Abstract
The green sea turtle (Chelonia mydas) has been a species of global concern for decades. In this study, heavy metals (mercury: Hg; Cadmium: Cd; Lead: Pb; Copper: Cu; and Zinc: Zn) were measured in blood and three egg fraction of green sea turtles nesting on the northern coast of Sea of Oman. Heavy metals concentrations in blood, yolk, albumen, and egg shell ranged between 0.16–36.78, 0.006–33.88, 0.003–4.02, and 0.002–6.85 μg/g (ww), respectively. According to the results, all heavy metals found in blood samples (n = 12) also were detected in the various parts of the eggs (n = 48). Moreover, there were no significant differences between concentrations of heavy metals in different clutches laid in a nesting season. However, Pb concentrations in blood samples significantly increased in later clutches (p < 0.05), whereas Cu concentrations in blood samples exhibit a declining trend (p < 0.05). These results reveal the existence of maternal transfer phenomenon in green sea turtles on the northern coast of Sea of Oman. Results of this study suggest that heavy metals could be one of the factors influencing reductions in fertilization and hatching success. Results also indicate that green sea turtle on the northern coast of Sea of Oman have high capacity in rapid response and detoxification of heavy metals and/or from the low exposure levels of these turtles to the heavy metals. Further research is required concerning the effects of heavy metals on green sea turtles, especially on their possible influence of fetal development of turtles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Marine ecosystems are contaminated by different pollutants, such as trace metals due to human activities (Sinaie et al. 2010). Populations of green sea turtles (Chelonia mydas) are distributed through the world’s tropical and subtropical marine habitat, facing serious anthropogenic threats, including poaching, fisheries impacts, pollution, and habitat loss. Consequently, all species of sea turtles have been classified as endangered or threatened and are listed in Appendix I of the Convention of International Trade in Endangered Species (IUCN 2003; Ehsanpour et al. 2014).
Heavy metals enter sea turtles’ bodies mainly through their food and water. Female sea turtles drink considerable amounts of water to decrease their body temperature during the nesting season and egg production (Kenyon et al. 2001). This phenomenon causes increased concentrations of heavy metals in their blood. In general, nonessential metals accumulate in tissues, such as the kidney, liver, and pancreas, of the sea turtle (Marco et al. 2004). However, eggs may be better indicators of recent exposure to heavy metal contaminants. Heavy metals also may be transferred from mother to eggs (Marco et al. 2004; Ehsanpour et al. 2014). The extent and quantity of heavy metal bioaccumulation in a turtle’s body is highly dependent on the species, status, and position of the species in the food chain, migration route and pattern, feeding and breeding areas, and natural activities, such as upwelling (Day et al. 2005). Furthermore, female sea turtles can transfer a part of accumulated contaminants in their bodies to the eggs and thus reduce the extent of contamination in their own bodies (Nagle et al. 2001). Egg production starts with vitellogenesis during which the area around each follicle is filled with fat as storage (Ehsanpour et al. 2014). This process is completed before turtles reach their nesting regions. Therefore, studying blood and eggs provides more comprehensive information about toxicokinetics of essential and nonessential metals in turtles’ bodies during the nesting season. Cu and Zn are important and necessary for growth and metabolism of cells and for suitable structure and function of proteins (Wallace et al. 2005). Therefore, transfer of these metals to eggs during the nesting season is necessary for fetal growth and development of offspring (Wallace et al. 2005).
Various species of sea turtles, especially juvenile turtles, have an opportunistic feeding strategy, which could lead to an increase in entering pollutants into their bodies. For example, juvenile green sea turtles can feed from animals at higher levels of food chain that can lead to entrance of the higher levels of pollution to their bodies (Kelly et al. 2008; Rauschenberger et al. 2004). Sea turtles are good indicators of environmental health status because of their longevity, position, and place in the food chain, mobility, and movement (Camacho et al. 2014). Thus, several studies have been performed in the field of ecotoxicology in various species of sea turtles around the world (Day et al. 2005; Guirlet et al. 2008; Kampalath et al. 2006; Sakai et al. 2000; Páez-Osuna et al. 2010; Marco et al. 2004; Nagle et al. 2001). Most studies on pollution levels in various species of sea turtles have been performed by collecting tissue samples of dead animals, which may not reflect realistic contaminant levels and health status of turtles (Ehsanpour et al. 2014). A nonlethal method for sampling blood from sea turtles was developed by Owens and Ruiz (1980). The development of nonlethal methods, such as collecting blood samples from live sea turtles, has been considered as an appropriate tool to evaluate health status and level of pollution (Van de Merwe et al. 2010; Day et al. 2007; Keller et al. 2004).
