Abstract
Stem rust resistance gene Sr22 transferred to common wheat from Triticum boeoticum and T. monococcum remains effective against commercially prevalent pathotypes of Puccinia graminis f. sp. tritici, including Ug99 and its derivatives. Sr22 was previously located on the long arm of chromosome 7A. Several backcross derivatives (hexaploid) possessing variable sized Sr22-carrying segments were used in this study to identify a closely linked DNA marker. Expressed sequenced tags belonging to the deletion bin 7AL-0.74–0.86, corresponding to the genomic location of Sr22 were screened for polymorphism. In addition, RFLP markers that mapped to this region were targeted. Initial screening was performed on the resistant and susceptible DNA bulks obtained from backcross derivatives carrying Sr22 in three genetic backgrounds with short T. boeoticum segments. A cloned wheat genomic fragment, csIH81, that detected RFLPs between the resistant and susceptible bulks, was converted into a sequence tagged site (STS) marker, named cssu22. Validation was performed on Sr22 carrying backcross-derivatives in fourteen genetic backgrounds and other genotypes used for marker development. Marker cssu22 distinguished all backcross-derivatives from their respective recurrent parents and co-segregated with Sr22 in a Schomburgk (+Sr22)/Yarralinka (−Sr22)-derived recombinant inbred line (RIL) population. Sr22 was also validated in a second population, Sr22TB/Lakin-derived F4 selected families, containing shortened introgressed segments that showed recombination with previously reported flanking microsatellite markers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stem rust caused by Puccinia graminis f. sp. tritici (Pgt) is the most damaging of three rust diseases of wheat. Frequent epidemics and severe losses have been reported in various wheat-growing regions (Leonard and Szabo 2005). In the last decade, a Pgt pathotype, Ug99, possessing virulence for the most commonly deployed genes including Sr31, was detected in Uganda (Pretorius et al. 2000). This pathotype spread to adjacent regions in North Eastern Africa, Yemen and by 2008 it reached Iran (Jin et al. 2008; Singh et al. 2008). As Sr31 is present in a very high proportion of wheat cultivars in many countries, the detection of this Sr31-virulent pathotype re-focused international attention on stem rust control. Amongst the various strategies for rust control, the deployment of diverse sources of resistance in new cultivars remains the most effective, practical and environmentally sustainable approach.
A majority of stem rust resistance genes that are effective against Ug99 come from the wild relatives of wheat (Singh et al. 2008). Sr22, transferred from diploid (AA) wheat Triticum boeoticum and T. monococcum into common hexaploid bread wheat (T. aestivum), conferred resistance against Ug99 (Jin et al. 2008, 2009; Singh et al. 2008). It was initially transferred into tetraploid wheat (Gerechter-Amitai et al. 1971). Subsequently, Sr22 was transferred to hexaploid wheat and was mapped on chromosome 7A (Kerber and Dyck 1973; The 1973). Although Sr22 provides resistance to all Pgt pathotypes (except the stem rust race 316 and 317 in Israel) tested in different wheat growing regions of the world, the Australian wheat cultivar Schomburgk remains the only commercially released cultivar that carries this gene (Gerechter-Amitai et al. 1971; Roelfs and Mcvey 1979; Leroux and Rijkenberg 1987; Knott 1990; Singh 1991; Khan et al. 2005).
Paull et al. (1994) detected T. boeoticum alleles in several backcross derivatives carrying Sr22 using RFLP probes distributed across wheat chromosome 7A and identified various sized T. boeoticum segments in these genotypes. Simple sequence repeat (SSR) markers cfa2123 and cfa2019 that flanked Sr22 (Khan et al. 2005) were used in a study aimed at recovering recombinants between wheat and T. boeoticum chromosome 7AmL in order to identify reduced T. boeoticum segments carrying Sr22 (Olson et al. 2010). Additional SSR markers wmc633 and gwm332 which are closer to Sr22 than cfa2019 were also used to characterise the shortened segments. Recombinants with the Sr22 resistance phenotype were identified but could not be detected using available wheat DNA markers.
This investigation reports the development of a robust molecular marker cssu22 that validated the presence of Sr22 in wheat germplasm and more significantly detected the occurrence of Sr22 in recombinants currently known to contain smaller T. boeoticum segments. The marker development process also facilitated the characterisation of Sr22 carrying backcross derivatives in several wheat backgrounds.
