Abstract
Key message
A novel allele-specific Rfo marker was developed and proved to be effective for MAS of Rfo gene in B. oleracea background and six Ogu-CMS fertility-restored interspecific hybrids were created for the first time.
Abstract
Ogura cytoplasmic male sterility (Ogu-CMS) has been extensively used for Brassica oleracea hybrid production. However, because of maternal inheritance, all the hybrids produced by CMS lines are male sterile and cannot be self-pollinated, which prohibits germplasm maintenance and innovation. This problem can be overcome by using the Ogu-CMS restorer line, but restorer material is absent in B. oleracea crops. Here, Rfo, a fertility-restored gene of Ogu-CMS, was transferred from rapeseed restorer lines into a Chinese kale Ogu-CMS line using interspecific hybridization combined with embryo rescue. Nine interspecific, triploid plant progenies were identified at morphological and ploidy level, with phenotypes intermediate between those of rapeseed and Chinese kale. Because the Rfo marker (Hu et al., Mol Breeding 22:663–674, 2008) cannot distinguish the Rfo and its homologies under a B. oleracea background, a novel allele-specific Rfo marker was developed based on the BLAST analysis of highly homologous Rfo sequences in B. oleracea. Screening using the novel Rfo marker found that six interspecific hybrids carrying Rfo were also fertile, although fertility varied during different flowering periods. Furthermore, BC1 offsprings with the Rfo gene were selected with the allele-specific Rfo marker and showed restored fertility. These results indicated that the novel allele-specific marker could be used for the MAS of Rfo gene in B. oleracea, and this study lays the foundation for the development of Ogu-CMS restorer material in cabbage and its related other subspecies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Male sterility, referring to the failure to produce functional pollen and viable male gametes, is a very widespread phenomenon in plants. It was first discovered by the German botanist Joseph Gottlieb Kolreuter in 1763 (Mayr 1986) and has since been reported in 320 species (Kaul 1988). Male sterility provides important tools for taking advantage of hybrid vigor, or heterosis, and provides useful models to study nuclear–cytoplasm interactions. According to differences in the male sterile gene location, male sterility is usually divided into cytoplasmic male sterility (CMS), which is controlled by mitochondrial genes and coupled nuclear genes, and genic male sterility (GMS), controlled by nuclear genes alone (Vedel et al. 1994). In contrast to CMS, male sterility traits in the GMS system cannot be efficiently maintained; therefore hybrid seed technologies based on the GMS system are not usually applicable. However, environmentally sensitive genetic male sterile mutants (EGMS), in which pollen fertility is sensitive to changes in the duration of day or temperature, are found in many species and enable GMS systems to be applicable in hybrid breeding (Virmani and Ilyas-Ahmed 2001), especially in cabbage (Brassica oleracea L. var. capitata) (Fang et al. 1997) and rice (Oryza sativa L.) (Li et al. 2007).
CMS systems are widely used in the seed production of commercial hybrids. An example is Ogura CMS (Ogu-CMS), discovered in radish (Raphanus sativus) and recognized as a stable type of male sterility (Ogura 1968). It is controlled by the mitochondrial gene, orf138, which consists of two co-transcribed open reading frames, orf138 and orfB (Bonhomme et al. 1991, 1992). The Ogura-type mitochondrial genome has four unique regions non-syntenic to the normal-type radish genome, and the orf138 gene is located at the edge of the largest unique region. These unique regions are composed of known Brassicaceae mitochondrial sequences, which suggests that unique regions may have been generated by integration and shuffling of pre-existing mitochondrial sequences, and novel genes such as orf138 were created through the shuffling process of the mitochondrial genome (Tanaka et al. 2012).
Brassica oleracea contains several subspecies, including cabbage, broccoli, cauliflower, Chinese kale and kohlrabi. They are important vegetable crops in the Brassicaceae family and widely cultivated throughout China. The heterosis is obvious, and self-incompatibility and male sterility are two main methods to exploit the heterosis. However, the lack of viable male sterility has prohibited the development of male sterile hybrids. Following the discovery of the Ogu-CMS type, many attempts have been made to introduce the Ogu-CMS from radish to cabbage through interspecific crosses and embryo rescue, or by protoplast fusion (Bannerrot et al. 1974; Walters et al. 1992). However, this type showed poor agronomic performance, such as abnormal pistils and lack of nectaries (Yang et al. 1997). Furthermore, Ogu-CMS material was improved via asymmetric protoplast fusion between the above Ogura material and broccoli (Brassica oleracea var. italica), which was widely used for hybrid breeding in cabbage (Fang et al. 2001; Wang et al. 2012).
Ogura CMS is a maternally inherited trait, and the cytoplasm of all progenies is derived from the female parent. Without a restorer line, the CMS hybrids cannot be self-pollinated or produce segregated progenies. For example, the cabbage variety “SG336” is resistant to club root disease, but it cannot be utilized for germplasm innovation owing to its Ogu-CMS characteristic. Therefore, the widespread use of Ogu-CMS prohibits germplasm innovation. Recently, more and more hybrids have been produced by Ogu-CMS in B. oleracea crops, and the problem associated with CMS hybrids becomes more serious. The most effective way to overcome this problem is to identify and use Ogu-CMS restorer lines. However, to date, the Ogura CMS restorer line has not been found in B. oleracea crops. Thus, there is an urgent need to create a cabbage Ogu-CMS restorer line.
Rapeseed (Brassica napus L.) is another Brassica crop grown worldwide. Hybrids are used extensively in its commercial production. Ogu-CMS and its corresponding restorer gene (Rfo) were transferred from radish to rapeseed through interspecific hybridization (Heyn 1976; Pelletier et al. 1983, 1988). Poor agronomic performance of these materials, such as high glucosinolate content and weak female fertility, has attracted lots of efforts subsequently to improve these materials (Delourme et al. 1991). By combining molecular marker-assisted selection (MAS) with extensive backcrossing, Delourme et al. (1995) obtained improved low glucosinolate lines, but the closely linked radish fragment also showed a remarkable negative agronomic effect on the restorer lines. In the early twenty-first century, Primard-Brisset et al. (2005) developed a low glucosinolate-restored line (R2000) through gamma-ray irradiation. This improved restorer line showed excellent agronomic performance in pollen vigor, transmission rate of Rfo, and fertility. Furthermore, Rfo allele-specific PCR markers were developed based on sequence differences between B. napus restorer and non-restorer lines (Hu et al. 2008). Since then, the Ogu-CMS/Rfo system has gradually been applied in Europe and North America, and has become a major technique in the seed production of hybrid rapeseed.
