Abstract
The stem, leaf and stripe rust resistance genes Sr31, Lr26 and Yr9, located on the short arm of rye chromosome 1, have been widely used in wheat by means of wheat-rye translocation chromosomes. Previous studies have suggested that these resistance specificities are encoded by either closely-linked genes, or by a single gene capable of recognizing all three rust species. To investigate these issues, two 1BL·1RS wheat lines, one with and one without Sr31, Lr26 and Yr9, were used as parents for a high-resolution F2 mapping family. Thirty-six recombinants were identified between two PCR markers 2.3 cM apart that flanked the resistance locus. In one recombinant, Lr26 was separated from Sr31 and Yr9. Mutation studies recovered mutants that separated all three rust resistance genes. Thus, together, the recombination and mutation studies suggest that Sr31, Lr26 and Yr9 are separate closely-linked genes. An additional 16 DNA markers were mapped in this region. Multiple RFLP markers, identified using part of the barley Mla powdery mildew resistance gene as probe, co-segregated with Sr31 and Yr9. One deletion mutant that had lost Sr31, Lr26 and Yr9 retained all Mla markers, suggesting that the family of genes on 1RS identified by the Mla probe does not contain the Sr31, Lr26 or Yr9 genes. The genetic stocks and DNA markers generated from this study should facilitate the future cloning of Sr31, Lr26 and Yr9.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The short arm of chromosome 1 (1RS) of rye (Secale cereale) carries important disease resistance genes that confer resistance to rust diseases (Puccinia spp.) and powdery mildew. Many successful wheat cultivars containing 1RS translocations from two different rye genotypes have been released, including the ‘Veery’ lines developed at CIMMYT (Rajaram et al. 1983). The Veery varieties were derived from crosses between a Mexican spring semi-dwarf and the winter wheat variety ‘Kavkaz’, which carries a 1BL·1RS translocation chromosome with the 1RS arm coming from ‘Petkus’ rye (Zeller 1973; Schlegel and Korzun 1997). This rye chromosome arm carries genes Sr31, Lr26, Yr9 and Pm8 conferring race-specific resistance to stem rust (caused by Puccinia graminis f. sp. tritici), leaf rust (Puccinia triticina), stripe rust (Puccinia striiformis f. sp. tritici) and powdery mildew (Blumeria graminis f. sp. tritici), respectively. The rust resistance genes Sr31, Lr26, and Yr9 from ‘Petkus’ rye co-segregated among 214 test-cross progeny and mapped to the end of chromosome 1RS, approximately 5 cM distal to the seed storage protein genes (Sec-1) (Singh et al. 1990; Lukaszewski 2000). In another study, the map location of Lr26 was confirmed to be distal to the Sec-1 locus of rye (Hsam et al. 2000). There is some evidence for the presence of Yr9 in triticale in the absence of Sr31 and Lr26 (McIntosh et al. 1995; Adhikari 1996).
In our previous work we identified DNA markers linked to the Sr31, Lr26 and Yr9 resistance genes on 1RS (Mago et al. 2002). Because suitable rust strains and genetic stocks for mapping these resistance genes directly in rye were not available, we used a wheat translocation line containing 1RS and a set of ph1-induced recombinants between 1RS and homoeologous wheat 1S arms (Lukaszweski 2000) to position DNA markers relative to the rust resistance genes. The DNA markers included resistance gene analogs (RGAs) of the nucleotide binding site–leucine rich repeat (NBS-LRR) class. We also developed a comparative map of a chromosomal region on 1S by combining previous Triticeae mapping data with results from rye and Aegilops tauschii (Mago et al. 2002).
Our objectives in this study were to lay the groundwork for map-based cloning of Sr31 and to determine whether Sr31, Lr26 and Yr9 could be separated by recombination or mutation. We therefore established a wheat mapping family using parental wheat translocation lines carrying 1RS from ‘Petkus’ rye (Sr31, Lr26,Yr9) and 1RS mostly derived from ‘King II’ rye (no rust resistance). This mapping family was used initially to identify DNA markers flanking Sr31, Lr26 and Yr9. Flanking DNA markers were then used to develop a high-resolution map of the Sr31, Lr26 and Yr9 region. A recombinant separating the leaf rust resistance gene Lr26 from Sr31 and Yr9 was obtained. No recombinants were recovered between Sr31 and Yr9. In addition we isolated mutants of the individual rust resistance genes thus separating the three rust resistance genes from each other.