There are more than 52 known habitats for marine turtles used for foraging, mating, and nesting on the northern boundaries of the Persian Gulf and the Sea of Oman (Pritchard and Mortimer 1999). Green and hawksbill turtles (Eretmochelys imbricata) have a large number of nesting sites in the regions of the northern coasts of the Persian Gulf and the Sea of Oman (Hesni et al. 2015; Tollab et al. 2015). The Sea of Oman includes important food and reproduction areas for Green turtles during different life stages, but rare number of green turtle come to beach for nesting. So, nesting ecology and toxicology information about green turtle is rare. During the past three decades, intensive efforts have been made to conserve these sea turtles that nest in large groups on the northern coast of the Persian Gulf. However, there is a lack of information on migrations to the northern coast of Sea of Oman. Populations of the green sea turtle in this area are facing low fertilization and hatching success (Mohammadizadeh and Soltanpour 2014). Heavy metals discharged into the marine ecosystem of the Sea of Oman have the potential to impact negatively the hatching success of this population. Overall, there is a clear need to improve knowledge of green turtles in Iranian territorial waters of the Sea of Oman. In line with these trends of research, this study was conducted:
-
1.
To assess the variations and relationships among trace metal concentrations in maternal blood and freshly laid eggs from the green sea turtle.
-
2.
To estimate the maternal transfer through the metal excretion rate on the basis of one nesting season of this turtle.
-
3.
To evaluate the effect of heavy metals on hatching success of the green sea turtle.
Materials and Methods
Study Area
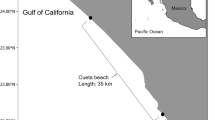
This study was performed on the Chabahar Beach on the northern coast of the Sea of Oman from June to October 2015. This beach has a semidiurnal tide (2 daily high tides). The beach is 300 km in length, and the width varies from 5 to 40 m, depending on the tide line. For this study, five areas along the beach were chosen, which are frequented by turtles (Fig. 1). These beaches have the highest densities of nesting green sea turtles (C.mydas) along the northern coast of the Sea of Oman. The beaches in these areas have fine sand and gentle slope. The beach length is rounded by a widespread sand system and also pebbles mixed with sand.
Sample and Data Collection
A complete visual physical examination was performed (n = 12) and curved carapace length (CCL) was measured. Health status of turtles was rated based on nest-building behavior and general body condition (Deem et al. 2006; Perrault et al. 2012). Turtles were tagged, according to the methodology described by the National Marine Fisheries Service/Southeast Fisheries Science Center (2008). Blood was collected from the interdigital vein of the hind flipper via a dorsal approach with the use of an 18-gauge, 3.7-cm needle and a 15-ml syringe Precoated with sodium heparin (heparin sodium injection, USP, PPC, Inc., C504730, Canada) or a nonheparin-coated syringe. Blood tubes were kept on wet ice in a cooler during the remaining time researchers were on the beach collecting samples (range 20 min to 2 h). Twelve blood samples were used in this study. Five eggs from each female were haphazardly collected before touching the sand during laying. We were taking eggs out as they were being laid. We were tracking females laying eggs in the nesting season. A total of 48 egg samples from 12 different nests were collected and transported to the laboratory where they were kept at 4 °C until analysis.
Chemical Analysis
Egg samples were rinsed with deionized water to remove any particulate matter that might have adhered. Next, eggs were weighed and sized and subsequently separated into shell, albumen, and yolk. The separation was carried out quickly to prevent thawing. This determination was carried out in composite pools of shell, albumen, and yolk of 4 eggs dissected from 12 turtles (3 samples, each constituted by egg fraction of 4 specimens).
