Abstract
The use of major resistance genes is a cost-effective strategy for preventing stem rust epidemics in wheat crops. The stem rust resistance gene Sr39 provides resistance to all currently known pathotypes of Puccinia graminis f. sp. tritici (Pgt) including Ug99 (TTKSK) and was introgressed together with leaf rust resistance gene Lr35 conferring adult plant resistance to P. triticina (Pt), into wheat from Aegilops speltoides. It has not been used extensively in wheat breeding because of the presumed but as yet undocumented negative agronomic effects associated with Ae. speltoides chromatin. This investigation reports the production of a set of recombinants with shortened Ae. speltoides segments through induction of homoeologous recombination between the wheat and the Ae. speltoides chromosome. Simple PCR-based DNA markers were developed for resistant and susceptible genotypes (Sr39#22r and Sr39#50s) and validated across a set of recombinant lines and wheat cultivars. These markers will facilitate the pyramiding of ameliorated sources of Sr39 with other stem rust resistance genes that are effective against the Pgt pathotype TTKSK and its variants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Puccinia graminis Pers. f. sp. tritici (Pgt), the causal agent of wheat stem rust, continues to threaten wheat crops worldwide. Stem rust has been effectively controlled in Australia through the deployment of diverse sources of resistance since the early 1980s (Bariana et al. 2004, 2007; Park 2007). Stem rust resistance in cultivars grown worldwide has been based primarily on major genes that are expected to follow gene-for-gene relationship. The sustainability of resistance is dependent upon the availability and careful management of resistance genes and continuous monitoring of Pgt populations for new virulence phenotypes. In 1999, a new pathotype of Pgt with virulence on the widely deployed rust resistance gene Sr31 was detected in Uganda. It was initially named as Ug99 (Pretorius et al. 2000). The name TTKSK was given based on the North American stem rust nomenclature system of 16 differential lines (Jin et al. 2008). Tests have shown that TTKSK carries virulence for several race-specific stem rust resistance genes currently used across the world and variants with virulence against commonly used stem rust resistance genes Sr24 (TTKST) and Sr36 (TTTSK) have also been detected (Jin and Singh 2006; Wanyera et al. 2006; Jin et al. 2008). TTKSK has been detected in Iran (Nazari et al. 2009) and may soon threaten wheat production in the Indian sub-continent (Singh et al. 2006; Stokstad 2007). Sr39 is effective against TTKSK (Jin et al. 2007) and its variants TTKST and TTTSK.
Sr39 was transferred to the hexaploid wheat cultivar Marquis from Aegilops speltoides (Kerber and Dyke 1990). The gene is located on a translocated segment of Ae. speltoides chromosome 2S to wheat chromosome 2B (translocation stock 2S#2; McIntosh et al. 1995; Friebe et al. 1996; Gill et al. 2008). The translocated segment carries the seedling stem rust resistance gene Sr39 and an adult-plant hypersensitive leaf rust resistance gene Lr35. No current Australian wheat cultivar carries Sr39, presumably because of negative agronomic effects of this large translocation. Labuschagne et al. (2002) reported that a South African wheat genotype (Karee*6/RL6082) with Lr35 (and also Sr39) showed significant increase in flour water absorption compared to the recurrent parent Karee. Recombination can be induced between the chromosome segment from a wild relative and a homoeologous wheat chromosome to remove deleterious effects associated with the alien chromatin and/or to restore wheat genes lost due to the translocation (Dundas and Shepherd 1994, 1996a, b, 1998; Dundas et al. 1999, 2001, 2004; Lukaszewski 2000; Anugrahwati et al. 2008). For example, Anugrahwati (2006) showed improvement in flour quality of wheat-1RS recombinants with shortened 1RS chromatin compared to the original 1DL.1RS translocation line. A preliminary report summarized recombination events between the wheat chromosome 2B and the Sr39/Lr35 carrying Ae. speltoides chromosome 2S (Dundas et al. 2007). These recombinants are described in detail in this paper. A simple set of PCR markers (Gold et al. 1999; Seyfarth et al. 1999; this work) was used to identify the presence of recombinants with shortened Sr39 carrying segments. These markers will serve as useful tools for transfer of the shortened Sr39 carrying Ae. speltoides translocation into future wheat cultivars individually as well as a component in gene combinations effective against TTKSK and its variants.
Materials and methods
Plant materials
The Sr39 translocation parent RL5711 was produced by Kerber and Dyke (1990) in the susceptible wheat ‘Marquis’ background. The original 2B-2S#2 translocation line with Sr39/Lr35 has been generously supplied to the Australian Cereal Rust Control Program (ACRCP) by Dr. Taing Aung (formerly of Agriculture Canada). Recombinants between Ae. speltoides and the wheat chromosome were produced as part of the continuing germplasm improvement effort of the ACRCP (Dundas et al. 2007).
