algal species as biosorbents for heavy metals. Environ Eng Manag J 6:237–251
Bücker-Neto L, Paiva ALS, Machado RD, Arenhart RA, Margis-Pinheiro M (2017) Interactions between plant hormones and heavy metals responses. Genet Mol Biol 40:373–386
Bulgariu D, Bulgariu L (2016) Potential use of alkaline treated algae waste biomass as sustainable biosorbent for clean recovery of cadmium (II) from aqueous media: batch and column studies. J Clean Prod 112:4525–4533
Cechinel MA et al (2016) Removal of metal ions from a petrochemical wastewater using brown macro-algae as natural cation-exchangers. Chem Eng J 286:1–15
Chairat M, Bremner JB (2016) Biosorption of lac dye by the red marine alga Gracilaria tenuistipitata: biosorption kinetics, isotherms, and thermodynamic parameters. Color Technol 132:472–480
Chang Y-C (2019) Microbial biodegradation of xenobiotic compounds. CRC Press, Boca Raton
Chmielewská E, Medved J (2001) Bioaccumulation of heavy metals by green algae Cladophora glomerata in a refinery sewage lagoon. Croatica Chemica Acta 74:135–145
Dao LH, Beardall J (2016) Effects of lead on growth, photosynthetic characteristics and production of reactive oxygen species of two freshwater green algae. Chemosphere 147:420–429
Darda S, Papalas T, Zabaniotou A (2019) Biofuels journey in Europe: currently the way to low carbon economy sustainability is still a challenge. J Clean Prod 208:575–588
Davis TA, Volesky B, Mucci A (2003) A review of the biochemistry of heavy metal biosorption by brown algae. Water Res 37:4311–4330
de Namor AFD, El Gamouz A, Frangie S, Martinez V, Valiente L, Webb OA (2012) Turning the volume down on heavy metals using tuned diatomite. A review of diatomite and modified diatomite for the extraction of heavy metals from water. J Hazard Mater 241:14–31
Dixit R et al (2015) Bioremediation of heavy metals from soil and aquatic environment: an overview of principles and criteria of fundamental processes. Sustainability 7:2189–2212
El Nemr A, El-Sikaily A, Khaled A, Abdelwahab O (2015) Removal of toxic chromium from aqueous solution, wastewater and saline water by marine red alga Pterocladia capillacea and its activated carbon. Arab J Chem 8:105–117
Fawzy MA, Issa AA (2016) Bioremoval of heavy metals and nutrients from sewage plant by Anabaena oryzae and Cyanosarcina fontana. Int J Phytoremediat 18:321–328
Furey PC, Deininger A, Liess A (2016) Substratum-associated microbiota. Water Environ Res 88:1637–1671
Furuhashi Y, Honda R, Noguchi M, Hara-Yamamura H, Kobayashi S, Higashimine K, Hasegawa HJBEJ (2019) Optimum conditions of pH, temperature and preculture for biosorption of europium by microalgae Acutodesmus acuminatus. Biochem Eng J 143:58–64
Gao F, Li C, Yang Z-H, Zeng G-M, Mu J, Liu M, Cui W (2016) Removal of nutrients, organic matter, and metal from domestic secondary effluent through microalgae cultivation in a membrane photobioreactor. J Chem Technol Biotechnol 91:2713–2719
Gharieb MM, Gadd GM (2004) Role of glutathione in detoxification of metal(loid)s by Saccharomyces cerevisiae. Biometals 17:183–188
Godlewska-Zylkiewicz B (2001) Analytical applications of living organisms for preconcentration of trace metals and their speciation. Crit Rev Anal Chem 31:175–189
Goher ME, Abdel-Satar A, Ali M, Hussian A, Napiorkowska-Krzebietke A (2016) Biosorption of some toxic metals from aqueous solution using non-living algal cells of Chlorella vulgaris. J Elementol 21:703–713
Gupta VK, Rastogi A, Nayak A (2010) Biosorption of nickel onto treated alga (Oedogonium hatei): application of isotherm and kinetic models. J Colloid Interface Sci 342:533–539