Owing to the closer relationship between B. napus and B. oleracea compared with radish, rapeseed restorer materials provide a promising resource for creating a B. oleracea restorer line. Moreover, previous studies have shown that the Rfo gene was introgressed into the linkage group 19 (chromosome 9 of C genome: C9) in B. napus (Feng et al. 2009; Hu et al. 2008), which increased the possibility of Rfo gene transfer from rapeseed restorer material into cabbage through distant hybridization.
Distant hybridization is an important method to create new types of plants, or introduce new important traits from different species or genera. It plays an important role in the breeding of Brassicaceae crops (Qiao 2012). As the genetic relationship is close, interspecific crosses between B. napus and B. oleracea are an important strategy in the transfer of beneficial agronomic traits or target genes (Li et al. 2014). To overcome the reproductive barriers in distant hybridization, numerous methods have been proposed including bridge parents, repeated pollination, ploidy change, and embryo rescue. Embryo rescue can break species limitations to an extent and increase the chance of genome recombination in different species. The plant embryo rescue system was initially established in the 1890s (Bridgen 1994), and since then numerous studies on the environment, media, and stages of embryo rescue have been carried out. Embryo rescue was applied in the distant hybridization between B. oleracea and other Brassica species, and many important traits and specific genes have been transferred to cabbage from wild species or related species, including male sterility (Bannerrot et al. 1974), triazine resistance (Ayotte et al. 1987), aphid resistance (Quazi 1988), and self-incompatible traits (Ripley and Beversdorf 2003). Furthermore, new Brassica species have been created, such as allotriploid and allodidiploid hybrids between B. oleracea and B. rapa or R. sativus (Nishi et al. 1959; Feng and Chen 1981; Fang et al. 1983; Chen 2006; Gu et al. 2006; Qiao 2012), and artificial resynthesized B. napus was obtained through interspecific cross between B. oleracea and B. rapa (Yin et al. 2004; Zhang et al. 2004; Wen et al. 2008).
Marker-assisted selection (MAS) has been widely used in the breeding of B. oleracea crops. At present, many markers closely linked to important agronomic traits have been developed and utilized, such as Ogu-CMS (Bonhomme et al. 1992), Fusarium wilt resistance (Pu et al. 2012; Lv et al. 2013), flowering time (Rahman et al. 2011), and petal color (Han et al. 2015). The aim of this study is to create the Ogu-CMS fertility-restored interspecific hybrids between B. oleracea and B. napus, and develop a novel allele-specific Rfo marker to distinguish the Rfo-homologous sequence of B. oleracea, which might assist in selecting the restored interspecific hybrids.
Materials and methods
Plant material
As Chinese kale (Brassica oleracea var. alboglabra) is easier to vernalize and has a shorter life cycle than cabbage and other cultivated subspecies, Chinese kale was used as a bridge to transfer the Rfo gene into B. oleracea. An Ogu-CMS Chinese kale (code: JL1) was used as the female parent, and three homozygous rapeseed restored lines (RfoRfo; codes: RF1, RF2, and RF3) and one heterozygous rapeseed restorer line (Rforfo; code: RF4) were used as the male parent. The Ogu-CMS Chinese kale was provided by Prof. Jianjun Lei from South China Agricultural University, which has been identified using orf138 specific primers (Bonhomme et al. 1992). The homozygous rapeseed lines were provided by Prof. Yunchang Li and Prof. Hanzhong Wang from the Oil Crops Research Institute, Chinese Academy of Agricultural Sciences. The heterozygous rapeseed line was provided by Prof. Wei Qian from the Southwest University, Chongqing, China. Cabbage material ‘JinZaosheng’ (code: GL1) was used as the non-restored control group. All plants used in this study were grown at the Institute of Vegetable and Flowers, Chinese Academy of Agricultural Sciences.
Distant hybridization and embryo rescue
To overcome the interspecific reproductive barrier, repeated pollination was performed during the bud period, and combined with embryo rescue, JL1 was crossed with RF1, RF2, RF3, and RF4. All crosses were made by hand pollination, twice, at approximately 9:00 a.m. and 3:00 p.m. For embryo rescue, two factors were designed: days after pollination (DAP) and days after pod culture in vitro (DAC); six treatments in all were used to find the optimal combinations. Siliques were removed from the plants at 5, 10, and 15 DAP, then surface-sterilized in 75 % ethanol for 30 s and in 8 % sodium hypochlorite for 12 min, followed by 10 min rinses three times in sterilized distilled water and placed in MS solid medium (Murashige and Skoog 1962). Immature embryos were excised from siliques and transferred into B5 solid medium (Gamborg et al. 1968) at 10 or 15 DAC. For each treatment in four combinations, two replications were performed. Culture conditions were as follows: light length 16 h/day, illumination intensity 2000 lx, and temperature 25 ± 2 °C. The number of embryo-derived interspecific progenies was scored. Analysis of variance (ANOVA) was performed between treatments using the one-way ANOVA of SPSS 11.0 for Windows (SPSS, Chicago, IL, USA).
Because the seeds of interspecific hybrids harvested directly from siliques (without embryo rescue) were abnormal and difficult to germinate normally, they were surface sterilized and then sown on MS medium as described above.
Once the seedling shoots had reached 1–2 cm in length, they were cut and placed in plant regeneration medium (MS medium with 1 mg l−1 6-BA and 0.1 mg l−1 NAA) for propagation and sub-culture. The propagated shoots were transferred into root medium (MS medium with 0.1 mg l−1 IBA and 0.1 mg l−1 NAA). Rooted seedlings were washed to remove adhered medium and transplanted directly to the field beneath a sun-shading net, as described by Liu et al. (2003).