Development of new recombinants
The wheat–Ae. speltoides chromosome 2S#2 recombinants were produced in the Cereal Cytogenetics Laboratory, Waite Campus, University of Adelaide. The first step was to determine the size of the 2S#2 chromosome segment in the translocation line RL5711. A range of group 2 DNA probes of both wheat and barley, previously mapped to the distal and proximal regions of short and long arms (GrainGenes: http://wheat.pw.usda.gov/), were obtained from the Australian Triticeae Mapping Initiative (ATMI) (maintained by Prof P. Langridge, University of Adelaide). They were labeled with 32P-dCTP by the random primer method and hybridized to genomic DNA of RL5711 (+Sr39) and Marquis (-Sr39) digested with BamHI, DraI, EcoRI, EcoRV or HindIII and transferred by blotting to Hybond N+ membrane (Amersham, UK). These probes were analyzed for the presence of polymorphic bands associated with the presence of the 2S#2 chromosome.
The second step was to introduce the ph1b mutant (Sears 1977) to induce homoeologous recombination. Line RL5711 (carrying the 2B-2S#2 translocation) was crossed as female with wheat line “Angas ph1bph1b” (pedigree: Eagle/Chinese Spring ph1bph1b/3/2*Angas) [the initial F3 plant of Eagle x CS was screened before backcrossing to Angas for absence of the Eagle ‘6Ae#1’ chromosome (ensuring the absence of gene Sr26), the presence of normal 6A chromosomes, the absence of Xpsr128-5B and the absence of the rust resistance gene Sr9g]. F2 seedlings were screened for a ph1bph1b genotype (negative for Xpsr128-5B), and also for the presence of both the normal 2B and 2B-2S#2 translocation chromosomes using probe MWG503 (DraI). Sixty-five F3 and 55 BC1 (backcross with Angas Ph1Ph1) seedlings were screened for dissociation of the 2S#2 markers with probes ABC252 (most distal marker on 2S#2 long arm), ABG2 (most distal marker on 2S#2 short arm), BCD111 (proximal marker on 2B long arm) and ABC454 (proximal marker on 2B short arm) (EcoRI). RFLP probe ABG358 (interstitial on the short arm) (Fig. 1) was used to further define the sizes of the 2S#2 chromosome segments. Plants showing dissociation of 2S#2 markers were progeny-tested and crossed as females with cv. Angas up to BC3.
Schematic representation of wheat–Aegilops speltoides chromosome 2S#2 recombinant lines showing the position of RFLP markers. Ae. speltoides chromatin is represented in black. Wheat chromatin is represented in white. Recombinant numbers 159,198 and 233 were susceptible to stem rust and numbers 151, 163, 220 and 247 were resistant
GISH analysis of recombinant lines
The original source 2B-2S#2 translocation line and the recombinants were also characterized by the genomic in situ hybridization (GISH). GISH followed the procedure described by Zhang et al. (2001). Total genomic DNA from Ae. speltoides (University of Sydney cytogenetics collection accession # C64.69) was labeled with biotin-14-dATP using BioNick DNA Labeling System (Invitrogen Life Science, Carlsbad, CA). Unlabeled total genomic DNA of wheat was used as a blocker. The probe:blocker ratio was ~1:50. Signals were detected with fluorescein avidin DN (Vector Laboratories, Burlingame, CA). Chromosomes were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen Life Science) and pseudocolored red. Slides were analyzed with a Zeiss Axio Imager epifluorescence microscope. Images were captured with a Retiga EXi CCD (charge-coupled device) camera (QImaging, Surrey, BC) operated with Image-Pro Plus 6.2 software (Media Cybernetics Inc., Bethesda, MD) and processed with Photoshop version 8.0 software (Adobe Systems, San Jose, CA).
Rust assays
The recombinant lines were rust tested for both stem rust and leaf rust at the University of Sydney Plant Breeding Institute, Cobbitty. Pathotype 34-1,2,3,4,5,6,7 (Culture no. 103) of Pgt and 104-1,2,3,(6),(7),11,13 (Culture no. 547) of P. triticina (Pt) were used. Rust test procedures were described in Bariana and McIntosh (1993).
PCR marker development
Development of new PCR-based markers for Sr39 was undertaken at CSIRO, Plant Industry, Canberra. Genomic DNA was isolated from leaves and DNA blot analysis was carried out according to Lagudah et al. (1991a, b). DNA was restricted with endonucleases under conditions recommended by the manufacturer (MBI Fermentas, Lithuania; NEB, USA).