Materials and methods
Plant material
Sr22 carrying backcross derivatives in common wheat backgrounds K441, IW562, Cranbrook, XL23, WT20/5, Warigal, Kiata, Lowan, CO1568, CO1213, Cocamba, K2001, RAC177 and Kulin were used (pedigree details can be found in Paull et al. 1994). The K441, CO1568 and CO1213 derivatives were identified as carrying shorter T. boeoticum segments (Paull et al. 1994). These backgrounds were chosen for RFLP analysis and the remaining material was used for validation of the newly developed PCR marker reported in this study. In addition, the recombinant wheat genotypes designated ‘U5616-20-154’ which carries the shortest T. boeoticum segment with Sr22 were included from Olson et al. (2010).
Ninety-two recombinant inbred lines from a cross between Schomburgk (+Sr22) and Yarralinka (−Sr22) were used for positioning the identified marker in relation to the previously reported flanking SSR markers (Khan et al. 2005).
Rust response test
The backcross derived lines and the Schomburgk/Yarralinka mapping population were tested in the seedling stage against the Pgt pathotype 40-1, 2, 3, 4, 5, 6, 7 (PBI culture number 383) at the University of Sydney Plant Breeding Institute, Cobbitty. The disease inoculation and scoring was performed using the procedure described by Bariana and McIntosh (1993).
DNA extraction
Genomic DNA from Sr22 carrying backcross derivatives and respective recurrent parents were extracted from young leaf tissue using the method described by Lagudah et al. (1991b). DNA from the Schomburgk/Yarralinka RIL population was extracted using the sap extraction method described by Clarke et al. (1989). DNA samples from backcross derivatives in K441, CO1213 and CO1568 (carrying shorter T. boeoticum segments) backgrounds were pooled and digested with 12 restriction enzymes (BamHI, BglII, DraI, EcoRI, EcoRV, HindIII, NcoI, NdeI, PstI, SacI, XbaI and XhoI). Similarly, DNA from respective recurrent parents were pooled and used as the negative control. RFLP analysis was performed using the procedure described by Lagudah et al. (1991a).
Wheat expressed sequenced tags
Based on the wheat composite and deletion bin map information from the Graingenes database (http://wheat.pw.usda.gov), the chromosome section spanning the orthologous Sr22 region was predicted to be in the deletion bin 7AL-0.74–0.86 (http://www.wheat.pw.usda.gov/cgi). A random set of expressed sequenced tags (ESTs) chosen from this bin included BF201318, BF483150, BG604514, BE605108 and BE442572. These EST sequences were blasted against rice sequence and primers were designed from the conserved sites to amplify the corresponding sequence from wheat genomic DNA. DNA of the CO1568 backcross derivative with Sr22 was used for PCR and the amplified fragment was cloned into pGEM-T vector and sequenced according to the protocol described in Seah et al. (1998). The fragment showing the highest similarity with the original EST sequence was then used for genomic blot hybridisation.
Chromosome group 7 specific probes
In addition to wheat ESTs, RFLP probes previously mapped on the long arm of chromosome group 7 were used to identify polymorphism between the two pooled DNA (with and without Sr22) samples (Paull et al. 1994; Boyko et al. 1999). Two RFLP probes glk750 (Liu and Tsunewaki 1991) and csIH81 (Lagudah et al. 1991a) used in the present study were sourced from the Australian Triticeae Mapping Initiative (ATMI) collection. The probes glk750 and csIH81 were originally cloned in pUC19 and pBR322 vectors, respectively, and the corresponding transformed E.coli cultures were maintained in glycerol stocks at −80°C. Vector plasmids were isolated, digested with PstI restriction enzyme and the inserts obtained were used as probes for genomic blot hybridisation.
csIH81 sequence analysis
In order to get the full-length sequence of the csIH81 DNA fragment, primers were designed on either side of the PstI restriction site in the recombinant pBR322 vector. The primer sequences are given below:
-
IH81Forward-5′TATATCGAGCATTCGGAC3′ and
-
IH81 Reverse-5′GTTTGTCGACATCGAACAGCC3′.
These primers were designed to amplify corresponding regions in group 7 chromosomes of Chinese Spring nulli-tetrasomic cytogenetic stocks and from diploids species T. boeoticum, T. monococcum, and T. urartu. Multiple sequence alignment (CLUSTAL—European Bioinformatics Institute—http://www.ebi.ac.uk/Tools/sequence.html) was used to identify polymorphic regions specific for chromosome 7A of common wheat and to design sequence specific primers.