DNA extraction and amplification with previous allele-specific Rfo primers in cabbage and Chinese kale
Genomic DNA was extracted from young leaves using the CTAB method (Saghai-Maroof et al. 1984). The DNA concentration was determined using a Nanodrop Spectrophotometer ND-100 (Thermo Fisher Scientific, Wilmington, DE, USA), diluted to a working concentration of 40–100 ng/μl, and stored at 4 °C. Previous allele-specific Rfo primers (Hu et al. 2008; Table 1) were tested to determine whether they could be used to select restored and non-restored materials in cabbage and Chinese kale. Each PCR contained 1 × ThermoPol Reaction buffer (Mg2+ included), 200 μM dNTPs, 0.2 μM forward primer, 0.2 μM reverse primer, 0.5 U of Taq DNA Polymerase, 200 ng of genomic DNA, and nuclease-free water to a total volume of 20 μl. The PCR amplification program was as follows: 95 °C for 3 min; 35 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 45 s; and a final extension at 72 °C for 5 min. PCR amplification products were separated in 1.2 % agarose gels in 1 × TBE buffer and visualized under UV light.
If the previous allele-specific Rfo primers amplified a non-specific fragment in the non-restored Ogu-CMS lines of cabbage and Chinese kale, the non-specific amplified fragments were cloned and sequenced.
Comparison of Rfo gene homologous sequences
Because the amplicon of BnRFO-AS2F/BnRFO-AS2R was only 247 bp, not long enough to design new primers, to get longer sequence of Rfo-homologous sequences, a conserved primer pair (Con-F/Con-R) was designed according to the multiple sequence alignment result (Table 1). The alignment was based on the genomic sequence of the radish Rfo gene (PPR-B) retrieved from the NCBI-GenBank database (GenBank Accession AY285674; Brown et al. 2003), and homologous sequences of B. oleracea var. capitata and B. rapa retrieved from the Brassica database (BRAD; http://brassicadb.org). Bands amplified by Con-F/Con-R from the Chinese kale parent were excised, purified, and then cloned into pEASY-T1 cloning vector, following manufacturer’s instructions (TransGen Biotech, Beijing, China). Positive clones were sequenced using M13 primers (Sangon Biotech, Shanghai, China). Based on the multiple sequence alignment of the amplicon Con-F/Con-R, a novel allele-specific Rfo primer was developed. PCR amplification was performed using the newly developed primers in the parents and F1 hybrids as described above. All obtained sequences were analyzed and aligned using the SeqMan program of DNASTAR (Madison, WI, USA) and the Web-based software ClustalW2, a multiple sequence alignment tool available on the EMBL-EBI website. Protein prediction from these Rfo-homologous sequences was performed using the Web-based tool AUGUSTUS (http://augustus.gobics.de/).
Morphological trait investigation and ploidy identification
Morphological characteristics, including leaf, bud, inflorescence, and flower of progenies were investigated. The ploidy of plant progenies was identified through flow cytometry (BD FACSCalibur™, BD Biosciences, San Jose, CA, USA). The DNA content was estimated according to the protocol of Dolezel et al. (2007). Chinese kale parental DNA content (2C) was measured as the reference. Its G1 peak was positioned on the abscissa (200 channels) by adjusting the instrument gain settings. The coefficient of variation (CV) of all samples was below 5 %.
Fertility level and fertility variation
At anthesis, the fertility of interspecific hybrids was investigated at different days after flowering (DAF). The fertility level and fertility trend were assessed from two aspects: relative amount of pollen grains and pollen viability. The relative amount of pollen grains was visually determined by the naked eye, using the maintainer line of JL1 as control (100 % pollen content) and was scored as follows: (1) no pollen or pollen less than 20 %; (2) pollen content from 20 to 40 %; (3) pollen content from 40 to 60 %; (4) pollen content from 60 to 80 %; and (5) pollen over 80 %. Relative amount of pollen grains was scored for newly opened flowers once every 2 days, and the average value was calculated once every 6 days.
Pollen viability was estimated using the aceto-carmine dyeing method. Pollen grains were collected from three newly opened flowers and dyed with the 1 % acetocarmine, and over 300 pollen grains were observed under the microscope in one replication. Pollen grain was considered viable if it turned deep pink and was plump. Pollen viability was observed once every 6 days. The average value (mean ± standard deviation) of viability pollen percentage was calculated from three replications. The standard deviation and ANOVA were performed using SPSS 11.0 for Windows.
Results
Analysis of Rfo-homologous sequence and development of specific marker
The previous Rfo specific primers BnRFO-AS2F/BnRFO-AS2R (Table 1; Hu et al. 2008) amplified the Rfo gene specifically in the rapeseed restorer lines RF1–4. However, two B. oleracea genotypes (Chinese kale line JL1 and cabbage line GL1) without Rfo gene were also positive for primers BnRFO-AS2F/BnRFO-AS2R, which meant that these primers had non-specific amplification in B. oleracea non-restorer lines (Fig. 1a). Clones of the non-specific amplified fragments were sequenced and showed 88 % identity to the Rfo fragment from radish (Fig. 2). Owing to the presence of non-specific amplifications, the primers BnRFO-AS2F/BnRFO-AS2R could not be used as a specific marker to differentiate B. oleracea restorer lines from non-restorer lines. Therefore, it was critical to design a novel allele-specific Rfo primer.
Rfo-homologous sequences of B. oleracea var. capitata and B. rapa, obtained from the BRAD database, shared over 89 % identity to the Rfo sequence (Supplementary Fig. S1). A highly conserved primer pair Con-F/Con-R, designed on the multiple sequence alignment, amplified an Rfo-homologous fragment of 600 bp, which covered the amplicon of BnRFO-AS2F/BnRFO-AS2R (247 bp). Sequence alignment of Con-F/Con-R amplicon revealed one homologous Rfo fragment in the Chinese kale non-restorer parent (Supplementary Fig. S2). Two insertion/deletion fragments at the nucleotide position of 63–65 and 475–486 were identified between Rfo gene and Chinese kale Rfo-homologous fragments (Supplementary Fig. S2). Based on the sequence difference, a novel specific primer BnRFO-NEW-R was designed (Table 1). The novel primer pair BnRFO-AS2F/BnRFO-NEW-R could be amplified in the rapeseed restorer materials, but not in the Ogu-CMS Chinese kale or cabbage non-restored lines (Fig. 1b). Thus, this novel primer pair BnRFO-AS2F/BnRFO-NEW-R could be used for the early selection of restorer individuals in interspecific hybrids of B. oleracea.