Blood samples were individually transported and processed. Glassware and plastic materials used for handling and transportation of samples were thoroughly acid-washed to prevent contamination of samples (Moody and Lindstrom 1977). Blood and pooled samples of eggs were freeze-dried (72 h at −49 °C and 133 9 10–3 mbar) and then powdered. Powdered samples (0.25 g) were digested with quartz-distilled concentrated nitric acid (5 mL) in hot plate equipment (HPA2235 M) under established conditions. The digested material was finally diluted to 50 mL using deionized water and stored in polyethylene containers for further analysis. All samples were analyzed in triplicate for Cd, Pb, Cu and Zn by Atomic Absorption Spectrophotometer (Lovibond 712005, Vermont, United States). Atomic Absorption Spectrophotometer operating condition for the studies metals are displayed in Table 1.
Total mercury levels were determined using cold vapor analysis technique. Powdered samples (0.25 g) was digested in 20 ml of 3:1 concentrated redistilled HNO3 and concentrated H2SO4 and then oxidized with 10 ml of saturated solution of KMnO4. Excess oxidizing agents and mercury ions were reduced by 10 ml of a reducing solution (3% NaBHa in 1% NaOH) in a hydride generator apparatus ((Lovibond 712005). Thereafter mercury was vaporized and measured in the atomic absorption spectrophotometer (Lovibond 712005). The instrument was pre-calibrated using standard solutions prepared from commercial Hg chemical of analytical grade. A blank (n = 3) was run in the same manner as that of samples. The stock solution was prepared daily. Mercury analysis was determined using standard prepared in the same acid matrix, which did not show significant metal contamination.
Nesting Parameters Data Collection
Hatching success was determined following the methodology described by Miller (1999) using records from all beaches. Hatching success was defined as “the proportion of hatchlings that hatched out of their shells” (Miller 1999).
Quality Control
Replicate samples, certified reference materials, and procedural blanks were used as quality control procedures. Reproducibility and recovery were high (>85%). To perform quality control, the procedural blanks were periodically analyzed for each batch of five samples. Quantitative analysis was done on a three-point linear calibration of heavy metal (Hg, Cd, Pb, Cu, and Zn) solution, obtained by dilution of the certified standard mixture of heavy metal (TraceCERT® CRMs, Sigma Aldrich). Satisfactory linearity was obtained, with values of the correlation coefficient R above 0.99.
Statistical Analysis
Statistical analyses of the data were conducted by using Statistical Package for Social Sciences (SPSS) software, version 20. All data are reported as mean ± standard deviation. Additionally, Microsoft Office Excel (2010) was applied to draw the diagrams and to estimate the linear regression coefficient between heavy metals (Hg, Cd, Pb, Cu, and Zn) concentration in the whole three egg fractions and blood measured in green sea turtles (C. mydas). The data possessed the homogeneity of variance and were normally distributed. One-way analysis of variance (ANOVA) was run followed by a Tukey’s test to compare the means (p < 0.05) between heavy metals (Hg, Cd, Pb, Cu, and Zn) contents determined in the green sea turtles blood samples and in the three egg fractions.
Results
Heavy Metals Concentrations
Concentrations of heavy metals (Hg, Cd, Pb, Cu, and Zn) determined in blood samples and three egg fractions of green sea turtles from the northern coast of the Sea of Oman are presented in Table 2. Percentages of heavy metals loads in the three egg fractions of green sea turtle are displayed in Fig. 2. Results showed that there are higher quantities of essential heavy metals (Cu, Zn) in blood and eggs of turtles compared to nonessential heavy metals (Cd, Hg, Pb). No significant difference was found between Trace metal concentrations in three egg fractions and hatching success (p > 0.01). There is a significant correlation between the quantities of Cd, Pb, and Zn in turtle eggs and blood. Concentrations of Cd, Zn, and Hg did not exhibit significant differences in the various clutches in a single nesting season.
Hatching Success
Hatching success (%) determined in the green sea turtle (C. Mydas) clutches laid are shown in Table 3. The results indicated that clutches at Lipar had the highest hatching success followed in order by Ramin, Kacho, and Tang. When comparing hatchling success among beaches, there were no significant differences between them (p ≥ 0.05).
Maternal Transfer of Metals
The results of spearman rank correlations between heavy metals (Hg, Cd, Pb, Cu, and Zn) concentration in the three egg fractions and blood measured in green sea turtles are summarized in Table 4. Heavy metal contents found in the blood samples were also found in the three egg fractions in the green sea turtles. The results showed that the amount of Pb concentration in the blood samples has increased significantly in the later egg laying times (p < 0.05). The amount of Cu concentration in the blood sample has dropped significantly in the later clutches laid in a nesting season (p < 0.05).