SSR and EST analyses of Sr39 lines
Primer sequences for the SSR markers from chromosome 2B were obtained from GrainGenes (http://wheat.pw.usda.gov/GG2/quickquery.shtml#microsats). ESTs in the deletion bin maps of chromosome 2B were identified from http://wheat.pw.usda.gov/cgi-bin/westsql/map_locus.cgi and the primers for amplification were designed using the Primer3 software (http://frodo.wi.mit.edu/).
Isolation of AFLP markers linked to Sr39
To isolate AFLP markers from the Sr39 carrying region, the seven dissociation lines with shortened Ae. speltoides chromosome segments (Dundas et al. 2007) along with Marquis (susceptible) and the resistant parent RL5711 were used. AFLP analysis was performed using the standard protocol (Vos et al. 1995). For selective amplification PstI and MseI primers with three additional nucleotides were used. Cloning and analysis of AFLP fragments were done as described in Mago et al. (2002).
Sequence-tagged site analysis
Three AFLP fragments P-ACA/M-GCT-842 (Sr39#22r), ACA/M-GAG-360 (Sr39#8r) and P-AAG/M-GTA-743 (Sr39#3r) associated with the presence of Sr39, and three AFLP markers P-ACA/M-GCT-911 (Sr39#2s), P-AAG/M-GTA-350 (Sr39#26s) and P-ACC/M-GCC-284 (Sr39#50s) associated with the susceptible genotype were sequenced using the dye terminator sequencing system and analyzed on an ABI prism system. Specific primers were designed for amplification of each of these fragments and used for PCR amplification of the diagnostic product. Primer sequences of the markers which amplified diagnostic polymorphism have been listed in Table 1. PCR products were separated on a 2% agarose gel.
Validation of marker–trait association
The marker–trait association was validated on a set of 65 F3 lines derived from a cross Cook/RL5711. This is the only family segregating for Sr39 available in Australia.
Results
Isolation of recombinants
Hybridization of ten RFLP probes previously mapped to the short arm and long arm of group 2 Triticeae chromosomes to DNA gel blots identified nine probes (ABG2, ABG358, ABG5, ABC454, BCD111, MWG503, MWG892, ABG72 and ABC252) that could distinguish the translocation line RL5711 from the background cultivar Marquis and establish the extent of the Ae. speltoides chromosome segment on the 2B-2S#2 translocation chromosome. Of these probes, only the most reliable markers were selected for screening for recombinants. The most widely spaced probes ABG2 and ABC252 were chosen for initial screening of 73 ph1bph1b seedlings for breakup of the 2S#2 chromosome. In addition, 23 plants were screened for dissociation of Xabc454-2S#2 − Xbcd111-2S#2, nine for Xbcd111-2S#2 − Xabc252-2S#2, seven for Xabc454-2S#2 − Xabc252-2S#2, seven for Xabg2-2S#2 − Xbcd111-2S#2 and one for Xabg2-2S#2 − Xabc454-2S#2. Six putative recombinants were found among the 73 plants screened for dissociation of the most separated markers Xabg2-2S#2 and Xabc252-2S#2 (8.2%) and one putative recombinant plant was found during screening of nine plants for dissociation of markers Xbcd111-2S#2 − Xabc252-2S#2. The seven dissociation lines were progeny tested to confirm their marker patterns and then tested with additional probes to characterize the recombination events. All seven lines were screened for low response to stem rust and leaf rust to ascertain their Sr39 and Lr35 genotypic status (Fig. 1). Consequently Sr39 was mapped adjacent to the locus Xabg358-2S#2. Lr35 was separated from Sr39 by recombination and was mapped to the Xabg358-2S#2-Xabc252-2S#2 interval. Two recombinants, #220 and #247, carried the shortest Ae. speltoides segments and both rust resistance genes.
Chromosome morphology
Figure 2 shows the in situ hybridization photos of the original 2B-2S#2 translocation chromosome and the recombinants. The interpretation of the chromosome structures is presented in an ideogram in Fig. 1.