Materials and methods
Plant material
A wheat F2 family of 143 individuals was derived from two parental lines each possessing a 1BL·1RS translocation. One parent, Federation *4/Kavkaz, carried a ‘Petkus’ 1RS with Sr31, Lr26 and Yr9. The other parent contained 1RS that was partly of ‘Petkus’ rye origin (the proximal region) and partly of ‘King II’ rye origin (the distal region) that included the Nor and Sec1 loci of ‘King II’ and the susceptible haplotype of Sr31, Lr26 and Yr9 (Fig. 1). The origin of the second parental line was described in Singh et al. (1990). The wheat line ‘Federation’, the recurrent parent of Federation*4/Kavkaz, was used as a control to establish that DNA markers originated from the 1RS arm backcrossed into ‘Federation’ from ‘Kavkaz’. Chinese Spring (CS) ditelo (Dt) 1BL was used to differentiate ‘King II’ 1RS markers from wheat 1AS or 1DS.
Structure of the short arm of rye chromosome 1 in the two parents used for generating the F2 mapping family. a Susceptible parent ‘King II’ derivative line. The ‘Petkus’– derived proximal region is shown in solid black and ‘King II’ distal region is shaded. b Resistant parent carrying the 1BL·1RS translocation from ‘Petkus’ rye, shown as solid black line. The inferred locations of the nucleolus organizer locus (Nor) and secalin seed storage gene locus (Sec1) are marked
To determine the Sr31, Lr26 and Yr9 genotypes of each of the 143 F2 plants, three lots of 25 F3 seedlings from each F2 plant were separately tested with cultures of the three rust pathogen species, namely P. graminis f. sp. tritici pathotype 98-1, 2, 3, 5, 6, P. triticina pathotype 104-2, 3, (6), (7), 11 and P. striiformis f. sp. tritici pathotype 110 E143 A+, respectively. The inoculation procedures were described in Mago et al. (2004).
The high-resolution mapping family consisted of 36 F2 individuals that were recombinant for PCR markers flanking Sr31. These were identified among 1,580 F2 individuals from the above cross. F3 progeny of the 36 recombinants were phenotyped for stem rust (Sr31), leaf rust (Lr26) and stripe rust (Yr9) response.
DNA isolation
Genomic DNA was isolated from leaves as described previously in Mago et al. (2002, 2004). For high-resolution mapping a half seed method was used. DNA was extracted by placing individual half seeds (without the embryo) in wells of a 96 well plate. The half seeds were crushed with a stainless steel ball bearing using a Retsch MM300 mixer mill (Retsch, Haan, Germany). After a short spin at 100 rpm, 300 μl of pre-warmed (65°C) extraction buffer (0.1 M Tris–HCl pH8.0, 0.05 M EDTA pH8.0 and 25% SDS) was added. The plate was sealed and incubated at 65°C for 1 hr then cooled at 4°C for 30 min. To each well, 150 μl of 6 M ammonium acetate was added, the plate was resealed and shaken vigorously before leaving it at 4°C for 30 min. The plate was centrifuged at 3,000 rpm for 30 min and the supernatant was transferred to a fresh deep well plate containing 180 μl of iso-propanol in each well, mixed thoroughly and left at room temperature for 5 min to precipitate before centrifugation at 3,000 rpm for 30 min. The supernatant was carefully discarded and the DNA washed with 250 μl of 70% ethanol. The pellet was air dried and resuspended overnight in 150 μl water at 4°C. The plate was centrifuged at 3,000 rpm for 30 min and 50 μl of the supernatant was transferred to a fresh microtiter plate for storage at -20°C.
Restriction fragment length polymorphism (RFLP), amplified fragment length polymorphism (AFLP) and PCR marker analysis
RFLP filters were made from 20 μg of DNA isolated from CS Dt1BL, the two parental lines, and from ‘Federation’, digested with 12 restriction enzymes (BamHI, BglII, DraI, EcoRI, EcoRV, HindIII, NcoI, NdeI, NsiI, SacI, XbaI and XhoI). Southern analysis was done under standard conditions using RFLP probes previously mapped in the region (Mago et al. 2002). DNA probes used for hybridization to blotted DNA digests were labeled with [32P]-CTP using the megaprime DNA labeling system (Amersham Pharmacia). When an enzyme was identified that gave a polymorphism with a probe, DNA from all the lines comprising the mapping family was digested with the same enzyme, hybridized with the probe and the RFLP scored. RFLP probes were mapped using the restriction enzymes BamHI (Mla-LRR, 540G23-T7, BF291707), DraI (BE196644, MWG2245), EcoRI (MWG68, MWG 837, BF478880, BE444266, BE405749), EcoRV (BE196644, Mla-LRR, MWG 60) and XbaI (MWG 36, P2M11). Marker Iag95 was mapped as a co-dominant PCR marker using primers and conditions described in Mago et al. (2002).