Simple and multiplex PCR primer reactions
Primer csIH81-BM (Forward-5′TTCCATAAGTTCCTACAGTAC3′ + Reverse-5′TAGACAAACAAGATTTAGCAC3′) was designed to amplify DNA sequence specific for Sr22 carrying segments of T. boeoticum and T. monococcum, whereas primers csIH81-AG (Forward-5′CTACCTCTGTCAATTTGAAC3′ + Reverse-5′GAAAAATGACTGTGATCGC3′) were designed to amplify corresponding fragments from Sr22 lacking genotypes. The PCR amplification conditions described by Lagudah et al. (2009) were followed. csIH81-BM and csIH81-AG primers were used under optimal annealing temperatures of 58 and 60°C, respectively. In order to optimise multiplex PCR conditions for use as a codominant marker assay, 10 μM concentration stocks of primers csIH81-BM and csIH81-AG were mixed according to the following volume ratios (BM:AG); 1 μl:1 μl, 1 μl:0.5 μl, 0.5 μl:0.5 μl and 0.5 μl:1 μl per total of 20 μl PCR reaction volume. Each combination was tested at annealing temperatures of 55, 58 and 62°C.
Mapping and validation
Previously reported SSR markers cfa2123 and cfa2019 were used for the initial screening of the Schomburgk/Yarralinka RIL population. The location of the genomic amplification product generated using the new multiplex primer was then determined in relation to the SSR markers cfa2123 and cfa2019 on chromosome 7AL. In addition, genomic DNA from the recombinant U5616-20-154 lines, from the cross between Sr22TB (a wheat genotype with Sr22) and Lakin, carrying the shortest T. boeoticum segment (Olson et al. 2010) without the previous SSR flanking markers were tested. The marker was also tested on Sr22 carrying backcross-derivatives in 14 common wheat backgrounds including the ones used for initial polymorphism analysis (Table 1).
Results
Phenotypic assessments
The rust resistance score of the backcross derived near-isogenic lines carrying Sr22 showed infection types (IT) that varied between IT2= and IT2− when tested against the Pgt pathotype 40-1, 2, 3, 4, 5, 6, 7 (Table 1). Recurrent parents produced seedling responses ranging from IT23 to IT3+. In the case of the Schomburgk/Yarralinka mapping population, the resistant parent Schomburgk and the resistant RILs showed IT2-, whereas the susceptible parent Yarralinka and the susceptible RILs produced IT3+ (Fig. 1). Of 92 RILs tested, 41 were resistant and 51 were susceptible. Chi-squared analysis supported the monogenic inheritance of Sr22 (χ 2 = 1.087 and table value of χ 2 is 3.84 and 6.64, at p = 0.05 and p = 0.01, respectively).
Identification of Sr22-linked DNA markers
Genomic DNA fragments corresponding to five ESTs located in the deletion bin 7AL-0.74–0.86 were screened as RFLP probes on resistant and susceptible DNA bulks. None of the probes showed polymorphism between the resistant and susceptible bulks. However, RFLP markers glk750 (Liu and Tsunewaki 1991; Paull et al. 1994) and csIH81 (Lagudah et al. 1991a) revealed restriction fragment differences when tested on the same panel. The RFLP obtained using the probe glk750 displayed a complex hybridisation pattern (Fig. 2a) expected from a multicopy genomic sequence. In contrast, the probe csIH81 produced a simpler RFLP pattern (Fig. 2b) typical of a single copy locus.
The ability of csIH81 to detect polymorphism between Sr22 and sr22 bulks prompted us to convert the RFLP to a PCR based STS marker. Primers designed at the terminal regions of the original csIH81 fragment derived from Aegilops tauschii, amplified corresponding regions from the other related sequence members of chromosome 7 in bread wheat and diploid ‘A’ genome species. Sequence information of T. boeoticum and T. monococcum were identical, whilst that of the nullisomic–tetrasomic stocks for chromosomes 7A, 7B and 7D were different and revealed the three genome specificities for the csIH81 sequence. The fragment amplified from T. urartu was identical to the 7A locus of the bread wheat cultivar Chinese Spring. Primers were designed in the polymorphic regions to amplify the 7A-specific products and those from T. boeoticum and T. monococcum, the source of Sr22. A specific amplification product was obtained with primer csIH81-BM in all the backcross derivatives that carry Sr22 and therefore served as a dominant marker (Fig. 3a). Conversely, the primer csIH81-AG amplified fragments only in wheat genotypes lacking Sr22 (Fig. 3b). Due to the lack of large sequence insertions or deletions, a multiplex PCR approach was adopted to develop a codominant marker with a pair of primers (csIH81-AG) targeting the 7A genome of common wheat and another pair of primers (csIH81-BM) the introgressed segment carrying Sr22.