In situ hybridization using Aegilops speltoides genomic DNA as probe on: a the original wheat–Ae. speltoides 2B-2S#2 translocation (+Sr39) from RL5711, and 2S#2 dissociation lines b #151, c #159, d #163, e #198, f #220, g #233 and h #247. The magnified images on chromosomes (all lines except #233) show the Ae. speltoides chromosome segment (fluorescent section). b–h Hybridization signals on the SAT chromosomes due to the highly similar repetitive rDNA sequences between wheat and Ae. speltoides. e The loss of most or the entire short arm of the translocation chromosome in RL5711
The original 2B-2S#2 translocation consists of a large segment of Ae. speltoides 2S#2 chromatin with a segment of non-labeled wheat chromatin on the distal end of the short arm (Fig. 2a). Approximately 80% of the physical length of the short arm is 2S#2 chromatin. Interpretation of structure of the long arm is more difficult because of the presence of labeled telomeres. However, chromosome pairing observations during backcrossing showed that ring bivalents were formed with this chromosome (T. Aung, personal communication), indicating that these labeled long-arm telomeres probably represent 2BL chromatin (Fig. 2a). If this assumption is correct, and the sub-terminal band on the long arm is of Ae. speltoides origin, then at least 77% of the long arm is 2S#2 chromatin.
Line #151 had lost the 2S#2 allele for the probe ABG2. The 2B allele for this probe was present indicating that recombination had occurred in the distal region of the short arm. The in situ photograph of this line shows labeling along most of the length of the chromosome (Fig. 2b). This line is resistant to stem rust (+Sr39) and leaf rust (+Lr35).
Line #159 had lost two 2S#2 RFLP markers ABG2 and ABG358 (Fig. 1). This line was susceptible to stem rust (−Sr39) and resistant to leaf rust (+Lr35). The in situ photograph shows that the distal end of the short arm has been replaced with non-labeled wheat chromatin extending to approximately 30% of the length of the arm (Fig. 2c). We have been unable to determine which wheat chromosome was involved in the recombination event.
Line #163 had lost the 2S#2 distal short arm allele for probe ABG2 (Fig. 1). This line was resistant to both stem rust and leaf rust (+Sr39 and +Lr35). In situ photographs show a chromosome with labeling on proximal portions of both arms (Fig. 2d). The long-arm telomeres (thought to be of 2B origin) and sub-terminal dots (thought to be Ae. speltoides origin), which were present in the original translocation, are no longer on this chromosome. Approximately 70% of both the short and long arms remain as 2S#2 chromatin. The apparent loss of the labeled 2BL telomeres from this chromosome suggests that another as yet unidentified wheat chromosome may have been involved in the recombination event.
Line #198 had lost the 2S#2 alleles for probes ABG2 and ABG358 (Fig. 1). It was susceptible to stem rust (−Sr39) and resistant to leaf rust (+Lr35). The proximal short-arm probe for ABC454 shows a 2S#2 band. In situ photos appear to show a telocentric chromosome consisting of the long arm of the original 2B-2S#2 translocation chromosome (Fig. 2e). Because of the presence of the 2S#2 allele for the group 2 short-arm probe ABC454, it is possible that the breakpoint on the translocation chromosome in line #198 is not at the centromere and a sub-microscopic portion of the short arm is retained. Therefore, it is possible that this is an acrocentric chromosome instead of telocentric.
Lines #220 and #247 showed similar loss of the distal long arm 2S#2 allele for probe ABC252 and the presence of the 2B allele for this probe (Fig. 1). All short-arm markers tested appear to have been retained. These lines are resistant to stem rust and leaf rust (+Sr39 and +Lr35). In situ photographs show loss of the sub-terminal dots on the long arm and also the insertion of non-labeling wheat chromatin so that the 2S#2 chromatin has been reduced from approximately 77 to 67% of the length of the long arm (Fig. 2f, h).
Line #233 retained only the 2S#2 allele for probe ABG2 (Fig. 1). All other 2S#2 alleles and resistance genes Sr39 and Lr35 were lost from this line. In situ photographs confirm loss of the large 2S#2 chromosome block of the original translocation (Fig. 2g). It is beyond the detection limit of the FISH technique to determine which wheat chromosome carries the remaining 2S#2 chromosome segment. Marker data suggest that the wheat 2B chromosome may not have been involved in the recombination event since both loci, Xabg2-2B and Xabg2-2S#2, are present in a line homozygous for the 2S#2 chromosome segment.
Further markers for characterization of recombinants and marker-assisted selection of Sr39/Lr35
A range of SSR markers from chromosome 2B were tested on the Sr39 parent and recombinants to look for polymorphisms. SSR markers BARC18, BARC183, BARC200 and wmc025 each amplified products from all stem rust susceptible lines but not from stem rust resistant lines, consistent with replacement of wheat 2B segment by the translocation (Fig. 3 and data not shown). The PCR product for SSR marker wmc025 in Chinese Spring (CS) (Fig. 3, lane 18) was smaller than in the other susceptible lines. SSR markers BARC7, BARC10, BARC45, BARC55, BARC101, BARC128, BARC160, gwm55, gwm319, wmc261 and wmc344 amplified monomorphic products from all lines (data not shown). Primers were also designed for chromosome 2B specific EST markers BF146002, BE443085, BE 404432, BE500705, BE398939, BI479116 and BQ280506. Only BE500705 amplified a diagnostic PCR product from stem rust susceptible lines (data not shown) and none of the other EST markers amplified from any of the lines.