Discussion
Results of the percentages of heavy metals (Cd, Cu, Zn, Pb, and Hg) in the various parts of eggs showed that all heavy metals have high levels of accumulation in egg vitellus except for Cu. This confirms and emphasizes the results of previous studies on the importance of the vitellus in the accumulation of heavy metals (Godley et al. 1999; Sakai et al. 2000; Páez-Osuna et al. 2010). Similar results have been observed in the Loggerhead turtle (Caretta caretta) (Sakai et al. 1995; Kaska and Furness 2001), the Olive Ridley turtle (Lepidochelis olivacea) (Páez-Osuna et al. 2010), and the hawksbill turtle (Ehsanpour et al. 2014).
The coefficients of variation (CV) of heavy metals in eggs of green sea turtles indicated the low extent of variations in these coefficients. Accordingly, one of the implications of this finding concerns the regulation of heavy metals suitably during the homeostatic and modifying processes and prevented their toxic effects (Páez-Osuna et al. 2010). This also may result from the high capacity of these turtles in rapid response and detoxification of heavy metals and/or from the low exposure levels of these turtles to the heavy metals.
The essential and nonessential heavy metal concentration results in green sea turtle’s egg and their comparison with those found by other researchers (Table 5) indicate that concentrations were similar and in the low ranges of contamination, suggesting no acute effects for fetuses. However, long-term exposure to such levels of heavy metals may influence reproduction (Lam et al. 2006; Sakai et al. 2000; Ehsanpour et al. 2014; Páez-Osuna et al. 2010). One of the main problems in the northern coasts of the Sea of Oman is the low hatching success and the high mortality rate of eggs before formation of fetuses (Mohammadizadeh and Soltanpour 2014). It seems that presence of heavy metals can be an influencing factor in reducing egg fertilization rates. On the other hand, eggs can absorb contaminants during the incubation period due to their permeable membranes, and this increases concentrations of contaminants in eggs, may have destructive effects on them, and thus can reduce hatching success. However, this research did not find any relationships between concentrations of heavy metals and hatching success in the studied nests. Further research is needed about heavy metals (Cd, Cu, Zn, Pb, and Hg) in this area.
The investigation of heavy metals in the blood samples of green sea turtles during the nesting season indicates the different toxicokinetic behaviors of the studied heavy metals. The results showed no significant differences between concentrations of Cd, Zn, and Hg in the different clutches. The results achieved in the present study could be explained by the low concentrations of these metals in the environment or from the short duration of time that these turtles were present in the region during the nesting season (Guirlet et al. 2008). Results indicated that an increased in the number of clutches laid in a nesting season will reduce the Cu concentration in the blood. This can be due to lower entry of contaminants through food, because turtles feed little or not at all during the nesting season. Moreover, it could be caused by shortage of stored Cu in the liver and kidney tissues of the turtle (Andreani et al. 2008). Contrary to Cu concentration, the results represented that concentration of Pb increases with an increase in the number of clutches laid in a nesting season. A possible justification for our findings might be due to the replacement of calcium (Ca) during egg formation (Bilinski et al. 2001). Calcium is an important element in formation of the bones and shells of baby turtles. Female turtles absorb Ca ions and store them in yolks and shells of the eggs (Bilinski et al. 2001). In vertebrates, Ca needs are satisfied by absorbing it from food, but female turtles obtain it from Ca reserves, such as bones, because they do not feed during the nesting season. Because Pb and Ca have similar kinetics, Pb can be transferred to blood together with Ca (Fossette et al. 2007; Caut et al. 2007). As a result of replacement of the Ca required for more than 300 eggs during the nesting season, considerable quantities of Pb enter the blood.
