Abstract
This study was conducted to determine trace metal concentrations (Cd, Cu, Zn, Pb and Hg) in blood and three egg fractions from Eretmochelys imbricata nesting on Qeshm Island in Iran. The results showed detectable levels of all analytes in all fractions. Pb and Hg were detectable in the blood and eggs, reflecting a maternal transfer. With the exception of Cu and Pb, analyzed elements in eggs were concentrated in yolk. Only Zn in blood had a significant correlation with the body size and weight (p < 0.01). It appears that Hawksbill sea turtles can regulate Zn concentrations through homeostatic processes to balance metabolic requirements. The relatively low concentrations of metals in blood support the knowledge that E. imbricata feed mainly on the low trophic levels. All essential and non-essential elements were detectable in blood and in eggs of the hawksbill, reflecting a maternal transfer. Consequently, movement patterns, home ranges of foraging grounds, and availability of food could explain variations in trace element concentrations among female turtles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
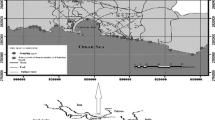
Iran has a coastline extending almost 1,800 km on the northern boundaries of the Persian Gulf and the Sea of Oman (Kami 1997) (Fig. 1). Qeshm Island, with about 1,495 km2, is the largest Island in the area.Some parts of Qeshm provide suitable habitat for nesting hawksbills and foraging green turtles. However, several classes of anthropogenic pollutants discharged into the marine ecosystem of the Persian Gulf have the potential to negatively impact the future growth and/or survival of this population. Heavy metal concentration in sea turtles is mainly determined by environmental exposure (Gardner et al. 2006). Sea turtles are of increasing interest as potential bioindicators for heavy metal accumulation in marine ecosystems. They are long-lived vertebrates that ingest organic and inorganic contaminants from food, sediment and water. According to the classical model, females migrate directly from the nesting beaches (Shibderaz in this study) to identifiable feeding areas (Hengam Island in this study) where they remain until they return to nesting beach again in 2–3 years. Based on this model, we suggest that by analyzing tissues from nesting hawksbills sampled in Shibderaz beach, Qeshm Island, we can quantify the concentration of metals from the hawksbill foraging grounds in the waters from the north coast of Persian Gulf region. Due to the wide geographic distribution that sea turtles cover during their life cycle, they may serve as meaningful ‘‘biomonitors’’ for overall ecosystem health. For this reason it is especially important to document and understand any intrinsic factors that affect survival or reproduction (Kampalath et al. 2006). All species of sea turtles are regarded as endangered or threatened and are listed in Appendix I of the Convention of International Trade in Endangered Species (IUCN 2003). In Iran, sea turtles are also classified as endangered species and, additionally, they are considered as priority species for conservation. We hypothesize that blood and eggs will be suitable indicators for monitoring trace metals and other contaminants in nesting turtles. It is of more interest to toxicologists and sea turtle conservationists to obtain information about the contamination of the live animals in a population. Therefore, we emphasize that we are contributing to a growing body of researching examining contaminants in healthy, free-range individuals, which will provide more robust information about the potential risk presented by such contaminants to endangered sea turtle populations. A non-lethal method for sampling blood from the dorsal cervical sinuses in the neck of sea turtles was developed by Owens and Ruiz (1980). The present study was conducted to assess the variations and relationships among trace metal concentrations in maternal blood and freshly laid eggs from each mother of E. imbricata. Additionally, the maternal transfer was estimated quallitaitvely through the metal excretion rate on the basis of one nesting season of this turtle that arrived at Shibderaz beaches in Qeshm Island, along the North coast of Persian Gulf.