Bulk segregant analysis was done on pooled DNA from ten homozygous resistant and ten homozygous susceptible F2 plants using the standard AFLP procedure with PstI (5′ GATGGATCCAGTGCAG 3′) and MseI (5′ GATGAGTCCTGAGTAA 3′) primer combinations with three additional nucleotides (Vos et al. 1995). CS Dt1BL and Federation were included to distinguish the 1RS bands from wheat bands of 1AS or 1DS origin. Cloning and sequencing of the polymorphic bands was done as described in Mago et al. (2002). One of the polymorphic bands amplified from Federation*4/Kavkaz, P-AGT/M-GTT-287 (P6M12), was cloned, sequenced and converted to a PCR-based marker (P6M12-P) that was amplified from the genomic DNA using the primers 5′ GTACTAGTATCCAGAGGTCACAAG 3′ and 5′CAGACAAACAGAGTACGGGC 3′. Amplification conditions were initial denaturation at 95°C for 3 min followed by 30 cycles of 94°C for 30 s, 57°C for 30 s and 72°C for 40 s, and then one cycle of 72°C for 10 min in 20 μl reactions. The marker was dominant: two P6M12-P fragments of 360 and 260 bp were amplified from the resistant parent Federation*4/Kavkaz and the susceptible parent was null for this marker.
DNA from Federation*4/Kavkaz and mutants in class 6, 7 and 8 was used to develop AFLP markers using the same procedure as described above. A mutant from class 1 with a deletion of the 1RS chromosome arm was included to identify the 1RS specific bands from any wheat AFLPs.
Four microliter of the DNA was used to perform a multiplex PCR using the primers for Iag95 and P6M12-P. Amplification conditions were initial denaturation at 95°C for 3 min followed by 30 cycles of 94°C for 30 s, 57°C for 1 min and 72°C for 1 min and then 1 cycle of 72°C for 10 min in 20 μl reaction. Recombinants were confirmed by repeating the PCR using the individual marker amplification. Recombinant individuals were recovered by germinating the retained half seed with the embryo and confirmed as recombinants by repeating the PCR on genomic DNA from the same plant.
Wheat and rice sequence comparisons and cloning of wheat ESTs
Synteny between wheat 1AS chromosome and rice was exploited in order to identify potential new markers in the region. Wheat ESTs previously mapped to deletion bin 1AS-3, which showed a nucleotide sequence identity with rice chromosome 5S BAC/PAC clones (Guyot et al. 2004; Spielmeyer and Richards 2004), were downloaded from http://wheat.pw.usda.gov/NSF/. Primers designed on the basis of published EST sequences were used to amplify CS genomic sequences that were gel purified (Qiagen, Germany) and cloned into pGemT-Easy Vector system (Promega). Insert identity was confirmed by DNA sequencing and cloned fragments were amplified by PCR to produce probes for DNA gel-blot analysis.
Linkage analysis
Segregations of the markers and rust resistance loci (Sr31, Lr26 and Yr9) were tested using chi-squared test for the expected 1:2:1 or 3:1 ratios. Linkage analysis and map construction for markers and resistance loci were performed with the MAPMAKER Version 2.0 (Lander and Green 1987). An LOD score of 3.0 was used to develop the linkage map and the Kosambi mapping function was used to convert recombination frequencies into centimorgans (cM).
Mutagenesis and mutant screening
Two sets of mutation experiments were conducted. In one experiment seed from wheat varieties ‘Pakistan 81’, ‘Sarhad 82’ and ‘Kohinoor 83’ that carry the ‘Petkus’ 1RS translocation were treated with ethyl methyl sulfonate (EMS). In the second mutagenesis experiment Federation*4/Kavkaz was mutagenized with EMS, γ-irradiation or sodium azide. EMS and γ-irradiation treatments were according to the methods described in Mago et al. (2004). For treatment with sodium azide seeds were soaked overnight in water then drained and treated for 2 h in 7 mM or 10 mM sodium azide in a pH 3.0 buffer with gentle shaking. The seeds were washed thoroughly under tap water overnight and dried on filter paper before sowing in soil. In the first experiment, M2 families from single ears of independent M1 plants were screened for either Lr26 or Yr9 mutants. In the second experiment, M2 families were screened for either Sr31 or Lr26 mutants. M3 families of each mutant were phenotyped with all three rust species (those detecting Sr31, Lr26 and Yr9).