All heavy metals studied in the blood of green sea turtles also were found in the various parts of their eggs. This indicates the phenomenon of maternal transfer of heavy metals to eggs among the turtles that lay eggs on the northern coasts of the Sea of Oman. The results acquired in the present study may be explained by the transformation of heavy metals to the eggs during the nesting season by being attached to transporter proteins called metalloprotein. In amphibians and fish, combination of metals, such as Cd and Zn, with vitellogenin is considered an important transfer mechanism (Falchuk and Montorzi 2001). In reptiles, the copper transporter protein (CTP) has been identified as an important agent in the absorption of Cu and its transfer to the eggs (Riggio et al. 2002). Unrine et al. (2006) also introduced lipovitellin as an important transporter protein for selenium. Numerous studies point out the role of metallothionein protein during the homeostasis and detoxification process, yet the role this protein plays in transferring heavy metals to the subsequent generation in mammals and reptiles is unclear (Riggio et al. 2003). A positive correlation between heavy metals in blood and eggs of turtles may be related to the active presence of transporter proteins, such as albumin and vetillogenin (Suzuki and Sasakura 1998), whereas lack of a significant correlation may indicate homeostasis of these elements in female sea turtles so that their transmission to offspring is not dependent on the level of contaminants to which the mother turtle is exposed to (Riggio et al. 2003). On the other hand, the phenomenon of maternal transfer is greatly dependent on the type of species, level of contamination, and the characteristics of the heavy metals (Guirlet et al. 2008). Study of the maternal transfer phenomenon during nesting season represented that there were no significant differences between concentrations of heavy metals and the times the eggs were laid. This indicates that the maternal transfer phenomenon is constant during the nesting season. Guirlet et al. (2008) also reported similar results in leatherback turtles (Dermochelys coriacea).
The present study provides useful initial information concerning concentrations of heavy metals (Cd, Cu, Zn, Pb, and Hg) in green sea turtles of the northern coasts of the Sea of Oman. The studied heavy metals had low concentrations, yet they could be detected in blood and various egg parts. This suggests the occurrence of maternal transfer. The low concentrations of heavy metals in green sea turtles may be due to their diet, because they consume large quantities of algae and plants from the low levels of the food chain. Results of this study indicate that heavy metals could be one of the factors influencing reductions in fertilization of turtle eggs and in their hatching success. However, more research concerning the effects of heavy metals on fetal development in turtles and on their hatch rates is required, and greater attention than any time before must be paid to environmental evaluation of industrial and development projects, especially on sea turtle nesting beaches.
References
Andreani G, Santoro M, Cottignoli S, Fabbri M, Carpenè E, Isani G (2008) Metal distribution and metallothionein in loggerhead (Caretta caretta) and green (Chelonia mydas) sea turtles. Sci Total Environ 390:287–294
Bilinski JJ, Reina RD, Spotila JR, Paladino FV (2001) The effects of nest environment on calcium mobilization by leatherback turtle embryos (Dermochelys coriacea) during development. Comp Biochem Physiol A: Mol Integr Physiol 130:151–162
Camacho MLD, Boada Jorge O, Pedro L, Manuel Z, Maira A, Octavio PL (2014) Monitoring organic and inorganic pollutants in juvenile live sea turtles: results from a study of Chelonia mydas and Eretmochelys imbricata in Cape Verde. Sci Total Environ 481:303–310
Caut S, Guirlet E, Jouquet P, Girondot M (2007) Influence of nest location and yolkless eggs on the hatching success of leatherback turtle clutches in French Guiana. Can J Zool 84:908–915