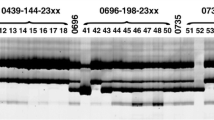
PCR amplification in wheat genotypes with and without Sr22 based on primers csIH81-BM (Fig. 4a) csIH81-AG (Fig. 4b) and a multiplex of csIH81-BM and AG (Fig. 4c). For each panel on the extreme left is a 1 kb ladder of a molecular marker, then from left to right are the wheat genotypes, Sr22/4*CO1568, CO1568, Sr22/4*CO1213, CO1213, Sr22/*4 Cranbrook and Cranbrook
Tests conducted with different primer pair concentration ratios and annealing temperatures showed that a primer mixture ratio of 2:1 (csIH81-BM: csIH81-AG) at 58°C gave clear amplification differences between resistant and susceptible lines. We designate the new csIH81-derived multiplex marker in bread wheat as cssu22 with an amplification product of 237 bp in Sr22 introgressed lines and 355 bp in sr22 genotypes (Fig. 3c).
Mapping and validation of cssu22 in diverse common wheat backgrounds
The cssu22 marker was used to screen different backcross derivatives of Sr22, which were phenotypically confirmed for resistance. The marker was able to identify the gene in all 14 diverse cultivar backgrounds (Table 1).
The RILs from the Schomburgk/Yarralinka population were initially screened with the previously reported SSR flanking markers, cfa2123 and cfa2019. Seven recombinants were detected between cfa2123 and Sr22 whilst five recombinants occurred between Sr22 and cfa2019. Marker cssu22 co-segregated with the Sr22 gene. In addition, the nine recombinant bread wheat lines that carry Sr22 but lack the flanking SSR markers cfa2123, wmc633, gwm332 and cfa2019 from the cross between Sr22TB and Lakin (Olson et al. 2010) also carried the cssu22 marker (Fig. 4). Hence, the newly developed marker is closer to the Sr22 gene than any of the previously published Sr22 markers.
Schematic diagram representing various Sr22 recombinant lines and position of molecular markers in chromosome 7A. Type 1 includes Schomburgk, Sr22/3* Kiata, Sr22/4* Lowan, Sr22/4*Wt20/5, Sr22/4*IW562 (Paull et al. 1994); Type 2 includes Sr22/4*CO1568; Type 3 includes Sr22/4*CO1213, Sr22/3* K441, Sr22/4*K2001 and Type 4 includes RAC177 and Sr22TB/Lakin-derived lines
Discussion
A robust Sr22-linked DNA marker, cssu22, was identified and validated across 14 Australian wheat backgrounds and some F4 families from a North American source with minimal T. boeoticum introgressed segment (Olson et al. 2010). The marker cssu22 co-segregated with Sr22 amongst 92 Schomburgk/Yarralinka-derived RILs. It comprises of two sets of primers and behaves as a codominant marker. Whilst the effectiveness of cssu22 across a range of backgrounds makes it a ‘breeder friendly’ molecular marker, it also has the ability to detect heterozygotes in segregating populations in early generations.
When ESTs from the targeted deletion bin on chromosome 7 failed to show polymorphism, a search for other RFLP markers predicted to be in the Sr22 region facilitated the marker identification process. From the sequence information of csIH81 region, short deletions and SNPs were identified between chromosome 7 homoelogs. Primers designed based on the diploid and hexaploid ‘A’ genome sequences enabled amplification products that distinguished Sr22Sr22, Sr22sr22 and sr22sr22 genotypes. The ability of the new marker, cssu22, to clearly differentiate the presence and absence of the gene in numerous genetic backgrounds should enable combining Sr22 with other stem rust resistance genes in marker assisted breeding towards achieving more durable rust resistant germplasm.
Further, screening of the Schomburgk/Yarralinka RIL population with previous flanking markers (Khan et al. 2005) revealed the presence of recombinants between Sr22 and linked SSRs. The present marker co-segregated with the gene indicating its utility in marker assisted selection over the previously reported markers. In addition, screening of recombinants from the cross between Sr22TB and Lakin with shortened T. boeoticum segments (without the previous flanking markers) provided further indication of the tight linkage of cssu22 with Sr22.