Gupta A, Joia J, Sood A, Sood R, Sidhu C (2016) Microbes as potential tool for remediation of heavy metals: a review. J Microb Biochem Technol 8:364–372
Gupta SK, Sriwastav A, Ansari FA, Nasr M, Nema AK (2017) Phycoremediation: an eco-friendly algal technology for bioremediation and bioenergy production. In: Bauddh K, Singh B, Korstad J (eds) Phytoremediation potential of bioenergy plants. Springer, Singapore, pp 431–456
Hallmann A (2015) Algae biotechnology-green cell-factories on the rise. Curr Biotechnol 4:389–415
He J, Chen JP (2014) A comprehensive review on biosorption of heavy metals by algal biomass: materials, performances, chemistry, and modeling simulation tools. Bioresour Technol 160:67–78
Henriques B et al (2017) Bioaccumulation of Hg, Cd and Pb by Fucus vesiculosus in single and multi-metal contamination scenarios and its effect on growth rate. Chemosphere 171:208–222
Iddou A, Hadj Youcef M, Aziz A, Ouali MS (2011) Biosorptive removal of lead (II) ions from aqueous solutions using Cystoseira stricta biomass: study of the surface modification effect. J Saudi Chem Soc 15:83–88
Issa AA, Fawzy MA, El-Deeb B (2016) Uptake of cadmium by the green alga Scenedesmus quadricauda in the presence of selenium nanoparticles. Int J Nano Chem 2:47–52
Jais NM, Mohamed R, Al-Gheethi A, Hashim MA (2017) The dual roles of phycoremediation of wet market wastewater for nutrients and heavy metals removal and microalgae biomass production. Clean Technol Environ Policy 19:37–52
Jalali-Rad R, Ghafourian H, Asef Y, Dalir S, Sahafipour M, Gharanjik B (2004) Biosorption of cesium by native and chemically modified biomass of marine algae: introduce the new biosorbents for biotechnology applications. J Hazard Mater 116:125–134
Jan S, Parray JA (2016) Approaches to heavy metal tolerance in plants. Springer, Singapore
Jasrotia S, Kansal A, Kishore V (2014) Arsenic phyco-remediation by Cladophora algae and measurement of arsenic speciation and location of active absorption site using electron microscopy. Microchem J 114:197–202
Joutey NT, Bahafid W, Sayel H, El Ghachtouli N (2013) Biodegradation: involved microorganisms and genetically engineered microorganisms. Biodegradation-life of science. InTech, Rijeka, pp 289–320
Juang YJ, Chang JS (2016) Applications of microfluidics in microalgae biotechnology: a review. Biotechnol J 3:327–335
Kanchana S, Jeyanthi J, Kathiravan R, Suganya K (2014) Biosorption of heavy metals using algae: a review. Int J Pharma Med Biol Sci 3:1
Khan MA, Ngabura M, Choong TS, Masood H, Chuah LA (2012) Biosorption and desorption of nickel on oil cake: batch and column studies. Bioresour Technol 103:35–42
Khan S, Shamshad I, Waqas M, Nawab J, Ming L (2017) Remediating industrial wastewater containing potentially toxic elements with four freshwater algae. Ecol Eng 102:536–541
Kleinübing SJ, Gai F, Bertagnolli C, da Silva MGC (2013) Extraction of alginate biopolymer present in marine alga Sargassum filipendula and bioadsorption of metallic ions. Mater Res 16:481–488
Kobielska PA, Howarth AJ, Farha OK, Nayak S (2018) Metal-organic frameworks for heavy metal removal from water. Coord Chem Rev 358:92–107
Kotrba P (2011) Microbial biosorption of metals—general introduction. In: Kotrba P, Mackova M, Macek T (eds) Microbial biosorption of metals. Springer, Dordrecht, pp 1–6
Krantev A, Yordanova R, Janda T, Szalai G, Popova L (2008) Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J Plant Physiol 165:920–931
Kumar KS, Dahms H-U, Won E-J, Lee J-S, Shin K-H (2015) Microalgae—a promising tool for heavy metal remediation. Ecotoxicol Environ Saf 113:329–352
Kumar D, Pandey LK, Gaur J (2016) Metal sorption by algal biomass: from batch to continuous system. Algal Res 18:95–109