Day RD, Christopher SJ, Becker PR, Whitaker DW (2005) Monitoring mercury in the loggerhead sea turtle, Caretta caretta. Environ Sci Technol 39:437–446
Day RD, Segars AL, Arendt MD, Lee AM, Peden-Adams MM (2007) Relationship of blood mercury levels to health parameters in the loggerhead sea turtle (Caretta caretta). Environ Health Perspect 115:1421–1428
Deem SL, Dierenfeld ES (2006) Blood values in free- ranging nesting leatherback sea turtles (Dermochelys coriacea) on the coast of the Republic of Gabon. J Zoo Wildl Med 37(4):464–471
Ehsanpour M, Afkhami M, Khoshnood R, Reich KJ (2014) Determination and maternal transfer of heavy metals (Cd, Cu Zn, Pb and Hg) in the Hawksbill Sea Turtle (Eretmochelys imbricata) from a nesting colony of Qeshm Island, Iran. Bull Environ Contam Toxicol. doi:10.1007/s00128-014-1244-3
Falchuk KH, Montorzi M (2001) Zinc physiology and biochemistry in oocytes and embryos. Biometals 14:385–395
Fossette S, Ferraroli S, Tanaka H, Ropert-Coudert Y, Arai N, Sato K, Naito Y, Le Maho Y, Georges JY (2007) Dispersal and dive patterns in gravid leatherback turtles during the nesting season in French Guiana. Mar Ecol Prog Ser 338:233–247
Godley BJ, Thompson DR, Furness RW (1999) Do heavy metal concentrations pose a threat to marine turtles from the Mediterranean Sea? Mar Pollut Bull 38(6):497–502
Guirlet E, Das K, Girondot M (2008) Maternal transfer of trace elements in leatherback turtles (Dermochelys coriacea) of French Guiana. Aquat Toxicol 88:267–276
Hesni MA, Tabib M, Ramaki AH (2015) Nesting ecology and reproductive biology of the hawksbill turtle, Eretmochelys imbricata, at Kish Island, Persian Gulf. J Mar Biol Assoc UK 96(7):1373–1378
IUCN (2003) IUCN red list of threatened species. Gland, Switzerland. http://www.redlist.org
Kampalath R, Gardner SC, Méndez-Rodríguez L, Jay JA (2006) Total and methylmercury in three species of sea turtles of Baja California Sur. Mar Pollut Bull 52:1784–1832
Kaska Y, Furness RW (2001) Heavy metals in marine turtle eggs and hatchlings in the Mediterranean. Zool Middle East 24:127–132
Keller JM, Kucklick JR, Harms CA, McClellan-Green PD (2004) Organochlorine contaminants in sea turtles: correlations between whole blood and fat. Environ Toxicol Chem 23:726–738
Kelly SM, Eisenreich KM, Baker JE, Rowe CL (2008) Accumulation and maternal transfer of polychlorinated biphenyls in snapping turtles of the upper Hudson River, New York, USA. Environ Toxicol Chem 27:2565–2574
Kenyon LO, Landry AM, Gill GA (2001) Trace metal concentrations in blood of the Kemp’s Ridley sea turtle (Lepidochelys kempii). Chelonian Conserv Biol 4:128–135
Lam JCW, Tanabe S, Chan SKF, Yuen EKW, Lam MHW, Lam PKS (2004) Trace element residues in tissues of green turtles (Chelonia mydas) from South China waters. Mar Pollut Bull 48:164–192
Lam JCW, Tanabe S, Chan SKF, Lam MHW, Martin M, Lam PKS (2006) Levels of trace elements in green turtle eggs collected from Hong Kong: evidence of risks due to selenium and nickel. Environ Pollut 144:790–801
Marco A, Lopez-Vicente M, Perez-Mellado V (2004) Arsenic uptake by reptile flexible-shelled eggs from contaminated nest substrates and toxic effect on embryos. Bull Environ Contam Toxicol 72:983–990
Miller JD (1999) Determining clutch size and hatching success. In: Eckert KL, Bjorndal KA, Abreu-Grobois FA (eds) Research and management techniques for the conservation of sea turtles. IUCN/SSC Marine Turtle Specialist Group Publication No 4, pp 124–129
Mohammadizadeh M, Soltanpour N (2014) Identification and prioritizing important nesting sites of green turtle in Iranian beaches of Oman Sea during 2008–2010. Bull Environ Pharmacol Life Sci 3:12–18
Moody JR, Lindstrom RN (1977) Selection and cleaning of plastic containers for storage of trace element samples. Anal Chem 49:2264–2267
Nagle RD, Rowe CL, Congdon JD (2001) Accumulation and selective maternal transfer of contaminants in the turtle Trachemys scripta associated with coal ash deposition. Arch Environ Contam Toxicol 40:531–536
National Marine Fisheries Service Southeast Fisheries Science Center (2008) Sea turtle research technical manual. NOAA Technical Memorandum. NMFS-SEFSC-579