Production of interspecific hybrids with embryo rescue
To transfer the Rfo gene from rapeseed to Chinese kale, the Ogu-CMS line JL1 was crossed with four rapeseed restorer lines. Details of the distant hybridizations are given in Table 2.
Without embryo rescue, 114 seeds were harvested from 6007 siliques (1.9 % seeds per pod; Table 2). Most of these were shriveled or malformed. Five out of 114 seeds germinated and grew into seedlings on the MS medium.
When using embryo rescue, most embryos gradually became brown, shriveled, or died during in vitro culture (Supplementary Fig. S3). A total of 1668 embryos (including collapsed, shriveled, or endosperm absent) were excised from 240 siliques, an average of 6.9 embryos per pod. In total, ten seedlings were obtained from the embryos, four from the cross JL1 × RF4 (Table 2).
Significant differences in the number of embryos excised from pods were observed among six treatments (Table 3, P < 0.05). Embryo rescue at treatment III (10 DAP + 10 DAC) had the highest tendency for seedling formation and the maximal number of embryos per pod in all cross combinations (Table 3, P < 0.05). In contrast, treatment VI (15 DAP + 15 DAC) had the lowest number of embryos per pod, most of which were brown or black and had collapsed or shriveled (Supplementary Fig. S3c, f), and no viable seedlings were obtained using this treatment. There were no significant differences among the treatments I, IV, and V (Table 3, P < 0.05). Younger embryos had lower survival when pods were removed from plants too early (e.g., treatment I). Treatment III (10 DAP + 10 DAC) provided the optimal conditions for embryo rescue in this study.
Morphology and ploidy identification of interspecific hybrids
Morphological observation and ploidy identification revealed that three hybrid plants were generated through repeated pollination without embryo rescue and six hybrid plants from embryo rescue were interspecific hybrids (Table 4). These plants grew more vigorously than their parents. Moreover, some morphological characteristics of the putative hybrid plants were intermediate between the two parents (Fig. 3a–d), and some of them were more similar to those of the rapeseed parents. For example, leaves were petiolated and lyrate, with one large-winged terminal lobe and one to three pairs of smaller lobes on the basal portion of the petiole (Fig. 3a); the leaf surfaces and petioles were pubescent, with hair more conspicuous on younger leaves (Fig. 3b). However, some other characteristics were more similar to the Chinese kale parent. For instance, the leaf margin was minutely denticulate, while it was denser in the Chinese kale parent. The leaf color was bright green with less wax deposition; marking it intermediate between the two parents. At anthesis, the inflorescence of the hybrids was also of intermediate type (Fig. 3c), with a slightly yellow flower and a full, slim bud (Fig. 3c, d). The flower size of the hybrids was larger than that observed in both parents, likely due to heterosis (Fig. 3d).
To determine ploidy level, the DNA content of hybrids was estimated using flow cytometry. The G1 peak position of nine interspecific hybrids was between the peaks of the two parents (Fig. 4) and close to the mean of the parents. This suggested that all hybrid plants were triploid plants. From the G1 peak position, it is proposed that the chromosome composition of interspecific hybrids was ACC (with some individual aneuploidy plants).
Marker-assisted selection for the restorer individual and fertility observation
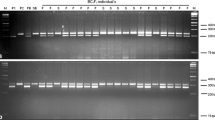
Nine interspecific hybrids were screened using primers BnRFO-AS2F/BnRFO-NEW-R. PCR results indicated that seven of the nine progenies harbored the Rfo gene, named as YL1–YL7 (Table 4; Fig. 5). YL1 contained the Rfo gene, but did not produce any pollen and only had abnormal stamens (Fig. 6b), which may be a result of a heterogenetic chromosome doubling disorder. Except for YL1, all the other six interspecific hybrids with the Rfo gene had pollen (Table 4; Fig. 6). Other than the seven Rfo-positive progenies, the remaining two interspecific hybrids that did not carry the Rfo gene had no pollen observed during the whole flowering period. Therefore, the novel designed primers proved useful for the selection of fertility-restored progenies.
At anthesis, there was a significant difference in fertility performance among the seven progenies containing the Rfo gene (Tables 5, 6, P < 0.05). The pollen performance differed, e.g., some had small amounts of pollen and short anthers (Fig. 6d–f), while others were full of pollen (Fig. 6c, g). Two progenies, YL2 and YL6, showed a better fertility performance than the other hybrid plants (Tables 5, 6), with more pollen grains (relative amount of pollen grains ≥4.4) and pollen viability (≥36.5 %). Furthermore, there was significant variation in fertility performance at different DAFs (Tables 5, 6, P < 0.05). Generally, all restored hybrids showed better fertility performance before 12 DAF, with the relative amount of pollen grains and pollen viability becoming lower after 12 DAF (Tables 5, 6). The fertility performances of some individuals (YL2 and YL6) are shown in Supplementary Fig. S4.
Furthermore, the BC1 progenies were obtained through backcross of the fertility-restored interspecific hybrid as the male parents and with JL1 as the female parent. Twenty-three BC1 progenies were obtained from YL2–3 (colchicine doubling individual from YL2), six from YL2 and two from YL6. No progeny was obtained from other F1 fertility-restored interspecific hybrids. Screening with the novel allele-specific Rfo marker identified 25 BC1 progenies that harbored the Rfo gene, and these had fertility restored (Fig. S5a, c, d). The mean pollen viability of some BC1 individuals was above 50 % higher than YL2 (36.8 %, Table 5), and 17 BC1 individuals above 70 %. It showed that fertility was further improved with one generation backcross. No pollen was observed in the remaining six BC1 progenies that did not carry the Rfo gene (Fig. S5b). This result further confirmed that the novel designed primers could improve the select efficiency of fertility-restored progenies.