Results
Segregation of resistance genes
For the molecular mapping of the rust resistance genes Sr31, Lr26 and Yr9, a total of 143 F3 families from the cross between the rust susceptible ‘King II’ derivative line and the rust resistant Federation*4/Kavkaz were tested for inheritance of the three rust resistances. Consistent with earlier analyses (Singh et al. 1990; Lukaszewski 2000) no recombination was observed between the three resistances. The observed segregation of 47 homozygous resistant, 65 heterozygous and 31 homozygous susceptible families is consistent with a 1:2:1 monogenic segregation ratio (χ2=4.81, P>0.05). Because the Sr31, Lr26 and Yr9 specificities co-segregated with each other, this study provided no evidence as to whether these specificities are determined by the same gene, or by separate, closely linked genes.
Genetic mapping
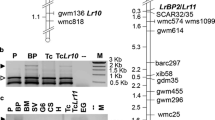
Twenty-five RFLP probes previously mapped on wheat chromosome 1S, barley 1HS (Wei et al. 1999) and rye 1RS (Mago et al. 2002), in combination with 12 restriction enzymes, were used to identify RFLPs. Only eight of the 25 probes detected a polymorphism between the parents. Seven of these RFLP markers, one AFLP marker (P6M12-P, see below), one RFLP-derived PCR marker (Iag95) and the rust resistance genes were mapped using the 143 F2 plants and their F3 progenies (Fig. 2). RFLP marker MWG60 mapped 4.5 cM distal and Iag95 1.7 cM distal to the rust resistance locus. Iag95 behaved as a co-dominant PCR marker. We previously showed that probes derived from the powdery mildew locus Mla in barley identified markers in the rust resistance region on 1RS (Mago et al. 2002, 2004). The Mla-LRR probe detected multiple restriction fragments and comparisons of ‘Federation’ and Federation*4/Kavkaz and CS Dt1BL and the King II derivative identified RFLPs derived from rye 1RS (Fig. 3). Some of these rye fragments were also polymorphic between the rust resistant and susceptible parents of the mapping family. These RFLPs co-segregated with rust resistance. RFLP marker MWG36, which maps distally to the Mla locus in barley (Wei et al. 1999) and a marker detected with barley EST BE196644 (Wei et al. 2002), also co-segregated with Mla-LRR markers and rust resistance. A barley BAC end marker 540G23-T7 and two RFLP probes, MWG68 and MWG837, which were previously mapped proximally to Mla in barley (Wei et al. 1999), were placed 0.35 cM proximal to the rust resistance locus. The map order of all these markers on 1RS was in agreement with previously published maps in other members of the Triticeae (Peng et al. 2004). Markers MWG36, MWG68 and MWG837 were dominant and efforts to convert MWG68 and MWG837 to PCR-based markers were not successful.
Genetic map of the genomic region carrying rust resistance genes Sr31, Lr26 and Yr9 on the short arm of rye chromosome 1 based on 143 F2 individuals. Genetic distances (cM) as well as number of recombinants (shown in brackets) observed for specific intervals are shown on the left hand side and genetic markers on the right
DNA gel blot hybridized with barley probe Mla-LRR. Lanes 1, CS Dt1BL, 2, ‘King II’ derivative line, 3, Federation*4/Kavkaz, 4, ‘Federation’. Twenty micrograms of genomic DNA per lane from each line was digested with the indicated restriction enzymes. The polymorphic rye specific bands in both the parents are indicated by an asterisk (*)
One AFLP marker (P6M12), which mapped proximal to the rust resistance locus and co-segregated with MWG68 and MWG837 (Fig. 2), was converted to a PCR-based marker (P6M12-P). None of the markers proximal to MWG68 or MWG837 in previously published wheat and barley maps showed polymorphism between the parents. For high-resolution mapping PCR markers Iag95 and P6M12-P, which flank the rust resistance gene(s), were used to identify individual F2 recombinants in the Sr31, Lr26 and Yr9 region.
Selection of recombinants for high-resolution mapping
DNAs from 1,580 F2 seeds were screened for recombination between the flanking PCR markers Iag95 and P6M12-P. Because Iag95 is a co-dominant marker and P6M12-P is dominant marker, only 50% of F2 individuals carrying a single recombinant chromosome and 50% of those carrying two recombinant chromosomes could be identified. Thirty-six F2 recombinants were recovered. Figure 4 shows the PCR amplification of Iag95 and P6M12-P in the parents and three recombinants and a schematic diagram representing the genotypes of the recombinants that could be recovered. The F3 progeny of the recombinants were phenotyped for rust resistance. One recombinant separated Lr26 from Sr31 and Yr9. This recombinant was of type 1 (Fig. 4) and was homozygous for Iag95-K, homozygous susceptible to leaf rust and heterozygous for Sr31, Yr9 and the RFLP probes Mla-LRR and BE196644.