Kwaansa-Ansah EE, Nti SO, Opoku F (2019) Heavy metals concentration and human health risk assessment in seven commercial fish species from Asafo Market, Ghana. Food Sci Biotechnol 28:569–579
Lahiri S, Ghosh D, Bhakta JN (2017) Role of microbes in eco-remediation of perturbed aquatic ecosystem. In: Bhakta J (ed) Handbook of research on inventive bioremediation techniques. IGI Global, Hershey, pp 70–107
Li M, Hu C, Zhu Q, Chen L, Kong Z, Liu Z (2006) Copper and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in the microalga Pavlova viridis (Prymnesiophyceae). Chemosphere 62:565–572
Liu Z-Y, Wang G-C, Zhou B-C (2008) Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour Technol 99:4717–4722
Lou Z et al (2015) Brown algae based new sorption material for fractional recovery of molybdenum and rhenium from wastewater. Chem Eng J 273:231–239
Luo F, Liu Y, Li X, Xuan Z, Ma J (2006) Biosorption of lead ion by chemically-modified biomass of marine brown algae Laminaria japonica. Chemosphere 64:1122–1127
Mahdavi H, Ulrich AC, Liu Y (2012) Metal removal from oil sands tailings pond water by indigenous micro-alga. Chemosphere 89:350–354
Mantzorou A, Navakoudis E, Paschalidis K, Ververidis F (2018) Microalgae: a potential tool for remediating aquatic environments from toxic metals. Int J Environ Sci Technol 16:1815–1830
Masood A, Khan MIR, Fatma M, Asgher M, Per TS, Khan NA (2016) Involvement of ethylene in gibberellic acid-induced sulfur assimilation, photosynthetic responses, and alleviation of cadmium stress in mustard. Plant Physiol Biochem 104:1–10
Matamoros V, Gutierrez R, Ferrer I, Garcia J, Bayona JM (2015) Capability of microalgae-based wastewater treatment systems to remove emerging organic contaminants: a pilot-scale study. J Hazard Mater 288:34–42
Miazek K, Iwanek W, Remacle C, Richel A, Goffin D (2015) Effect of metals, metalloids and metallic nanoparticles on microalgae growth and industrial product biosynthesis: a review. Int J Mol Sci 16:23929–23969
Moroney J, Bartlett S, Samuelsson G (2001) Carbonic anhydrases in plants and algae. Plant Cell Environ 24:141–153
Mrudula V, Vijaya T, Mouli KC, Jyothi UN, Aishwarya S, Reddy VD (2016) Novel method for removal of heavy metals by using low cost absorbents. Indo Am J Pharm Res 6:5472–5480
Namdeti R, Pulipati K (2014) Lead removal from aqueous solution using Ficus hispida leaves powder. Desalin Water Treat 52:339–349
Nowicka B, Pluciński B, Kuczyńska P, Kruk J (2016) Physiological characterization of Chlamydomonas reinhardtii acclimated to chronic stress induced by Ag, Cd, Cr, Cu and Hg ions. Ecotoxicol Environ Saf 130:133–145
Oyetibo GO, Miyauchi K, Huang Y, Chien M-F, Ilori MO, Amund OO, Endo G (2016) Biotechnological remedies for the estuarine environment polluted with heavy metals and persistent organic pollutants. Int Biodeterior Biodegrad 119:614–625
Park DM et al (2016) Bioadsorption of rare earth elements through cell surface display of lanthanide binding tags. Environ Sci Technol 50:2735–2742
Parmar M, Thakur LS (2013) Heavy metal Cu, Ni and Zn: toxicity, health hazards and their removal techniques by low cost adsorbents: a short overview. Int J Plant Anim Environ Sci 3:2231–4490
Perales-Vela HV, Pena-Castro JM, Canizares-Villanueva RO (2006) Heavy metal detoxification in eukaryotic microalgae. Chemosphere 64:1–10
Piotrowska-Niczyporuk A, Bajguz A, Zambrzycka E, Godlewska-Żyłkiewicz B (2012) Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Plant Physiol Biochem 52:52–65
Pokethitiyook P, Poolpak T (2016) Biosorption of heavy metal from aqueous solutions. Phytoremediation. Springer, Cham, pp 113–141