Discussion
Restorer line development in Brassica crops
In Brassica crops, CMS plays an important role in heterosis utilization. In the past 30 years, a number of CMS types and corresponding restorer genes have been reported in different Brassica species (Budar et al. 2004). For example, Pol CMS (Fu et al. 1989) and its restorer gene Rfp (Liu et al. 2012), Nap CMS (Thompson 1972) and its restorer gene Rfn (Brown 1999), and Shan 2A CMS (Li 1980) were all found in B. napus. Hau CMS (Jing et al. 2012) and CMS-orf220 (Yang et al. 2010) were found in B. juncea. These CMS types are widely used in the hybrid production of Brassica crops. In addition to the CMS types originated in Brassica crops, some CMS types have been transferred to Brassica species from other relative genera, e.g., Ogura CMS in R. sativus. Ogura CMS has previously been transferred into B. oleracea and B. napus from radish, through interspecific crosses (Bannerrot et al. 1974) and subsequent protoplast fusion (Pelletier et al. 1983), respectively. Although an Ogu-CMS rapeseed restorer line has been developed (Heyn 1976; Delourme et al. 1998, 1999; Primard-Brisset et al. 2005; Chen et al. 2013), no restorer material has been found or developed in B. oleracea crops.
In this study, six fertility-restored interspecific hybrids carrying the Rfo gene were created for the first time. Furthermore, some BC1 fertility-restored individuals, closer to the genetic background of Chinese kale parent, were obtained by backcrossing, which was confirmed by genetic background selection and morphological observation in our recent researches. In addition, we have screened other 15 genotypes of B. oleracea using the novel Rfo specific marker. No non-specific amplified fragments were detected, so this novel marker could be used in other genotypes of B.olerecea. The novel marker and the fertility-restored interspecific hybrids lay the foundation for the development of Ogu-CMS restorer material in cabbage.
Application of embryo rescue in the development of interspecific hybrids
As B. oleracea and B. napus belong to different species of the Brassica genus, it is difficult to obtain hybrids through hand pollination alone. Although great effort has been put in obtaining interspecific hybrids, only a few have been produced from thousands of pollinations (Honma and Summers 1976; Quazi 1988; Li et al. 2014). Consistent with previous results, only three interspecific hybrids (0.05 %) were obtained when using hand pollination in this study.
Embryo rescue has been proved to be an effective technique to enhance the frequency of interspecific hybrid production. Since it was first used in Brassica (Nishi et al. 1959), numerous interspecific hybrids have been created by embryo rescue (Ayotte et al. 1987; Chiang et al. 1977; Quazi 1988; Inomata 1979; Wen et al. 2008). In this study, six interspecific hybrids (2.5 %) were obtained using embryo rescue, which was about 50 times higher than when embryo rescue was not used (Table 4). Thus, use of embryo rescue greatly improved the efficiency of interspecific hybrid development.
Additionally, the stage when an embryo is excised from siliques is crucial. When resistance to Plasmodiophora brassicae was transferred from B. napus to cabbage using embryo rescue, it was suggested that a minimum time point of 20 DAP was essential for embryo development and that embryo breakdown occurred beyond 30 DAP (Chiang et al. 1977). Quazi (1988) reported that approximately 98 % of embryos had collapsed after 22 DAP when using embryo rescue to obtain interspecific hybrids between B. napus and B. oleracea. The optimal embryo-excise stage is different for crosses in different species or species crosses, e.g., 14–15 DAP in interspecific crosses between B. napus and B. oleracea (Ayotte et al. 1987), and 16-18 DAP in crosses between B. rapa and B. oleracea (Wen et al. 2008). In this study, treatment III (10 DAP + 10 DAC; 20 days total) was the optimal stage for embryo rescue; this is consistent with the above-mentioned reports. In addition to the nine interspecific hybrids, another six hybrid progenies showed only maternal characteristics; these might be produced by parthenogenesis or somatic embryogenesis. Eenink (1974a, b) suggested that parthenogenesis in Cruciferae occurred after interspecific or intergeneric pollination. Furthermore, matromorphic progenies can be produced from testa remaining from the embryo rescue process (Quazi 1988).
Rfo-homologous sequence analysis
The radish restorer gene Rfo belongs to the pentatricopeptide repeat (PPR) protein family (Desloire et al. 2003). These are characterized by tandem degenerate repeats of a 35 amino acid motif; most are thought to function as sequence-specific RNA binding proteins that modulate mitochondrial and chloroplast gene expression through post-transcriptional processes (Schmitz-Linneweber and Small 2008). Rfo (PPR-B) is flanked in the radish genome by two highly similar PPR proteins: PPR-A and PPR-C (Desloire et al. 2003). PPR-C is a pseudogene, whereas PPR-A is expressed at lower levels than Rfo. Both PPR-A and PPR-C proteins have a deletion removing four amino acids from one of the PPR domains and display a complete inability to restore fertility (Uyttewaal et al. 2008). Moreover, an apparent threshold level of Rfo expression must be achieved for fertility restoration (Qin et al. 2014). The PPR-B protein is localized to mitochondria and can bind target mRNA of the Ogu-CMS-associated gene orf138. Transgenic introduction of a single copy of PPR-B into Ogu-INRA CMS plants completely restores male fertility (Uyttewaal et al. 2008). In this study, BLAST analysis of B. oleracea and B. rapa genome sequences indicated that Rfo-homologous sequences were present in these species. Whole genome sequencing of B. napus was recently completed (Chalhoub et al. 2014); two Rfo-homologous sequences were present in the A and C genome of B. napus. All retrieved sequences were highly similar (> 88 %) to the Rfo sequence (Supplementary Fig. S1). The predicted putative proteins shared over 80 % identity with PPR-B. Interestingly, these proteins had the same four-amino acid deletion as PPR-A in their PPR domains; this is likely to destroy the PPR structure and prevent the restoration function (Supplementary Fig. S6). In B. oleracea, we found that four-amino acid deletion was common in Rfo-homologous sequences based on the analysis of the re-sequencing data, which is consistent with the fact that fertility-restored material for Ogu-CMS is absent in B. oleracea. The discovery of Rfo-homologous sequences in Brassica species suggests that PPR-B may have originated from genes present in the ancestor of Brassicaceae crops. One possibility is that PPR-B may have evolved in response to Ogura CMS in radish and is derived from a reservoir of PPR-B-like genes. Another possibility is that the function of Rfo genes inherited from the ancestor is redundant in Brassica crops, and so it was lost through deletion. It will be interesting to investigate the role of PPR-B-like proteins if they are active in Brassica.