PCR amplification of the SSR markers on wheat–Aegilops speltoides recombinants. Lanes 1–9 show BARC200 and 10–18 show wmc025 amplification. Lanes 1 and 10, #151; 2 and 11, #159; 3 and 12, #163; 4 and 13, #198; 5 and 14, #220; 6 and 15, #247; 7 and 16, Marquis; 8 and 17, RL 5711; 9 and 18, Chinese Spring
AFLP markers for Sr39/Lr35
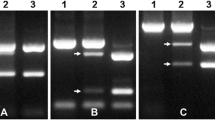
We used the AFLP technique with PstI/MseI primer sets on the wheat near isogenic line (NIL) pairs Marquis (S)–RL5711 (R) and recombinants #151 (R) and #159 (S) (see Fig. 1) to develop additional markers. With 144 primer combinations, 6 AFLPs distinguished the stem rust resistant and susceptible recombinants. Three markers P-ACA/M-GAG-360 (Sr39#8r), P-ACA/M-GCT-842 (Sr39#22r) and P-AAG/M-GTA-743 (Sr39#3r) were linked with resistance and three markers P-ACA/M-GCT-911 (Sr39#2s), P-AAG/M-GTA-350 (Sr39#26s) and P-ACC/M-GCC-284 (Sr39#50s) were associated with susceptibility. The AFLP fragments were cloned, used as RFLP probes and mapped to chromosome 2B. Only, probes Sr39#22r, Sr39#3r, Sr39#2s and Sr39#50s showed polymorphism between the stem rust susceptible and resistant recombinants. Figure 4 shows the hybridization of two of the AFLP-derived markers Sr39#50s and Sr39#3r to genomic DNA of the wheat–Ae. speltoides recombinant lines. Similar results were seen for markers Sr39#2s and Sr39#22r (data not shown). The hybridization of Sr39#50s to line #198 is caused by background 2B chromosomes in this line.
Hybridization of AFLP-derived markers (a) Sr39#50s, BamHI (b) Sr39#3r, EcoRV, to wheat–Ae. speltoides recombinant lines. Lanes 1 Marquis (susceptible parent), 2 RL 5711 (resistant parent), 3 Recombinant #151, 4 #159, 5 #163, 6 #198, 7 #220, and 8 #247. Arrow indicates the position of polymorphic band
PCR markers for Sr39/Lr35
AFLP fragments Sr39#22r, Sr39#3r, Sr39#2s and Sr39#50s that showed polymorphic bands were sequenced. The sequence information was used to design primers to develop simple PCR markers. Markers Sr39#3r and Sr39#2s which were polymorphic as RFLP probes did not amplify any PCR product diagnostic for the resistant and susceptible lines (not shown). Marker Sr39#22r amplified a diagnostic band from all the resistant recombinants (Fig. 5a). Interestingly Sr39#50s that was initially cloned from an AFLP marker for the susceptible #159 recombinant, amplified a single band from susceptible lines and two bands from the resistant recombinant lines (Fig. 5b). Both markers (Sr39#22r and Sr39#50s) were sequenced from the recombinant lines to confirm their identity. Figure 5c shows the amplification of a previously described PCR marker developed by Gold et al. (1999) for the parent and recombinant lines. This marker amplified PCR products from all recombinants including the susceptible recombinants #159 and #198 but not from #233. Similar results were also obtained using the marker of Seyfarth et al. (1999). Although these earlier markers were diagnostic for the translocations, they were more distantly linked to Sr39 than Sr39#22r and Sr39#50s.