Sr22 produces IT2= to 2− similar to stem rust resistance genes Sr9e, Sr13, Sr24, Sr26, Sr39, Sr45 (McIntosh et al. 1995). In the absence of closely linked markers, the presence of Sr22 in combination with the aforementioned genes cannot be easily determined. Markers for SrR, Sr24, Sr26, Sr33, Sr39 and Sr45 have been reported (Mago et al. 2005; Bariana et al. 2007; Sambasivam et al. 2008; Mago et al. 2009). Combinations of Sr22 with stem rust resistance genes SrR, Sr26, Sr33, Sr39 and Sr45 that are effective against the Pgt pathotype Ug99 and its derivatives can be achieved. Sr22 has not widely used in commercial cultivars worldwide presumably due to the availability of linked rust resistance genes Sr31/Lr26/Yr9, Sr24/Lr24 and Sr38/Lr37/Yr17. Sr24, Sr31 and Sr38 are all ineffective against the Ug99-derivative, TTKST. Therefore, Sr22 represents an effective source of resistance for deployment in combination with other genes in new wheat cultivars.
Given the evidence for homologous recombination between the A genome of T. aestivum and the Sr22 introgressed segment from this study, coupled with the identification of the cssu22 marker, there are encouraging signs for the prospects of developing resources to enable cloning of Sr22.
References
Bariana HS, McIntosh RA (1993) Cytogenetic studies in wheat. XV. Location of rust resistance genes in VPM1 and its genetic linkage with other disease resistance genes in chromosome 2A. Genome 32:476–482
Bariana HS, Brown GN, Bansal UK, Miah H, Standen GE, Lu M (2007) Breeding triple rust resistant wheat cultivars for Australia using conventional and marker-assisted selection technologies. Aust J Agric Res 58:576–587
Boyko EV, Gill KS, Mickelson-Young L, Nasuda S, Raupp WJ, Ziegle JN, Singh S, Hassawi DS, Fritz AK, Namuth D, Lapitan NLV, Gill BS (1999) A high-density genetic linkage map of Aegilops tauschii, the D-genome progenitor of bread wheat. Theor Appl Genet 99:16–26
Clarke BC, Moran LB, Appels R (1989) DNA analyses in wheat breeding. Genome 32:334–339
Gerechter-Amitai ZK, Wahl I, Vardi A, Zohary D (1971) Transfer of stem rust seedling resistance from wild diploid einkorn to tetraploid durum wheat by means of a triploid hybrid bridge. Euphytica 20:281–285
Jin Y, Szabo LJ, Pretorius ZA, Singh RP, Ward R, Fetch T Jr (2008) Detection of virulence to resistance gene Sr24 within race TTKS of Puccinia graminis f. sp. tritici. Plant Dis 92:923–926
Jin Y, Szabo LJ, Rouse MN, Fetch T, Pretorius ZA, Wanyera R, Njau P (2009) Detection of virulence to resistance gene Sr36 within the TTKS race lineage of Puccinia graminis f. sp tritici. Plant Dis 93:367–370
Kerber ER, Dyck PL (1973) Inheritance of stem rust resistance transferred from diploid wheat (Triticum monococcum) to tetraploid and hexaploid wheat and chromosome location of the gene involved. Can J Genet Cytol 15:397–409
Khan RR, Bariana HS, Dholakia BB, Naik SV, Lagu MD, Rathjen AJ, Bhavani S, Gupta VS (2005) Molecular mapping of stem and leaf rust resistance in wheat. Theor Appl Genet 111:846–850
Knott DR (1990) Near-isogenic lines of wheat carrying genes for stem rust resistance. Crop Sci 30:901–905
Lagudah ES, Appels R, McNeil D (1991a) The Nor-D3 locus of Triticum tauschii: natural variation and linkage to chromosome 5 markers. Genome 34:387–395
Lagudah ES, Appels R, Brown AHD, McNeil D (1991b) The molecular-genetic analysis of Triticum tauschii-the D genome donor to hexaploid wheat. Genome 34:375–386
Lagudah ES, Krattinger SG, Herrera-Foessel S, Singh RP, Huerta-Espino J, Spielmeyer W, Brown-Guedira G, Selter LL, Keller B (2009) Gene-specific markers for the wheat gene Lr34/Yr18/Pm38 which confers resistance to multiple fungal pathogens. Theor Appl Genet 119:889–898
Leonard KJ, Szabo LJ (2005) Stem rust of small grains and grasses caused by Puccinia graminis. Mol Plant Pathol 6:99–111