In our study, the interspecific hybrids possessed not only part of the Chinese kale genome, but also part of the rapeseed genome. The novel Rfo specific primers were successfully applied in the MAS of interspecific hybrid and BC generation, and can be used to distinguish the B. oleracea background and rapeseed background. In addition, the novel primers BnRFO-AS2F/BnRFO-NEW-R can amplify the Rfo gene in the rapeseed restored lines, but not the homologous fragments mentioned in Hu et al. (2008) (data not shown), which meant that the newly designed primer can also be used as an allele-specific marker in the rapeseed restorer lines.
Author contribution statement
YH and ZY conceived and designed the research. YH conducted experiments and wrote the manuscript. All authors read and approved the manuscript.
References
Ayotte R, Harney PM, Machado VS (1987) The transfer of triazine resistance from Brassica napus L. to B. oleracea L. I. Production of F1 hybrids through embryo rescue. Euphytica 36(2):615–624
Bannerrot H, Boulidard L, Couderon Y, Temple J (1974) Transfer of cytoplasmic male sterility from Raphanus sativus to Brassica oleracea. In: Wills AB, North C (eds) Proc Eucarpia Meet Cruciferae. Scottish Hortic Res Inst, Invergavrie, pp 52–54
Bonhomme S, Budar F, Ferault M, Pelletier G (1991) A 2.5 kb NcoI fragment of Ogura radish mitochondrial DNA is correlated with cytoplasmic male-sterility in Brassica cybrids. Curr Genet 19:121–127
Bonhomme S, Budar F, Lancelin D, Small I, Defrance MC, Pelletier G (1992) Sequence and transcript analysis of the Nco2.5 Ogura-specific fragment correlated with cytoplasmic male sterility in Brassica cybrids. Mol Gen Genet 235:340–348
Bridgen MP (1994) A review of plant embryo culture. HortScience 29:1243–1246
Brown GG (1999) Unique aspects of cytoplasmic male sterility and fertility restoration in Brassica napus. J Hered 90:351–356
Brown GG, Formanova N, Jin H, Wargachuk R, Dendy C, Patil P et al (2003) The radish Rfo restorer gene of Ogura cytoplasmic male sterility encodes a protein with multiple pentatricopeptide repeats. Plant J 35:262–272
Budar F, Delourme R, Pelletier G (2004) Male sterility. In EC Pua, Douglas (eds) Biotechnology in agriculture and forestry 54. Springer, Berlin Heidelberg New York, pp 43–64
Chalhoub B, Denoeud F, Liu SY et al (2014) Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 345:950
Chen HG (2006) Studies on fertility and crossability of amphidiploids between Raphanus sativus and Brassica alboglabra. Dissertation, Wuhan; Huazhong Agricultural University
Chen WJ, Li M, Wang TH, Hui RK, Tu JX, Fu TD (2013) Development of New Restorer Materials with Ogu CMS in Brassica napus. Agri Sci Technol 14:18–25
Chiang MS, Chiang BY, Grant WF (1977) Transfer of resistance to race 2 of plasmodiophora Brassica from Brassica napus to cabbage (B. oleracea var. capitata). I. Interspecific hybridization between B. napus and B. oleracea var. capitata. Euphytica 26:319–336
Delourme R, Eber F, Renard M (1991) Radish cytoplasmic male sterility in rapeseed: breeding restorer lines with a good female fertility. Proc 8th Int Rapeseed Congr. University Extension Press, University of Saskatchewan, Saskatoon, pp 1506–1510
Delourme R, Eber F, Renard M (1995) Breeding double low restorer lines in radish cytoplasmic male sterility of rapeseed (Brassica napus L.). In: Proc 9th Int Rapeseed Congr, vol 1. Cambridge, UK, pp 6–8
Delourme R, Foisset N, Horvais R, Barret P, Champagne G, Cheung WY, Landry BS, Renard M (1998) Characterisation of the radish introgression carrying the Rfo restorer gene for the Ogu-INRA cytoplasmic male sterility in rapeseed (Brassica napus L.). Theor Appl Genet 97:129–134
Delourme R, Horvais R, Valle´e P, Renard M (1999) Double low restored F1 hybrids can be produced with the Ogu-INRA CMS in rapeseed. In: Proc 10th Int Rapeseed Congr. Canberra, pp 26–29
Desloire S, Gherbi H, Laloui W, Marhadour S, Clouet V, Catolico L et al (2003) Identification of the fertility restoration locus, Rfo, in radish, as a member of the pentatricopeptide-repeat protein family. EMBO Rep 4:588–594
Dolezel J, Greilhuber J, Suda J (2007) Estimation of nuclear DNA content in plants using flow cytometry. Nat Protoc 2:2233–2244
Eenink AH (1974a) Matromorphy in Brassica oleracea L. I. Terminology, parthenogenesis in Cruciferae and the formation and usability of matromorphic plants. Euphytica 23:429–433
Eenink AH (1974b) Matromorphy in Brassica oleracea L. II. Differences in parthenogenetic ability and parthenogenesis inducing ability. Euphytica 23:435–445
Fang ZY, Sun PT, Liu YM (1983) A Preliminary study on distant hybridization between Raphanus sativus L. and Brassica oleracea L. Acta Horticulturae Sinica 3:008
Fang ZY, Sun PT, Liu YM, Yang LM, Wang XW, Hou AF, Bian CS (1997) A male sterile line with dominant gene (MS) in cabbage (Brassica oleracea var. capitata) and its utilization for hybrid seed production. Euphytica 97:265–268
Fang ZY, Sun PT, Liu YM et al (2001) Investigation of different types of male sterility and application of dominant male sterility in cabbage. China Veg 1:6–10
Feng W, Chen YH (1981) Artificial amphidiploids in an interspecific cross (Brassica oleracea L. × B. pekinensis Rupr.). Acta Horticulturae Sinica 8:37–40