Amplification of PCR-based markers developed in this study on wheat–Aegilops speltoides recombinants. a Marker Sr39#22r. b Sr39#50s. Lanes 1 Marquis (susceptible parent), 2 RL 5711 (resistant parent), 3 Recombinant #151, 4 #159, 5 # 163, 6 #198, 7 #220, 8 #247 and 9 #233. c PCR amplification of the previously published marker (Gold et al. 1999) on wheat–Ae. speltoides recombinants. Lanes 1–9 Marquis; RL5711; wheat–Ae. speltoides recombinants #151; #159; #163; #198; #220; #247 and #233
Validation of PCR markers for marker-assisted selection
Validation of markers across diverse genetic backgrounds is important for their successful implementation in breeding programs. The PCR markers were validated using a set of Australian wheat cultivars and breeder’s materials. Marker Sr39#50s amplified the susceptible allele (Fig. 6a) and Sr39#22r showed no amplification from any of the wheats lacking Sr39 (not shown). The other susceptibility-linked markers wmc025 and BARC200 were not consistent in amplification from the susceptible wheats and thus proved useless. Figure 6b shows the amplification of markers Sr39#50s and Sr39#22r from several lines of a Cook/RL5711-derived F3 population segregating for the Sr39/Lr35 translocation. Although Sr39#50s distinguished resistant and susceptible recombinants, it did not behave as a co-dominant marker and it failed to differentiate between heterozygous and homozygous susceptible families (Fig. 6b). However, using the two marker combination (Sr39#50s and Sr39#22r) it was possible to differentiate homozygous and heterozygous families. Since Sr39#50s is a wheat-derived marker, it is possible that it may recombine in some wheat backgrounds. The previously developed Sr39 markers (Gold et al. 1999; Seyfarth et al. 1999) can be used to identify the resistant lines in this segregating population, but will not be able to distinguish between the homozygous and heterozygous lines.
Discussion
Two types of resistance genes have been used to control stem rust in Australia and other parts of the world. The predominant group includes race-specific or major genes that provide high levels of resistance at both seedling and adult plant stages. The expression of resistance in this group is often associated with hypersensitivity. The other type involves adult plant resistance (APR) genes that are seedling susceptible and provide partial resistance and, Sr2 belongs to this group. The success of stem rust control in Australian wheats relied on deployment of various combinations of stem rust resistance genes including Sr2 (Bariana et al. 2007). The availability of diverse sources of resistance is essential to achieve long-term protection against the stem rust pathogen. Such sources of stem rust resistance have been transferred from wild relatives of wheat to commercially acceptable genetic backgrounds (McIntosh et al. 1995). Some of these sources require amelioration of negative effects prior to their use in breeding programs (Dundas et al. 2007).
This investigation reports the cytogenetic characterization of the shortened Ae. speltoides/wheat 2S-2B translocation chromosome carrying linked rust resistance genes Sr39 and Lr35 and development of DNA markers that can differentiate homozygotes from heterozygotes. DNA markers and rust bioassays were used to select recombinants with reduced Ae. speltoides chromatin and retention of one or both of the rust resistance genes. Comparison of recombinants #151 and #159 that appeared to differ in the distal part of the short arm of chromosome 2 and by the presence and absence of Sr39, respectively, allowed the development of a PCR marker Sr39#22r for the shortened chromosome segment carrying Sr39 (Fig. 1). These markers were validated on a selected set of F3 lines segregating for Sr39. Although a relatively high frequency of wheat–Ae. speltoides recombination was observed in this study, the lines retaining Sr39 still carry large segments of Ae. speltoides chromatin. Recombination between the 2B-2S#2 translocation chromosome and wheat homoeologues may have been influenced by the distal short- and long-arm segments of the translocation chromosome which are thought to be 2B chromatin. Further shortening of Sr39-carrying wheat–Ae. speltoides recombinants with smaller 2S#2 chromatin might be possible through the combined use of markers developed in this study and those previously reported (Gold et al. 1999; Seyfarth et al. 1999), however, it would require a large population.
The shortest recombinant from the current study, #247, which carries both Sr39 and Lr35, has been released to Australian wheat breeders for assessment of its potential as a new source of rust resistance. The markers Sr39#50s and Sr39#22r used together will allow the recovery of homozygous lines in early generations. In addition, it is now possible to stack shortened versions of Sr39, Sr26 and SrR, all of which are effective against the Ug99 (TTKSK group) and all other known Pgt pathotypes using the PCR markers developed in this study and earlier studies by Mago et al. (2002, 2005). The presence of three effective stem rust resistance genes should ensure durability for resistance to stem rust. However, durability of these sources will depend on the international wheat breeding community adhering to a hitherto unachieved level of cooperation to prevent the widespread release of these genes singly in future cultivars to avoid the step-wise accumulation of virulence in the stem rust pathogen.