Materials and Methods
Sampling
Eggs and blood of E. imbricata were sampled (permit Hormozgan Environmental Organization No. 25992) from Shibderaz, Qeshm Island, Iran during the nesting season between April and June 2011(Fig. 1). Twelve female Hawksbill individuals were haphazardly selected assuming to provide a representative distribution of size classes; the total weight of individual turtles was determined and curved carapace length (CCL) and width (CCW) were measured after nesting was concluded. After biometric measurements, blood and egg samples were collected from each female turtle; blood samples were taken from the dorsal cervical sinus using a sterile plastic syringe and needle in order to collect 5–10 mL that were immediately placed in an acid-washed (Moody and Lindstrom 1977) polyethylene tube. During blood extraction from each individual, careful cleaning of the neck region (with ethanol and deionized water) prior to sampling was practiced. Four eggs from each female were haphazardly collected at the time of oviposition before they touched sand. All samples were kept under fresh conditions (4°C) and were transported to the laboratory.
Trace Metal Analyses
Egg samples were rinsed with deionized water to remove any particulate matter that might have adhered. Next, eggs were weighed and sized and subsequently separated into shell, albumen, and yolk. The separation was carried out quickly to prevent thawing. For the analysis, eggs were separated into three fractions and randomly groped in pools of 2; that is, each individual was represented by two pools (A and B) containing the three fractions of eggs (four eggs from each turtle); fractions were randomly dispersed in the two groups. Blood samples were individually transported and processed. Glassware and plastic materials used for handling and transportation of samples were thoroughly acid-washed to prevent contamination of samples (Moody and Lindstrom 1977). Blood and pooled samples of eggs were freeze-dried (72 h at – 49°C and 133 × 10−3 mbar) and then powdered. Powdered samples (0.25 g) were digested with quartz-distilled concentrated nitric acid (5 mL) in hot plate equipment (PC 420D) under established conditions (MESL 1997). The digested material was finally diluted to 50 mL using deionized water and stored in polyethylene containers for further analysis. All samples were analyzed in triplicate for Cd, Pb, Cu and Zn by Atomic Absorption Spectrophotometer (Shimadzu, model SOLAAR M5, England) and for Hg by LECO AMA254 Advanced Mercury Analyzer.
Data Analyses
Normality and homoscedasticity of data were assessed by a Kolmogorov–Smirnov test respectively. In order to define statistical differences (p < 0.05) among mean metal concentrations in blood and the three fractions of eggs, a one way ANOVA test was used. Correlations of metal concentrations of egg fractions and blood with body size (CCL) and weight were determined separately using simple regression analyses, establishing correlation coefficients (significant when p < 0.05) as the indicator of correlation. Statistical analyses were carried out using SPSS version 16.0.
Results and Discussion
Biometric Data
The mean size of female turtles(N = 12) was 43.02 ± 12.6 kg (CCL 63.54 ± 10.23 cm; CCW 57.17 ± 9.05 cm); mean egg diameter and egg weight (N = 48) was 3.64 ± 0.01 cm and 29.77 ± 3.73 g, respectively.
Metal Concentrations
Trace metal concentrations of egg fractions and blood of E. imbricata are presented in Table 1. For comparative purposes, data that were originally presented on a wet weight basis were converted into dry weights using the mean water content of each egg fraction correspondent; water content determined in this study was used [albumen, 97.3 % ± 1.9 % (n = 48); yolk, 62.5 % ± 2.9 % (n = 48); eggshell, 59.0 % ± 5.6 % (n = 48)]. Blood concentrations of Cu and Zn found in E. imbricata are less than those found in D. coriacea (Guirlet et al. 2008), L. Kempii (Kenyon et al. 2001) and L. olivacea (Pa′ez-Osuna et al. 2010), but the levels of Cd was within of the range of concentrations reported for D. coriacea (Guirlet et al. 2008), L. Kempii (Kenyon et al. 2001) and L. olivacea (Pa′ez-Osuna et al. 2010). Day et al. (2005) found significant positive correlations between blood mercury levels and mercury concentrations in the muscle and spinal cord of C. caretta. Similarly, Keller et al. (2004) found strong correlations between blood and carapace fat for a number of organochlorine compounds. Females seem to ingest a significant volume of water to decrease their body temperature