PCR amplification of the flanking co-dominant marker Iag95 (a) and dominant marker P6M12-P (b) used for high resolution mapping of genomic region carrying rust resistance genes on the rye chromosome arm 1RS. Lanes R (resistant parent), Federation*4/Kavkaz; S (susceptible parent), ‘King II’ substitution line derivative; Type 1–3, three types of recombinants. (c) Crossing scheme used to generate the recombinants and the different types (1–3) that were recovered using PCR markers Iag95 and P6M12-P. Numbers of recombinants recovered of each type are indicated in brackets. The ‘Petkus’ alleles are designated Iag95-P and P6M12-P and the ‘King II’ derived alleles are Iag95-K and P6M12-K
All the RFLP markers that mapped to the Iag95-P6M12-P region (Fig. 2) were mapped in the high-resolution mapping family (Fig. 5). Iag95 mapped 1.99 cM distal to Lr26. Of the 36 recombination events identified between Iag95 and P6M12, 30 were located between Iag95 and Lr26. RFLP marker MWG36, which previously co-segregated with the rust loci, was separated from Lr26 by 13 recombination events (within ~1 cM; Fig. 5). Two recombination events were identified between the barley BAC end marker 540G23-T7, which maps proximal to the Mla locus in barley (Wei et al. 1999; Mago et al. 2004) and the Sr31/Yr9 locus (0.13 cM). The polymorphisms detected with the barley Mla-LRR probe using restriction enzymes BamHI, EcoRI and EcoRV, co-segregated with Sr31 and Yr9, but recombined with Lr26. Barley EST marker BE196644 also co-segregated with Sr31 and Yr9. The barley BAC end probes derived from ‘Morex’ in the region distal to the Mla locus (Wei et al. 1999) were either monomorphic or did not hybridize to the 1RS region of the parents. In barley, this region contains several retro transposon-like elements and may have significantly diverged from the orthologous rye region (Wei et al. 2002).
High-resolution genetic map of the distal portion of the short arm of rye chromosome 1S harboring resistance genes Sr31, Lr26 and Yr9 and comparison to the BAC/PAC contig of distal end of short arm of rice chromosome 5. Genetic distances (cM) as well as number of recombinants (shown in brackets) observed for specific intervals on the high-resolution map are shown on the left and genetic markers are shown on the right
Identification of AFLP markers associated with the resistance genes
To increase the number of markers in the region of the rust resistance genes, we screened AFLPs across bulk DNA from homozygous resistant and homozygous susceptible F2 plants as described previously. A total of 144 PstI–MseI primer combinations identified 15 AFLPs specific to the rye chromosome arm 1RS. Eight of these originated from the susceptible parent and seven from the resistant parent. Only four of the 15 markers mapped to the Iag95-P6M12-P interval and one of these P-ATG/M-GTG (P8M11) co-segregated with Iag95 (Fig. 5). Three AFLP markers P-ACC/M-GTG, P-AGT/M-GAC and P-ATC/M-GAC (P2M11, P6M22 and P7M2) co-segregated with RFLP probes MWG68 and MWG837. No AFLP marker mapped between the rust locus and the distal RFLP marker MWG36.
Wheat – rice synteny in the Sr31, Lr26 and Yr9 region and generation of additional markers
To find extra markers in the region we explored the synteny of wheat 1S to rice. Guyot et al. (2004) and Spielmeyer and Richard (2004) demonstrated that genes on BAC/PAC contigs from rice chromosome 5S were related to genes in wheat chromosome 1S deletion bin 1AS3-0.86-01.00. Wheat RFLP marker MWG2245, which co-segregated with Iag95 (Fig. 5), provides a link between these wheat genes, the Sr31, Lr26, Yr9 region and the rice genome. We designed primers to the 21 wheat ESTs that were mapped proximally to MWG2245 by Guyot et al. (2004) and Spielmeyer and Richard (2004). PCR products amplified from wheat were used as probes and the resultant RFLPs were mapped. Four RFLPs (BF291707, BE405749, BE444266 and BF478880) mapped to the Iag95-P6M12-P region (Fig. 5) but none were closer to the resistance genes than previously mapped DNA markers. Comparison of the map location of these markers on 1RS and the rice 5S BAC/PAC contig is shown in Fig. 5. The order of the distal markers BF291707 and BE405749 is reversed and the proximal markers BF478880 and BE444266, which co-segregate in our high-resolution mapping family, are 600 kb apart in rice. The markers BE405749 and BF478880 that are 2.1 cM apart in rye are separated by 200 kb in rice. If there is synteny between rye and rice in this region, then rice orthologs of rust resistance genes could be expected to occur in this interval. No clear candidates for Sr31, Lr26 and Yr9 were identified in this rice interval, similar to a previous study looking for orthologs of the barley stem rust resistance gene Rpg1 in rice (Kilian et al. 1997).