References
Anugrahwati DR (2006) Isolation, characterization and quality testing of 1DS/1RS wheat-rye recombinants. PhD thesis. Discipline of Plant and Pest Science, School of Agriculture, Food and Wine, The University of Adelaide, Australia, 177 p
Anugrahwati DR, Shepherd KW, Verlin DC, Zhang P, Mirzaghaderi G, Walker E, Francki MG, Dundas IS (2008) Isolation of wheat-rye 1RS recombinants that break the linkage between stem rust resistance gene SrR and secalin. Genome 51:341–349
Bariana HS, McIntosh RA (1993) Cytogenetic studies in wheat. XV. Location of rust resistance genes in VPM1 and their genetic linkage with other resistance genes in chromosome 2A. Genome 36:476–482
Bariana HS, Willey N, Venkata BP, Lehmenseik A, Standen G, Lu M (2004) Breeding methodology to achieve durability for rust resistance in wheat, Cereals 2004. In: Black CK, Panozzo JF, Rebetzke GJ (eds) Proceedings 54th Australian cereal chemistry conference and 11th wheat breeders assembly, Canberra, ACT, 21–24 September 2004, pp 8–12
Bariana HS, Brown GN, Bansal UK, Miah H, Standen GE, Lu M (2007) Breeding triple rust resistant wheat cultivars for Australia using conventional and marker-assisted selection technologies. Aust J Agric Res 58:576–587
Dundas IS, Shepherd KW (1994) Progress towards improving the yield of wheat varieties carrying stem rust resistance gene Sr26 using cytological methods. In: Paull J, Dundas IS, Shepherd KJ, Hollamby GJ (eds) Proceedings of the 7th assembly wheat breed society of Australia: Wheat breeding—into the second century. Adelaide, SA, pp 129–132
Dundas IS, Shepherd KW (1996a) Towards yield improvement of stem rust resistant wheat varieties carrying Sr26. In: Richards RA, Wrigley CW, Rawson HM, Rebetzke GJ, Davidson JL, Brettell RIS (eds) Proceedings of the of 8th assembly of wheat breeding society of Australia, pp 201–203
Dundas IS, Shepherd KW (1996b) Towards yield improvement of wheat with stem rust resistance gene Sr26 by cytogenetic methods using molecular-based marker selection. In: Abstracts of the second international crop science congress, New Delhi, India
Dundas IS, Shepherd KW (1998) Shortening the Agropyron chromosome segment carrying gene Sr26 utilizing chromosome engineering and molecular markers. In: Slinkard AE (ed) Proceedings of the 9th international wheat genetics symposium university extension press, University of Saskatoon, pp 35–37
Dundas IS, Shepherd KW, McIntosh RA (1999) Progress towards improving the performance of wheat lines carrying alien stem rust resistance genes Sr26, Sr32 and Sr37 using molecular cytogenetics. In: Williamson PW (ed) Proceedings of the 9th assembly of the wheat breeding society of Australia. Toowoomba, 27 September–1 October, pp 141–143
Dundas IS, Bariana HS, Park RF, Islam AKMR, McIntosh RA, Shepherd KW (2001) New rust resistance genes for wheat improvement. In: Eastwood R, Hollamby G, Rathjen A, Gororo N (eds) Proceedings of the 10th assembly of the wheat breeding society of Australia. Mildura, 16–21 September, pp 44–47
Dundas IS, Verlin DC, Park RF, Bariana HS, Anugrahwati DR, Shepherd KW, McIntosh RA, Islam AKMR (2004) Progress in development of new rust resistant wheat using chromosomes from uncultivated relatives. In: Black CK, Panozzo JF, Rebetzke GJ (eds) Proceedings of the 54th Australian cereal chemistry conference and 11th wheat breeders assembly, pp 122–124
Dundas IS, Anugrahwati DR, Verlin DC, Park RF, Bariana HS, Mago R, Islam AKMR (2007) New sources of rust resistance from alien species: meliorating linked defects and discovery. Aust J Agric Res 58:545–549
Friebe B, Jiang J, Raupp WJ, McIntosh RA, Gill BS (1996) Characterization of wheat-alien translocations conferring resistance to diseases and pests: current status. Euphytica 91:59–87
Gill BS, Huang L, Kuraparthy V, Raupp WJ, Wilson DL, Friebe B (2008) Alien genetic resources for wheat leaf rust resistance, cytogenetic transfer, and molecular analysis. Aust J Agric Res 59:197–205
Gold J, Harder D, Townley-Smith F, Aung T, Procunier J (1999) Development of a molecular marker for rust resistance genes Sr39 and Lr35 in wheat breeding lines. Electronic J Biotech 2. http://www.ejbiotechnology.info/content/vol2/issue1/full/1/index.html