Poo K-M, Son E-B, Chang J-S, Ren X, Choi Y-J, Chae K-J (2018) Biochars derived from wasted marine macro-algae (Saccharina japonica and Sargassum fusiforme) and their potential for heavy metal removal in aqueous solution. J Environ Manag 206:364–372
Priyadarshini E, Priyadarshini SS, Pradhan N (2019) Heavy metal resistance in algae and its application for metal nanoparticle synthesis. Appl Microbiol Biotechnol 7:1–20
Quan Q, Chen Y, Ma Q, Wang F, Meng X, Wang B (2016) The impact of atmospheric deposition of cadmium on dominant algal species in the East China Sea. J Ocean Univ China 15:271–282
Raeesossadati M, Ahmadzadeh H, McHenry M, Moheimani N (2014) CO2 bioremediation by microalgae in photobioreactors: impacts of biomass and CO2 concentrations, light, and temperature. Algal Res 6:78–85
Raikova S et al (2016) Assessing hydrothermal liquefaction for the production of bio-oil and enhanced metal recovery from microalgae cultivated on acid mine drainage. Fuel Process Technol 142:219–227
Rajamani S, Siripornadulsil S, Falcao V, Torres M, Colepicolo P, Sayre R (2007) Phycoremediation of heavy metals using transgenic microalgae. Adv Exp Med Biol 616:99–109
Rijstenbil JW, Gerringa LJA (2002) Interactions of algal ligands, metal complexation and availability, and cell responses of the diatom Ditylum brightwellii with a gradual increase in copper. Aquat Toxicol 56:115–131
Rodrigo WD, Eder CS, de Roberta PM, Alexandra L, Marcelo M, Paulo AH, Zenilda LB (2012) Effects of cadmium on growth, photosynthetic pigments, photosynthetic performance, biochemical parameters and structure of chloroplasts in the agarophyte Gracilaria domingensis (Rhodophyta, Gracilariales). Am J Plant Sci 3:1077–1084
Salama E-S, Kurade MB, Abou-Shanab RA, El-Dalatony MM, Yang I-S, Min B, Jeon B-H (2017) Recent progress in microalgal biomass production coupled with wastewater treatment for biofuel generation. Renew Sustain Energy Rev 79:1189–1211
Sargın İ, Arslan G, Kaya M (2016) Efficiency of chitosan-algal biomass composite microbeads at heavy metal removal. React Funct Polym 98:38–47
Schiewer S (1999) Modelling complexation and electrostatic attraction in heavy metal biosorption by Sargassum biomass. J Appl Phycol 11:79–87
Schiewer S, Wong M (2000) Ionic strength effects in biosorption of metals by marine algae. Chemosphere 41:271–282
Sheng PX, Tan LH, Chen JP, Ting YP (2005) Biosorption performance of two brown marine algae for removal of chromium and cadmium. J Dispers Sci Technol 25:679–686
Singare P, Lokhande R, Pathak P (2010) Study on physico-chemical properties and heavy metal content of the soil samples from Thane Creek of Maharashtra, India. Interdisip Environ Rev 11:38–56
Singh N, Raghubanshi A, Upadhyay A, Rai U (2016) Arsenic and other heavy metal accumulation in plants and algae growing naturally in contaminated area of West Bengal, India. Ecotoxicol Environ Saf 130:224–233
Singh N, Upadhyay A, Rai U (2017) Algal technologies for wastewater treatment and biofuels production: an integrated approach for environmental management. In: Gupta S, Malik A, Bux F (eds) Algal biofuels. Springer, Cham, pp 97–107
Sooksawat N, Meetam M, Kruatrachue M, Pokethitiyook P, Inthorn D (2016) Equillibrium and kinetic studies on biosorption potential of charophyte biomass to remove heavy metals from synthetic metal solution and municipal wastewater. Bioremediat J 20:240–251
Sunda W (2012) Feedback interactions between trace metal nutrients and phytoplankton in the ocean. Front Microbiol 3:204
Sweetly J (2014) Macroalgae as a potentially low-cost biosorbent for heavy metal removal: a review. Int J Pharm Biol Arch 5:17–26