in warm waters of nesting tropical beaches (Southwood et al. 2005) and to ensure egg production (albumin is mainly composed of water; Wallace et al. 2006) which would explain the heavy metals are higher in blood than egg fractions. Blood is a physiologic medium of interchange and transport of substances among the tissues of organisms; its flux in the different organs and tissues varies significantly (Guyton 1977). Day et al. (2005) proposed the use of blood for monitoring trace metal exposure in marine turtles because it is possible to predict the load in internal tissues. The decreasing order of metal concentrations in the analyzed materials in E. imbricata from Qeshm Island was as follows: for Zn, blood > Yolk > eggshell > albumen; for Cu, Egg shell > blood > albumen > yolk; for Cd, albumen > yolk > eggshell > blood; for Pb, egg shell > yolk > albumen > blood and for Hg, blood > albumen > eggshell > yolk.Trace metal concentrations, including standard deviation, in the eggshell, albumen, yolk, and blood of E. imbricata turtle are shown in Fig. 2. In general, Zn concentrations varied among sampled individuals (Fig. 2), the CV (SD × 100/mean) was consistently the lowest (<10.6 %) found in all analyzed egg fractions. Therefore, it appears that Hawksbill sea turtle can regulate Zn concentrations through homeostatic processes in balance between metabolic requirements and prevention against toxic effects. On the other hand, the relatively high CV was found for Cd (21.4 %–175.8 %), Hg (27.9 %–100.0 %), Pb (13.7 %–75.6 %) and Cu (15.3 %–46.5 %), at the three egg fractions, it implying that such elements are not actively controlled by female turtles and probably their levels change as a function of the degree of exposure (Pa′ez-Osuna et al. 2010).
The multiple comparisons of means of metals in egg fractions produced various significant (p < 0.05) differences: The Zn mean concentration was higher in the yolk than the other tissues; the presented data indicate that shell and albumen were not significantly different from each other but both were significantly lower than yolk (Fig. 2). Cu and Pb mean concentrations were highest in the egg shell than the other tissues. The Cd concentration was higher in the albumen, whereas Hg concentration was higher in blood. Similar metal results were found in Caretta caretta by Sakai et al. (1995), Kaska and Furness (2001) and L. olivacea by Pa´ez-Osuna et al. (2010). Non-essential metals include Mercury (Hg), Lead (Pb) and Cadmium (Cd) although several essential metals, notably Zinc (Zn) and Copper (Cu), can act as toxicants at elevated concentrations in organisms (Devkota and Schmidt 1999). Cu had a significant concentration in eggshell and albumen. The concentration of essential metals in yolk are important because they contribute to the physiological processes for development of the embryo, such metals being transferred from mother to eggs, whereas for nonessential metals, the transference is more limited (Storelli and Marcotrigiano 2003).
Considering the proportions of each egg fraction (albumen, 3.85 %; yolk, 81.2 %; eggshell, 14.95 % in dry weight) and concentrations of each metal in each case, it is observed that the highest load or percentage of Cd and Cu was incorporated in the yolk (Fig. 3); in the eggshell, Cu and Pb contribute with a load of 51.9 % and 41.5 %, respectively. Yolk represents the higher portion of egg in weight (81.2 %) and constitutes, with the exception of Cu, the main fraction where the highest load of the analyzed metals occurs. This confirms the importance of the yolk in the accumulation of trace metals in marine turtles (Godley et al. 1999; Sakai et al. 2000; Pa´ez-Osuna et al. 2010). In contrast, considering the weights and proportions of metals in each egg fraction, the trace metal content (mg/kg dry weight) of whole eggs (i.e., albumen + yolk + eggshell) were calculated: 12.8 ± 5.2 for Cu, 43.2 ± 14.4 for Zn, 1.2 ± 1.2 for Cd, and 0.009 ± 0.01 for Hg. Zn concentrations were lower than those reported by Sakai et al. (1995), Guirlet et al. (2008) and Pa´ez-Osuna et al. (2010) in eggs of C. caretta, D. coriacea and L. olivacea respectively; Cu and Cd were lower than levels in eggs of C. caretta reported by Sakai et al. (1995) but resulted to be comparable levels in eggs of L. olivacea (Pa´ez-Osuna et al. 2010). Such differences could be attributed to the diet of Persian Gulf hawksbills, and the bioaccumulation of metals by sponges and other benthic invertebrates which comprise the majority of the hawksbill diet. Sea turtles are opportunistic