Owens DW, Ruiz GJ (1980) New methods of obtaining blood and cerebrospinal fluid from marine turtles. Herpetologica 36:17–20
Páez-Osuna F, Calderón-Campuzano MF, Soto-Jiménez MF, RuelasInzunza JR (2010) Lead in blood and eggs of the sea turtle, Lepidochelys olivacea, from the Eastern Pacific: concentration, isotopic composition and maternal transfer. Mar Poll Bull 60:433–439
Perrault JR, Miller DL, Eads E, Johnson C, Merrill A (2012) Maternal health status correlates with nest success of leatherback sea turtles (Dermochelys coriacea) from Florida. PLoS ONE 7(2):e31841. doi:10.1371/journal.pone.0031841
Pritchard PCH, Mortimer JA (1999) Taxonomy, external morphology and species identification. In: Eckert KL, Bjorndal KA, Abreu-Grobois FA (eds) Research and management techniques for the conservation of sea turtles. iucn/Species Survival Commission Marine Turtles Specialist Group Publication, No 4, Washington, DC
Rauschenberger RH, Sepulveda MS, Wiebe JJ, Szabo NJ, Gross TS (2004) Predicting maternal body burdens of organochlorine pesticides from eggs and evidence of maternal transfer in Alligator mississippiensis. Environ Toxicol Chem 23:2906–2915
Riggio M, Lee J, Scudiero R, Parisi E, Thiele DJ, Filosa S (2002) High affinity copper transport protein in the lizard Podarcis sicula: molecular cloning, functional characterization and expression in somatic tissues, follicular oocytes and eggs. Biochim Biophys Acta-Gene Struct Expr 1576:127–135
Riggio M, Trinchella F, Filosa S, Parisi E, Scudiero R (2003) Accumulation of zinc, copper, and metallothionein mRNA in lizard ovary proceeds without a concomitant increase in metallothionein content. Mol Reprod Dev 66:374–382
Sakai H, Ichihashi H, Suganuma H, Tatsukawa R (1995) Heavy metal monitoring in sea turtles using eggs. Mar Pollut Bull 30(5):347–353
Sakai H, Saeki K, Ichihashi H, Suganuma H, Tanabe S, Tatsukawa R (2000) Species-specific distribution of heavy metals in tissues and organs of loggerhead turtle (Caretta caretta) and green turtle (Chelonia mydas) from Japanese coastal waters. Mar Pollut Bull 40:701–709
Sinaie M, Darvish Bastami K, Ghorbanpour M, Najafzadeh H, Shekari M, Haghparast S (2010) Metallothionein biosynthesis as a detoxification mechanism in mercury exposure in fish, spotted scat (Scatophagus argus). Fish Physiol Biochem. doi:10.1007/s10695-010-9403-x
Suzuki KT, Sasakura C (1998) Biological interaction between transition metals (Ag, Cd and Hg), selenide/sulfide and selenoprotein P. J Inorg Biochem 71(3–4):159–162
Tollab MA, Dakhteh MH, Ghorbanzadeh Zaferani G, Askari Hesni M, Ahmadi F, Shojaei Langari M, Alavian Z, Rezaie-Atagholipour M (2015) The Olive Ridley Turtle, Lepidochelys olivacea, in the Persian Gulf: a review of the observations, including the first nesting of the species in the area. Chelonian Conserv Biol 14(2):192–196
Unrine JM, Jackson BP, Hopkins WA, Romanek C (2006) Isolation and partial characterization of proteins involved in maternal transfer of selenium in the western fence lizard (Sceloporus occidentalis). Environ Toxicol Chem 25:1864–1867
Van de Merwe JP, Hodge M, Olszowy HA, Whittier JM, Lee SY (2010) Using blood samples to estimate persistent organic pollutants and metals in green sea turtles (Chelonia mydas). Mar Pollut Bull 60:579–588
Wallace BP, Williams CL, Paladino FV, Morreale SJ, Lindstrom RT, Spotila JR (2005) Bioenergetics and diving activity of internesting leatherback turtles Dermochelys coriacea at Parque Nacional Marino las Baulas, Costa Rica. J Exp Biol 208:3873–3884
Acknowledgements
Financial support for this study was obtained from the Iranian Department of the Environment. Special thanks to Hosseini, Arbabi, and Soltanpour, who were important in the data collection process.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sinaei, M., Bolouki, M. Metals in Blood and Eggs of Green Sea Turtles (Chelonia mydas) from Nesting Colonies of the Northern Coast of the Sea of Oman. Arch Environ Contam Toxicol 73, 552–561 (2017). https://doi.org/10.1007/s00244-017-0421-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-017-0421-x