Jin Y, Singh RP (2006) Resistance in U.S. wheat to recent eastern African isolates of Puccinia graminis f. sp. tritici with virulence to resistance gene Sr31. Plant Dis 90:476–480
Jin Y, Singh RP, Ward RW, Wanyera R, Kinyua M, Njau P, Fetch T, Pretorius ZA, Yahyaoui A (2007) Characterization of seedling infection types and adult plant infection responses of monogenic Sr gene lines to race TTKS of Puccinia graminis f. sp. tritici. Plant Dis 91:1096–1099
Jin Y, Szabo LJ, Pretorius ZA, Singh RP, Ward R, Fetch T Jr (2008) Detection of virulence to resistance gene Sr24 within race TTKS of Puccinia graminis f. sp. tritici. Plant Dis 92:923–926
Kerber ER, Dyke PL (1990) Transfer to hexaploid wheat of linked genes for adult-plant leaf rust and seedling stem rust resistance from amphiploid of Aegiolops speltoides × Triticum monoccum. Genome 33:530–537
Labuschagne MT, Pretorius ZA, Grobbelaar B (2002) The influence of leaf rust resistance genes Lr29, Lr34, Lr35 and Lr37 on bread making quality in wheat. Euphytica 124:65–70
Lagudah ES, Appels R, McNeil D (1991a) The Nor-D3 locus of Triticum tauschii: natural variation and linkage to chromosome-5 markers. Genome 34:387–395
Lagudah ES, Appels R, Brown AHD, McNeil D (1991b) The molecular-genetic analysis of Triticum tauschii—the D genome donor to hexaploid wheat. Genome 34:375–386
Lukaszewski AJ (2000) Manipulation of the 1RS.1BL translocation in wheat by induced homoeologous recombination. Crop Sci 40:216–225
Mago R, Spielmeyer W, Lawrence GJ, Lagudah ES, Ellis JG, Pryor A (2002) Identification and mapping of molecular markers linked to rust resistance genes located on chromosome 1RS of rye using wheat-rye translocation lines. Theor Appl Genet 104:1317–1324
Mago R, Bariana HS, Dundas IS, Spielmeyer W, Lawrence GJ, Pryor AJ, Ellis JG (2005) Development of broadly useful PCR-markers for the wheat stem rust resistance genes Sr24 and Sr26. Theor Appl Genet 111:496–504
McIntosh RA, Wellings CR, Park RF (1995) Wheat rusts: an atlas of resistance genes. CSIRO Publications, Victoria
Nazari K, Mafi M, Yahyaoui A, Singh RP, Park RF (2009) Detection of wheat stem rust race (Puccinia graminis f. sp. tritici) TTKSK (Ug99) in Iran. Plant Dis 93:317
Park RF (2007) Stem rust of wheat in Australia. Aust J Agric Res 58:558–566
Pretorius ZA, Singh RP, Wagoire WW, Payne TS (2000) Detection of virulence to wheat stem rust resistance gene Sr31 in Puccinia graminis f. sp. tritici in Uganda. Plant Dis 84:203
Sears ER (1977) An induced mutation with homoeologous pairing in wheat. Can J Genet Cytol 19:585–593
Seyfarth R, Feuillet C, Schachermayr G, Winzeler M, Keller B (1999) Development of a molecular marker for the adult plant leaf rust resistance gene Lr35 in wheat. Theor Appl Genet 99:554–560
Singh RP, Hodson DP, Jin Y, Huerta-Espino J, Kinyua MG, Wanyera R, Njau P, Ward RW (2006) Current status, likely migrations and strategies to mitigate the threat to wheat production from race Ug99 (TTKS) of stem rust pathogen. CAB Rev Perspectives Agric Veterinary Sci Nutr Nat Resour 1(054):13p
Stokstad E (2007) Deadly wheat fungus threatens world’s breadbaskets. Science 315:1786–1787
Vos P, Hogers R, Bleeker M, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Wanyera R, Kinyua MG, Jin Y, Singh RP (2006) The spread of stem rust caused by Puccinia graminis f. sp. tritici, with virulence on Sr31 in wheat in eastern Africa. Plant Dis 90:113
Zhang P, Friebe B, Lukaszewski AJ, Gill BS (2001) The centromere structure in Robertsonian wheat-rye translocation chromosomes indicates that centric breakage-fusion can occur at different positions within the primary constriction. Chromosoma 110:335–344
Acknowledgments
The authors are thankful to Xiaodi Xia for providing excellent technical assistance. The authors are thankful to the Grains Research and Development Corporation, Australia for financial support through the Australian Cereal Rust Control Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B. Keller.
Rights and permissions
About this article
Cite this article
Mago, R., Zhang, P., Bariana, H.S. et al. Development of wheat lines carrying stem rust resistance gene Sr39 with reduced Aegilops speltoides chromatin and simple PCR markers for marker-assisted selection. Theor Appl Genet 119, 1441–1450 (2009). https://doi.org/10.1007/s00122-009-1146-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-009-1146-7