omnivorous, consuming whatever is available. Hatchlings and pelagic turtles typically consume what is available at the surface, whereas older, larger benthic turtles consume food throughout the water column, with a greater emphasis on benthos. Hawksbill sea turtles are omnivorous, feeding in coastal waters on a diet that consists primarily of sponges. They are selective feeders choosing only certain species of sponges of which are toxic to other animals. Sea jellies and other coelenterates are also common prey for hawksbill turtles. They also eat mollusks, fish, marine algae, crustaceans and other sea plants and animals (Meylan 1988). Coastal habitats are often in close proximity to sources of persistent organic pollutants (POPs) and heavy metals, which make their way into the marine environment from industrial, domestic and agricultural sources (Newman and Unger 2003). These chemicals accumulate in marine animals nearly exclusively through their diet (Langston and Spence 1995). An additional factor that should be considered is the elevated mobility of E. imbricata turtles across the Persian Gulf; potentially enhanceing exposure to environmental toxicants. Coastal waters in the northern part of Hormuz Strait in the Persian Gulf, receive large inputs of anthropogenic pollutants through industrial and urban discharges, atmospheric deposition, and terrestrial drainage (Mohammadizadeh et al. 2013), Thus, movement patterns as well as availability of food could both contribute to variations in trace element concentrations among turtles.The monitoring of metals in E. imbricata therefore provides vital information about the health of individuals and populations and is an important area of sea turtle conservation research.
Metal Correlations
Because no reliable age determination methods exist for sea turtles (Bjorndal et al. 1998), we used the body size parameters CCL, CWL, and weight to evaluate growth related variations in trace element concentrations of egg fractions and blood. Zn content in blood showed positive correlation with the body size and weight (as a proxy for age) (p < 0.01). Independently of such a result, the expected behavior is that bigger organisms will accumulate more Zn which indicates bioaccumulation. Weights of eggs had no significant correlation with concentration of metals analyzed in each fraction of eggs (p > 0.05).
In the case of load of metal in the blood of the nesting females versus the metal levels found in the fractions of eggs, Zn had positive significant correlation (p < 0.05). This indicates that when Zn is increased in the female turtles, the concentration of Zn is increased in each fractions of egg. Few studies have reported data on trace elements in sea turtle blood and eggs because most of the available data involve stranded turtles and consequently maternal transfer of trace elements to eggs in sea turtles is poorly known and should be examined to assess risk for incubation success (Guirlet et al. 2008).
Maternal Transfer of Metals
In birds, amphibians and reptiles, eggs receive their initial burden of POPs and heavy metals with maternal transfer during egg formation (Hopkins et al. 2006). After migration to the nesting site, parental investment is limited to the nutrients and energy invested in the yolk that will support embryonic development and the post natal period of the hatchlings (Hewavisenthi and Parmenter 2002). In reptiles, while Cu and Zn have a paramount role in the growth and the tissue development of embryo, Cd, Hg and Pb are particularly toxic at this key period of development (Wolfe et al. 1998). In our study, concentrations of essential elements are higher in eggs and blood compared to non-essential elements, reflecting the lower exposure of hawksbill turtles in our study to these toxic metals. All essential and non-essential elements were detectable in blood and in eggs of the hawksbill, reflecting a maternal transfer.
Permeability of eggshells to soil contaminants should also be considered as a way of contamination that could affect hatching success of the nest for reptile species with permeable eggshells deposited in contaminated substrate (Marco et al. 2004). However, Nagle et al. (2001) found that slider turtles (T. scripta) inhabiting contaminated basins accumulated multiple contaminants, including Cd, without transferring it to eggs, while our results clearly show a maternal transfer of cadmium to eggs. Therefore, maternal transfer is likely to depend on the species, the level of contamination and the nature of the element considered.