Torres EM, Hess D, McNeil BT, Guy T, Quinn JC (2017) Impact of inorganic contaminants on microalgae productivity and bioremediation potential. Ecotoxicol Environ Saf 139:367–376
Tüzün I, Bayramoğlu G, Yalçın E, Başaran G, Celik G, Arıca MY (2005) Equilibrium and kinetic studies on biosorption of Hg(II), Cd (II) and Pb(II) ions onto microalgae Chlamydomonas reinhardtii. J Environ Manage 77:85–92
Ungureanu G, Filote C, Santos SC, Boaventura RA, Volf I, Botelho CM (2016) Antimony oxyanions uptake by green marine macroalgae. J Environ Chem Eng 4:3441–3450
Vilar VJ, Botelho CM, Boaventura RAJPB (2005) Influence of pH, ionic strength and temperature on lead biosorption by Gelidium and agar extraction algal waste. Process Biochem 40:3267–3275
Vogel M, Günther A, Rossberg A, Li B, Bernhard G, Raff J (2010) Biosorption of U (VI) by the green algae Chlorella vulgaris in dependence of pH value and cell activity. Sci Total Environ 409:384–395
Volesky B (2007) Biosorption and me. Water Res 41:4017–4029
Wang J, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27:195–226
Wang S, Vincent T, Faur C, Guibal E (2016a) Alginate and algal-based beads for the sorption of metal cations: Cu (II) and Pb(II). Int J Mol Sci 17:1453
Wang X-X, Wu Y-H, Zhang T-Y, Xu X-Q, Dao G-H, Hu H-Y (2016b) Simultaneous nitrogen, phosphorous, and hardness removal from reverse osmosis concentrate by microalgae cultivation. Water Res 94:215–224
Warmate A, Ideriah T, ARI IT, Inyang UU, Ibaraye T (2011) Concentrations of heavy metals in soil and water receiving used engine oil in Port Harcourt, Nigeria. J Ecol Nat Environ 3:54–57
Wu Y-C, Xiao Y, Wang Z-J, Zhao F (2016) Performance of bioelectrochemical systems inoculated with Desmodesmus sp. A8 under different light sources. Bioremed J 20:233–239
Yang J, Cao J, Xing G, Yuan H (2015) Lipid production combined with biosorption and bioaccumulation of cadmium, copper, manganese and zinc by oleaginous microalgae Chlorella minutissima UTEX2341. Bioresour Technol 175:537–544
Yang I-S et al (2016) Cultivation and harvesting of microalgae in photobioreactor for biodiesel production and simultaneous nutrient removal. Energy Convers Manage 117:54–62
Yi Q, Fan R, Xie F, Zhang Q, Luo Z (2016) Recovery of palladium (II) from nitric acid medium using a natural resin prepared from persimmon dropped fruits residues. J Taiwan Inst Chem Eng 61:299–305
Yu Q, Kaewsarn P (1999) Fixed-bed study for copper (II) removal from aqueous solutions by marine alga Durvillaea potatorum. Environ Technol 20:1005–1008
Zabochnicka-Świątek M, Krzywonos M (2014) Potentials of biosorption and bioaccumulation processes for heavy metal removal. Mercury 6(1):145
Zeraatkar AK, Ahmadzadeh H, Talebi AF, Moheimani NR, McHenry MP (2016) Potential use of algae for heavy metal bioremediation, a critical review. J Environ Manage 181:817–831
Zhang X, Zhao X, Wan C, Chen B, Bai F (2016) Efficient biosorption of cadmium by the self-flocculating microalga Scenedesmus obliquus AS-6-1. Algal Res 16:427–433
Zhu M, Wachs IE (2016) Determining number of active sites and TOF for the high-temperature water gas shift reaction by iron oxide-based catalysts. ACS Catal 6:1764–1767
Acknowledgements
This research was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (Grant No. 2017R1A2B2004143), and by a Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korean government (MSIP) (Grant No. KETEP-20163010092250).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salama, ES., Roh, HS., Dev, S. et al. Algae as a green technology for heavy metals removal from various wastewater. World J Microbiol Biotechnol 35, 75 (2019). https://doi.org/10.1007/s11274-019-2648-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-019-2648-3