Isolation and analysis of rust susceptible mutants
Two independent mutation experiments were conducted on wheat varieties carrying ‘Petkus’ 1RS. In the first experiment, using EMS, 70 mutants were recovered from 6,737 M2 families. Thirty-six mutants were for Yr9 alone, 32 were for Lr26 alone and two were mutant for both Lr26 and Yr9. Sr31 mutants were not selected in this experiment. In the second experiment, a total of 43 mutants were recovered from 4,478 M2 families. These include 35 from radiation treatment (from 2,540 M2 families) and two from EMS treatment (980 M2 families). Treatment with sodium azide yielded six mutants (958 M2 families).The rust resistance genotypes of the mutants were confirmed by analyzing their M3 progenies using all three rust species. Forty-one of the 43 mutants were mutant for Sr31, Lr26 and Yr9. Two irradiation mutants were mutant for Sr31 only. No Lr26 or Lr26, Yr9 double mutants were recovered in experiment 2. All mutants that lost more than one resistance were deletions. These experiments indicate that these resistances can be mutated independently and are probably controlled by separate genes.
DNA markers from 1RS (Mago et al. 2002 or present study) were used to examine the majority of the mutants. Ten classes of mutants were distinguished and these are depicted in Fig. 6 together with the number of mutants analyzed in each class. Mutants in classes 1–3 are likely terminal deletions of varying sizes with some of those in class 1 possibly being whole arm or entire chromosome deletions. These included the two Lr26, Yr9 double mutants from experiment 1, which had not been characterized for their Sr31 phenotype. Mutants in classes 4–6 are interstitial deletions that lost all resistance genes and at least two DNA markers. Although the mutant in class 7 had lost no DNA marker it is assumed to be an interstitial deletion because it lacks all three rust resistances. The two mutants in class 8, which lack only Sr31, could be point mutations or involve small interstitial deletions not identified by any of the markers used in the study. Other evidence (see below) indicates that mutants in classes 7 and 8 do involve interstitial deletions. Hybridization of the Mla-LRR probe with the genomic DNA of mutants in classes 7 and 8 digested with multiple restriction enzymes (6 enzymes for class 7 and 16 enzymes for class 8; data not shown) did not detect loss of any members of the complex Mla cluster on 1RS. The barley EST probe BE196644 was also present in mutants in classes 7 and 8 but was deleted in all other classes. Mutants in classes 9 and 10, all derived from EMS treatment, may be point mutations as they lack none of the molecular markers and retain Sr31.
Diagrammatic representation of the various classes of rust susceptible mutants. Numbers of mutants analyzed in each class are in brackets. Class 1 mutants may include aneuploids and complete 1RS deletions. Mutants in class 4–8 were interstitial deletions and are represented as empty box. Asterisk the order of Sr31 and Yr9 with respect to Mla-LRR could not be determined
AFLP analysis using 144 PstI–MseI primer combinations of mutants in classes 6, 7 and 8 and their resistant and susceptible parents, identified five new markers P-ACC/M-GAG-384, P-ACG/M-GCG-339, P-ACG/M-GCG-411, P-ACA/M-GCG-553 and P-ATG/M-GTC-456 that were absent in one or more of the five independent mutant lines. The sequences of these AFLP markers are available in the GenBank (Accession Nos. DQ167393–DQ167397). Four of the AFLPs P-ACC/M-GAG-384, P-ACG/M-GCG-339, P-ACG/M-GCG-411 and P-ATG/M-GTC-456 were absent in mutant classes 6, 7 and 8 while P-ACA/M-GCG-553 was absent in classes 6 and 7 but present in class 8. Loss of four markers in class 8 mutants suggests that they also contain deletions. Mutant classes 9 and 10 have not been analyzed. Importantly the AFLPs were present in rust resistant sibs of each mutant class. Figure 7 shows the amplification of P-ACC/M-GAG-384. The marker was amplified in both parents but not in any of the mutants of class 1, 6 and 8. None of the AFLPs that distinguish the mutants from the resistant parent could be mapped in our mapping family because they failed to identify polymorphisms between the resistant and susceptible parents either as AFLP, RFLP or PCR markers. However loss of these AFLPs in class 6 mutants, together with the mapped molecular markers detected by the Mla-LRR and BE196644 probes (Fig. 6), indicates that they map to the rust resistance gene region. Additionally, loss of these markers in class 7 and 8 mutants, which have not lost any of the mapped molecular markers (Fig. 6), indicates that these mutants also result from deletion linked to, and most likely including, the rust resistance genes.