Conclusions
The present study provides the first data on baseline trace element concentrations in wild hawksbill turtles from North coast of Persian Gulf. Whole blood has proven useful for measuring trace element levels in turtles. Levels of toxic metals such as Hg, Cd and Pb were low in the turtles sampled for this study but always detectable in blood and eggs suggesting a maternal transfer. The relatively low metal concentrations found in the blood samples may be attributed to dietary specialization; E. imbricata feed heavily on sponges which occupy a low trophic level. Our findings, in combination with information from the referenced studies (Wolfe et al. 1998; Sakai et al. 1995; Pa´ez-Osuna et al. 2010), suggest that Cd and Hg levels examined are relatively low in adult E. imbricata, these levels may pose a hazard to the developing embryo after maternal transfer. Further investigations are needed to better understand the exact role of trace elements in sea turtle development as well as their potential relationship and adverse effects on embryonic development and subsequent hatching success due to maternal and environmental contamination.
References
Bjorndal K, Bolten A, Bennet R, Jacobson ER, Wronski TJ, Valeski JJ, Eliazar PJ (1998) Age and growth in sea turtles: limitations of skeletochronology for demographic studies. Copeia 1:23–30
Day RD, Christopher SJ, Becker PR, Whitaker DW (2005) Monitoring mercury in the loggerhead sea turtle, Caretta caretta. Environ Sci Tech 39:437–446
Devkota B, Schmidt GH (1999) Effects of heavy metals (Hg2+, Cd2+, Pb2+) during the embryonic development of acridid grasshoppers (Insecta Caelifera). Arch Environ Contam Toxicol 36:405–414
Gardner SC, Fitzgerald SL, Vargas BA, Rodríguez LM (2006) Heavy metal accumulation in four species of sea turtles from the Baja California peninsula, Mexico. Biometals 19:91–99
Godley BJ, Thompson DR, Furness RW (1999) Do heavy metal concentrations pose a threat to marine turtles from the Mediterranean Sea? Mar Pollut Bull 38(6):497–502
Guirlet E, Das K, Girondot M (2008) Maternal transfer of trace elements in leatherback turtles (Dermochelys coriacea) of French Guiana. Aquat Toxicol 88:267–276
Guyton AC (1977) Tratado de fisiologı´a me´dica. Nueva Editorial Interamericana, Me´xico
Hewavisenthi S, Parmenter CJ (2002) Egg components and utilization of yolk lipids during development of the flatback turtle Natator depressus. J Herpetol 36:43–50
Hopkins WA, DuRant SE, Staub BP, Rowe CL, Jackson BP (2006) Reproduction, embryonic development, and maternal transfer of contaminants in the amphibian Gastrophryne carolinensis. Environ Health Perspect 114:661–666
IUCN (2003) IUCN red list of threatened species. Gland, Switzerland: IUCN, 2003. http://www.redlist.org
Kami Haji Goli (1997) First record of the Olive Ridley Turtle in Iranian Coastal waters. Zool Middle East 15:67–70
Kampalath R, Gardner SC, Méndez-Rodríguez L, Jay JA (2006) Total and methylmercury in three species of sea turtles of Baja California Sur. Mar Pollut Bull 52:1784–1832
Kaska Y, Furness RW (2001) Heavy metals in marine turtle eggs and hatchlings in the Mediterranean. Zool Middle East 24:127–132
Keller JM, Kucklick JR, Harms CA, McClellan-Green PD (2004) Organochlorine contaminants in sea turtles: correlations between whole blood and fat. Environ Toxicol Chem 23:726–738
Kenyon LO, Landry AM, Gill GA (2001) Trace metal concentrations in blood of the kemp’s ridley sea turtle (Lepidochelys kempii). Chelonian Conserv Biol 4:128–135