Feng J, Primomo V, Li Z, Zhang Y, Jan CC, Tulsieram L, Xu SS (2009) Physical localization and genetic mapping of the fertility restoration gene Rfo in canola (Brassica napus L.). Genome 52:401–407
Fu TD, Yang XN, Yang GS (1989) Development and research of Polima cytoplasmic Male Sterility in Brassica napus. J Huazhong Agri Univ 8:201–207
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Expt Cell Res 50:151–158
Gu AX, Shen SX, Chen XP, Zhang CH, Li XF (2006) Allotriploid hybrids obtained from interspecific hybridization between Chinese cabbage and cabbage and the preliminary research on reproductive characters. Acta Horticulturae Sinica 33:73–77
Han FQ, Yang C, Fang Z et al (2015) Inheritance and InDel markers closely linked to petal color gene (cpc-1) in Brassica oleracea. Mol Breeding 35:160
Heyn FW (1976) Transfer of restorer genes from Raphanus to cytoplasmic male sterile Brassica napus. Cruciferae Newsl 1:15–16
Honma S, Summers WL (1976) Interspecific hybridization between Brassica napus L. (Napobrassica group) and B. oleracea L. (Botrytis group). J Am Soc Hortic Sci 101:299–302
Hu X, Sullivan-Gilbert M, Kubik T, Danielson J, Hnatiuk N, Marchione W, Greene T, Thompson S (2008) Mapping of the Ogura fertility restorer gene Rfo and development of Rfo allele-specific markers in canola (Brassica napus L.). Mol Breeding 22:663–674
Inomata N (1979) Production of interspeciWc hybrids in Brassica campestris × B. oleracea by culture in vitro of excised ovaries II. Development of excised ovaries on various culture media. Jpn J Breed 29:115–120
Jing B, Heng S, Tong D, Wan Z, Fu T et al (2012) A male sterility-associated cytotoxic protein ORF288 in Brassica juncea causes aborted pollen development. J Exp Bot 63:1285–1295
Kaul MLH (1988) Male Sterility in Higher Plants. Springer, Berlin
Li DR (1980) Preliminary report on breeding of three-line system in Brassica napus. Shanxi J Agri Sci 1:26–29
Li S, Yang D, Zhu Y (2007) Characterization and use of male sterility in hybrid rice breeding. J Integr Plant Biol 49:791–804
Li Q, Zhou Q, Mei J, Zhang Y, Qian W et al (2014) Improvement of Brassica napus via interspecific hybridization between B. napus and B. oleracea. Mol Breeding 34:1955–1963
Liu XP, Liu ZW, Tu JX, Chen BY, Fu TD (2003) Improvement of microspores culture techniques in Brassica napus L. Hereditas (Beijing) 25:433–436 (in Chinese with English summary)
Liu Z, Liu P, Long FR, Hong DF, He QB, Yang GS (2012) Fine mapping and candidate gene analysis of the nuclear restorer gene Rfp for pol CMS in rapeseed (Brassica napus L.). Theor Appl Genet 125:773–779
Lv HH, Yang LM, Kang JG et al (2013) Development of InDel markers linked to Fusarium wilt resistance in cabbage. Mol Breeding 32(4):961–967
Mayr E (1986) Joseph Gottlieb Kolreuter’s contributions to biology. Osiris 2:135–176
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol 15:473–497
Nishi S, Kawata J, Toda M (1959) In the breeding of interspecific hybrids between two genomes “c” and “a” of Brassica through the application of embryo culture techniques. Jpn J Breed 5:215–222
Ogura H (1968) Studies on the new male sterility in Japanese radish, with special reference to the utilization of this sterility towards practical raising of hybrid seed. Mem Fac Agric Kagoshima Univ 6:39–78
Pelletier G, Primard C, Vedel F, Chetrit P, Remy R, Rousselle P, Renard M (1983) Intergeneric cytoplasmic hybridization in Cruciferae by protoplast fusion. Mol Gen Genet 191:244–250
Pelletier G, Primard C, Ferault M, Vedel F, Chetrit P, Renard M, Delourme R (1988) Uses of protoplasts in plant breeding: cytoplasmic aspects. Plant Cell Tiss Org Cult 12:173–180
Primard-Brisset C, Poupard JP, Horvais R, Eber F, Pelletier G, Remard M, Delourme R (2005) A new recombined double low restorer line for the Ogu-INRA cms in rapeseed (Brassica napus L.). Theor Appl Genet 111:736–746
Pu Z, Shimizu M, Zhang Y, Tomohiko N, Takeshi H, Hidetaka M, Ryo F, Keiichi O (2012) Genetic mapping of a fusarium wilt resistance gene in Brassica oleracea. Mol Breeding 30(2):809–818
Qiao HY (2012) Study on the interspecific Hybrids between Brassica rapa and Brassica oleracea. Dissertation, Chinese Academy of Agricultural Sciences, Beijing
Qin X, Warguchuk R, Arnal N, Gaborieau L, Mireau H, Brown G (2014) In vivo functional analysis of a nuclear restorer PPR protein. BMC Plant Biol 14:313
Quazi MH (1988) Interspecific hybrids between Brassica napus L. and B. oleracea L. developed by embryo culture. Theor Appl Genet 75:309–318
Rahman MH, Bennett RA, Yang RC et al (2011) Exploitation of the late flowering species Brassica oleracea L. for the improvement of earliness in B. napus L.: an untraditional approach. Euphytica 177(3):365–374
Ripley VL, Beversdorf WD (2003) Development of self-incompatible Brassica napus (I) introgression of S-alleles from Brassica oleracea through interspecific hybridization. Plant Breeding 122:1–5
Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW (1984) Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci USA 81:8014–8018
Schmitz-Linneweber C, Small I (2008) Pentatricopeptide repeat proteins: a socket set for organelle gene expression. Trends Plant Sci 13:663–670