An example of AFLP analysis of mutants used to develop markers in the Sr31, Lr26 and Yr9 region. Lanes 1, Susceptible parent (‘King II’ derivative), 2, Federation*4/Kavkaz, 3, Mutant class1, 4, Mutant Class 6, 5, Mutant Class 8. Arrow shows the AFLP P-ACC/M-GAG-384 in the parents (lanes 1 and 2) and is absent in interstitial deletion mutants in classes 1, 6 and 8
Discussion
Wild relatives of common wheat, Triticum aestivum, and related cultivated species like rye, provide a source of genes for wheat improvement (Friebe et al. 1996) and many disease and pest resistance genes have been introgressed into wheat from alien sources by translocation. The short arm of rye chromosome 1 (1RS) from ‘Petkus’ rye has been the most widely used alien translocation in wheat because of the agronomic and disease resistance advantages it has provided over a wide geographical area. A major difficulty for map-based cloning from alien sources is that normally the alien chromatin does not recombine with its wheat homoeologues. Consequently, it is difficult to develop genetic stocks to facilitate the cloning of such genes. Recombination between alien translocations and a wheat homoeologous chromosome can be achieved to some extent by inducing recombination in a ph-1 mutant background. Lukaszewski (2000) induced recombination between wheat and rye in a ph-1 mutant background which allowed limited mapping of DNA markers and the rust resistance genes on 1RS (Mago et al. 2002). In another approach to map resistance genes on the wheat rye translocation chromosome 1BL·1RS, Singh et al. (1990) testcrossed a heterozygote with 1BL·1RS derived from ‘Petkus’ rye (Sr31, Lr26, Yr9) and 1R from King II rye (sr31, lr26, yr9) with CS Dt1BL. Recombination between the two 1RS arms permitted a map to be generated in which the rust resistance genes co-segregated. With the aim to develop tools to clone Sr31 we used two 1BL·1RS lines, one with and one without Sr31, Lr26 and Yr9, as parents to establish an F2 mapping family. Initial mapping in a low resolution family based on 143 F2s showed that chromosomes from the two rye segments (‘Petkus’ and ‘King II’) recombined and also confirmed the co-segregation of Sr31, Lr26 and Yr9.
High-resolution mapping, which gives precise placement of the target gene amongst closely linked markers, is essential for map-based cloning projects in cereals (Blair et al. 2003; Yan et al. 2003; Bulgarelli et al. 2004; Yahiaoui et al. 2004). We identified 36 F2 individuals recombinant for the two markers 2.3 cM apart that flanked the rust resistance genes. One of these recombinants separated Lr26 from Sr31 and Yr9 (0.06 cM) providing a partial answer to the question of whether one or more genes located on the chromosome arm 1RS confer resistance against the three rust species. As no recombinants between Sr31 and Yr9 were obtained, we used mutagenesis to further investigate whether Sr31 and Yr9 are different genes. The recovery of several mutants for Sr31, Lr26 or Yr9 alone indicates that they are different closely linked distinct loci (Fig. 6).
Previously we mapped the stem rust resistance gene SrR on 1RS from ‘Imperial’ rye using deletion mutation analysis (Mago et al. 2004). Unlike Sr31, SrR is not linked to any leaf rust or stripe rust resistance gene. It is presently unknown whether Sr31 and SrR express the same or different resistance specificities which could indicate whether they might be the same or different genes. An analysis of SrR mutants revealed that it maps in the same region as Sr31 and that homologues of the barley powdery mildew resistance gene Mla were candidates for SrR (Mago et al. 2004). An Mla-LRR probe identified about 20 fragments on 1RS and some interstitial deletion mutants showed loss of only three of these bands (Mago et al. 2004). In the present study, we were interested in the relationship between the rye Mla homologues and Sr31, Lr26 and Yr9. High-resolution mapping of the region also showed that all Mla RFLPs on 1RS co-segregated with Sr31and Yr9 resistance genes and recombined with Lr26. However, the retention of all Mla restriction fragments in the single deletion mutant in class 7, that has lost all three rust resistance genes, and the two mutants in class 8, that have lost only Sr31, suggest that a rye homologue of barley Mla is unlikely to be a candidate for any of the three resistance genes of ‘Petkus’ origin. Two other RGA classes (RGH2 and RGH3; Wei et al. 1999) at the barley Mla locus failed to identify any rye homologues in our study. Nevertheless, the mapping information and mutant stocks provided here will be important resources for the future cloning of these genes by either map-based or candidate–gene cloning approaches.