Lam JCW, Tanabe S, Chan SKF, Lam MHW, Martin, M, Lam PKS (2006) Levels of trace elements in green turtle eggs collected from Hong Kong: evidence of risks due to selenium and nickel. Environ Pollut 144:790–801
Lam JCW, Tanabe S, Chan SKF, Yuen EKW, Lam MHW, Lam PKS (2004) Trace element residues in tissues of green turtles (Chelonia mydas) from South China waters. Mar Pollut Bull 48:164–192
Langston WJ, Spence SK (1995) Biological factors involved in metal concentrations observed in aquatic organisms. In: Tessier A, Turner DR (eds) Metal speciation and bioavailability in aquatic systems. Wiley, Chichester, pp 407–478
Marco A, Lopez-Vicente M, Perez-Mellado V (2004) Arsenic uptake by reptile flexible-shelled eggs from contaminated nest substrates and toxic effect on embryos. Bull Environ Contam Toxicol 72:983–990
Meylan A (1988) Spongivory in hawksbill turtles: a diet of glass. Science 239:393–395
Mohammadizadeh M, Darvish Bastami K, Kazaali A, Ehsanpour M, Afkhami M (2013) Effects of PAHs on blood thyroidal hormones of Liza klunzingeri in the northern part of Hormuz strait (Persian Gulf). Comp Clin Pathol. doi:10.1007/s00580-013-1725-5
Moody JR, Lindstrom RN (1977) Selection and cleaning of plastic containers for storage of trace element samples. Anal Chem 49:2264–2267
Nagle RD, Rowe CL, Congdon JD (2001) Accumulation and selective maternal transfer of contaminants in the turtle Trachemys scripta associated with coal ash deposition. Arch Environ Contam Toxicol 40:531–536
Newman MC, Unger MA (2003) Fundamentals of ecotoxicology. Lewis, Boca Raton
Owens DW, Ruiz GJ (1980) New methods of obtaining blood and cerebrospinal fluid from marine turtles. Herpetologica 36:17–20
Pa´ez-Osuna F, Caldero´n-Campuzano MF, Soto-Jime´nez MF, Ruelas- Inzunza JR (2010) Lead in blood and eggs of the sea turtle, Lepidochelys olivacea, from the Eastern Pacific: concentration, isotopic composition and maternal transfer. Mar Poll Bull 60:433–439
Sakai H, Ichihashi H, Suganuma H, Tatsukawa R (1995) Heavy metal monitoring in sea turtles using eggs. Mar Pollut Bull 30(5):347–353
Sakai H, Saeki K, Ichihashi H, Suganuma H, Tanabe S, Tatsukawa R (2000) Species-specific distribution of heavy metals in tissues and organs of loggerhead turtle (Caretta caretta) and green turtle (Chelonia mydas) from Japanese coastal waters. Mar Pollut Bull 40:701–709
Southwood AL, Andrews RD, Paladino FV, Jones DR (2005) Effects of diving and swimming behavior on body temperatures of Pacific leatherback turtles in tropical seas. Physiol Biochem Zool 78:285–297
Storelli MM, Marcotrigiano GO (2003) Heavy metal residues in tissues of marine turtles. Mar Pollut Bull 46:397–400
van de Merwe JP, Hodge M, Olszowy HA, Whittier JM, Lee SY (2010) Using blood samples to estimate persistent organic pollutants and metals in green sea turtles (Chelonia mydas). Mar Pollut Bull 60:579–588
Wallace BP, Sotherland PR, Tomillo PS, Bouchard SS, Reina RD, Spotila JR, Paladino FV (2006) Egg components, egg size, and hatchling size in leatherback turtles. Comp Biochem Physiol A Mol Integr Physiol 145:524–532
Wolfe MF, Schwarzbach S, Sulaiman RA (1998) Effects of mercury on wildlife: a comprehensive review. Environ Toxicol Chem 17:146–160
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ehsanpour, M., Afkhami, M., Khoshnood, R. et al. Determination and Maternal Transfer of Heavy Metals (Cd, Cu, Zn, Pb and Hg) in the Hawksbill Sea Turtle (Eretmochelys imbricata) from a Nesting Colony of Qeshm Island, Iran. Bull Environ Contam Toxicol 92, 667–673 (2014). https://doi.org/10.1007/s00128-014-1244-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-014-1244-3