Tanaka Y, Tsuda M, Yasumoto K, Yamagishi H, Terachi T (2012) A complete mitochondrial genome sequence of Ogura-type male-sterile cytoplasm and its comparative analysis with that of normal cytoplasm in radish (Raphanus sativus L.). BMC Genom 13:352
Thompson KF (1972) Cytoplasmic male sterility in oilseed rape. Heredity 29:253–257
Uyttewaal M, Arnal N, Quadrado M, Martin-Canadell A, Vrielynck N, Hiard S, Gherbi H, Bendahmane A, Budar F, Mireau H (2008) Characterization of Raphanus sativus pentatricopeptide repeat proteins encoded by the fertility restorer locus for Ogura cytoplasmic male sterility. Plant Cell 20:3331–3345
Vedel F, Pla M, Vitart V, Gutierres S, Chetrit P, De Paepe R (1994) Molecular basis of nuclear and cytoplasmic male sterility in higher plants. Plant Physiol Biochem 32:601–608
Virmani SS, Ilyas-Ahmed M (2001) Environment-sensitive genic male sterility (EGMS) in crops. Adv Agron 72:139–195
Walters WT, Mutschler AM, Earle DE (1992) Protoplast fusion-derived Ogura male-sterile cauliflower with cold tolerance. Plant Cell Rep 10:624–628
Wang QB, Zhang YY, Fang ZY, Liu YM, Yang LM, Zhuang M (2012) Chloroplast and mitochondrial SSR help to distinguish allo-cytoplasmic male sterile types in cabbage (Brassica oleracea L. var. capitata). Mol Breeding 30:709–716
Wen J, Tu JX, Li ZY, Fu TD, Ma CZ, Shen JX (2008) Improving ovary and embryo culture techniques for efficient resynthesis of Brassica napus from reciprocal crosses between yellow-seeded diploids B. rapa and B. oleracea. Euphytica 162:81–89
Yang LM, Liu YM, Wang XW, Sun PT, Zhuang M, Fang ZY (1997) Preliminary observation on main botanical characteristics of cytoplasmic male sterile cabbage. China Veg 6:24–25
Yang J, Liu X, Yang X, Zhang M (2010) Mitochondrially-targeted expression of a cytoplasmic male sterility-associated orf220 gene causes male sterility in Brassica juncea. BMC Plant Biol 10:231
Yin J, Chen S, Tang Z, Chen L, Li J (2004) Resynthesis of Brassica napus through interspecific hybridization between yellow-seeded B. oleracea var. acephala and B. campestris. South West China J Agri Sci 2:149
Zhang GQ, Tang GX, Song WJ, Zhou WJ (2004) Resynthesizing Brassica napus from interspecific hybridization between Brassica rapa and B. oleracea through ovary culture. Euphytica 140:181–187
Acknowledgments
The authors thank Prof. Jianjun Lei, Prof. Yunchang Li, Prof. Hanzhong Wang, and Prof. Wei Qian for providing materials. This work was supported by grants from the National Natural Science Foundation of China (31272180), the Major State Basic Research Development Program (973 Program, 2012CB113906), the National High Technology Research and Development Program of China (863 Program, 2012AA100102), the Key Projects in the National Science and Technology Pillar Program during the Twelfth Five-Year Plan Period (2012BAD02B01), and the earmarked fund for the Modern Agro-Industry Technology Research System, China (nycytx-35-gw01). This work was performed in the Key Laboratory of Biology and Genetic Improvement of Horticultural Crops (North China), Ministry of Agriculture, Beijing 100097, People’s Republic of China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Richard G.F. Visser.
Electronic supplementary material
Below is the link to the electronic supplementary material.

122_2016_2728_MOESM1_ESM.jpg
Supplementary Fig. S1 Multiple sequence alignment of Rfo sequences with Rfo-homologous sequences in B.oleracea, B.napus, B.rapa (JPEG 2678 kb)

122_2016_2728_MOESM2_ESM.jpg
Supplementary Fig. S2 Sequence alignment of Rfo fragment with Rfo-like fragment amplified by primers Con-F/Con-R. The red arrows represent the location of the primers BnRFO-AS2F/BnRFO-NEW-R (JPEG 4826 kb)

122_2016_2728_MOESM3_ESM.jpg
Supplementary Fig. S3 Embryo development during the embryo rescue process. (a) Freshly removed pods. (b) Excised embryos. (c) Collapsed embryos. (d) Development of pods during the embryo rescue process. (e) Development of embryos in the early period (< 15 days after pollination). (f) Development of embryos in the late period (> 25 days after pollination) (JPEG 631 kb)

122_2016_2728_MOESM4_ESM.jpg
Supplementary Fig. S4 Fertility performance of (a) YL2, and (b) YL5 at different days after flowering (DAF). The red arrows represent the variation of pollen at different DAFs. Left panels: pollen performance; Right panels: pollen viability (JPEG 290 kb)

122_2016_2728_MOESM5_ESM.jpg
Supplementary Fig. S5 (a) PCR amplification in partial BC1 progenies using the novel allele-specific Rfo marker. Lanes 1-8: non-restored BC1 progenies, lanes 9-16: restored- fertility BC1 progenies. Pollen performance in non-restored BC1 progenies (b) and restored- fertility BC1 progenies (c,d) (JPEG 146 kb)

122_2016_2728_MOESM6_ESM.jpg
Supplementary Fig. S6 Protein sequence alignment of Rfo with Rfo homologous sequences in B.oleracea, B.napus, B.rapa (JPEG 2342 kb)
Rights and permissions
About this article
Cite this article
Yu, Hl., Fang, Zy., Liu, Ym. et al. Development of a novel allele-specific Rfo marker and creation of Ogura CMS fertility-restored interspecific hybrids in Brassica oleracea . Theor Appl Genet 129, 1625–1637 (2016). https://doi.org/10.1007/s00122-016-2728-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-016-2728-9