Leroux J, Rijkenberg FHJ (1987) Occurrence and pathogenicity of Puccinia graminis f. sp. tritici in South Africa during the period 1981-1985. Phytophylactica 19:456–472
Liu Y-G, Tsunewaki K (1991) Restriction fragment length polymorphism (RFLP) analysis in wheat. II. Linkage maps of the RFLP sites in common wheat. Jpn J Genet 66:617–633
Mago R, Miah H, Lawrence GJ, Wellings CR, Spielmeyer W, Bariana HS, McIntosh RA, Pryor AJ, Ellis JG (2005) High resolution mapping and mutation analysis separate the rust resistance genes Sr31, Lr26 and Yr9 on the short arm of rye chromosome 1. Theor Appl Genet 112:41–50
Mago R, Zhang P, Bariana HS, Verlin DC, Bansal UK, Ellis JG, Dundas IS (2009) Development of wheat lines carrying stem rust resistance gene Sr39 with reduced Aegilops speltoides chromatin and simple PCR markers for marker-assisted selection. Theor Appl Genet 119:1441–1450
McIntosh RA, Wellings CR, Park RF (1995) Wheat rusts: an atlas of resistance genes. CSIRO Press, Victoria
Olson E, Brown-Guedira G, Marshall D, Stack E, Bowden RL, Jin Y, Rouse M, Pumphrey MO (2010) Development of wheat lines having a small introgressed segment carrying stem rust resistance gene Sr22. Crop Sci (in press)
Paull JG, Pallotta MA, Langridge P, The TT (1994) RFLP markers associated with Sr22 and recombination between chromosome 7A of bread wheat and the diploid species Triticum boeoticum. Theor Appl Genet 89:1039–1045
Pretorius ZA, Singh RP, Wagoire WW, Payne TS (2000) Detection of virulence to wheat stem rust resistance gene Sr31 in Puccinia graminis f. sp. tritici in Uganda. Plant Dis 84:203
Roelfs AP, McVey DV (1979) Low infection types produced by Puccinia graminis f. sp. tritici and wheat lines with designated genes for resistance. Phytopathology 69:722–730
Sambasivam PK, Bansal UK, Hayden MJ, Dvorak J, Lagudah ES, Bariana HS (2008) Identification of markers linked with stem rust resistance genes Sr33 and Sr45. In: Appels R, Eastwood R, Lagudah E, Langridge P, Mackay M, McIntyre L, Sharp P (eds) Proceedings of 11th international wheat genetics symposium, Sydney University Press, Sydney, Australia, pp 351–353
Seah S, Sivasithamparam K, Karakousis A, Lagudah ES (1998) Cloning and characterisation of a family of disease resistance gene analogs from wheat and barley. Theor Appl Genet 97:937–945
Singh RP (1991) Pathogenicity variations of Puccinia recondita f. sp. tritici and Puccinia graminis f. sp. tritici in wheat-growing areas of Mexico during 1988 and 1989. Plant Dis 75:790–794
Singh RP, Hodson DP, Huerta-Espino J, Jin Y, Njau P, Wanyera R, Herrera-Foessel SA, Ward RW (2008) Will stem rust destroy the world’s wheat crop? Adv Agron 98:271–309
The TT (1973) Chromosome location of genes conditioning stem rust resistance transferred from diploid to hexaploid wheat. Nat New Biol 241:256
Acknowledgments
The first author acknowledges the University of Sydney for providing IPRS scholarship. We are grateful to Sutha Chandramohan, Libby Viccars and Hanif Miah for excellent technical support and research funding from the Australian Centre for International Agricultural Research (ACIAR). We thank Dr. A. J. Rathjen for provision of Schomburgk/Yarralinka RIL population and Dr. PJ Sharp for supplying clone csIH81 and glk750. We also thank Dr. Rana Munns (CSIRO) and Dr. Yuri Shavrukov (ACPFG) for providing DNAs of diploid accessions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by X. Xia.
Rights and permissions
About this article
Cite this article
Periyannan, S.K., Bansal, U.K., Bariana, H.S. et al. A robust molecular marker for the detection of shortened introgressed segment carrying the stem rust resistance gene Sr22 in common wheat. Theor Appl Genet 122, 1–7 (2011). https://doi.org/10.1007/s00122-010-1417-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-010-1417-3