References
Adhikari KN (1996) Genetic studies of stem rust resistance in oat and trititicale. PhD Thesis, The University of Sydney, Australia
Blair MW, Garris AJ, Iyer AS, Chapman B, Kresovich S, McCouch SR (2003) High resolution genetic mapping and candidate gene identification at xa5 locus for bacterial blight resistance in rice (Oryza sativa L.). Theor Appl Genet 107:62–73
Bulgarelli D, Collins NC, Tacconi G, Dellaglio E, Brueggeman R, Kleinhofs A, Stanca AM, Valè G (2004) High-resolution genetic mapping of the leaf stripe resistance gene Rdg2a in barley. Theor Appl Genet 108:1401–1408
Friebe B, Jiang J, Raupp WJ, McIntosh RA, Gill BS (1996) Characterization of wheat-alien translocations conferring resistance to diseases and pests: current status. Euphytica 91:59–87
Guyot R, Yahiaoui N, Feuillet C, Keller B (2004) In silico comparative analysis reveals a mosaic conservation of genes within a novel colinear region in wheat chromosome 1AS and rice chromosome 5S. Funct Integr Genomics 4:47–58
Hsam SLK, Mohler V, Hartl L, Wenzel G, Zeller FJ (2000) Mapping of powdery mildew and leaf rust genes on the wheat-rye translocated chromosome T1BL·1RS using molecular and biochemical markers. Plant Breed 119:87–89
Kilian A, Chen J, Han F, Steffenson B, Kleinhofs A (1997) Towards map-based cloning of the barley stem rust resistance genes Rpg1 and Rpg4 using rice as an intergenomic cloning vehicle. Plant Mol Biol 35:187–195
Lander ES, Green P (1987) Construction of multilocus genetic linkage maps in humans. Proc Natl Acad Sci USA 84:2363–2367
Lukaszewski AJ (2000) Manipulation of the 1RS·1BL translocation in wheat by induced homoeologous recombination. Crop Sci 40:216–225
Mago R, Spielmeyer W, Lawrence GJ, Lagudah ES, Ellis JG, Pryor A (2002) Identification and mapping of molecular markers linked to rust resistance genes located on chromosome 1RS of rye using wheat-rye translocation lines. Theor Appl Genet 104:1317–1324
Mago R, Spielmeyer W, Lawrence GJ, Ellis JG, Pryor A (2004) Resistance genes for rye stem rust (SrR) and barley powdery mildew (Mla) are located in syntenic regions on short arm of chromosome 1. Genome 47:112–121
McIntosh RA, Wellings CR, Park RF (1995) Wheat rusts: an atlas of resistance genes. CSIRO, Australia
Peng JH, Zadeh H, Lazo GR, Gustafson JP, Chao S, Anderson OD, Qi LL, Echalier B, Gill BS, Dilbirligi M, Sandhu D, Gill KS, Greene RA, Sorrells ME, Akhunov ED, Dvorak J, Linkiewicz AM, Dubcovsky J, Hossain KG, Kalavacharla V, Kianian SF, Mahmoud AA, Miftahudin, Conley EJ, Anderson JA, Pathan MS, Nguyen HT, McGuire PE, Qualset CO, Lapitan NL (2004) Chromosome bin map of expressed sequence tags in homoeologous group 1 of hexaploid wheat and homoeology with rice and Arabidopsis. Genetics 168:609–623
Rajaram S, Mann CHE, Ortiz-Ferrara G, Mujeb-Kazi A (1983) Adaptation, stability and high yield potential of certain 1B/1R CIMMYT wheats. In: Sakamoto S (ed) Proceedings of the 6th International Wheat Genet Symposium, Kyoto, Japan, pp 613–621
Schlegel R, Korzun V (1997) About the origin of 1RS·1BL wheat-rye chromosome translocations from Germany. Plant Breed 116:537–540
Singh NK, Shepherd KW, McIntosh RA (1990) Linkage mapping of genes for resistance to leaf, stem and stripe rusts and ω-secalins on short arm of rye chromosome 1R. Theor Appl Genet 80:609–616
Spielmeyer W, Richard RA (2004) Comparative mapping of wheat chromosome 1AS which contains the tiller inhibition gene (tin) with rice chromosome 5S. Theor Appl Genet 109:1303–1310
Vos P, Hogers R, Bleeker M, Reijans M, Van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acid Res 23:4407–4414
Wei F, Gobelman-Werner K, Morroll SM, Kurth J, Mao L, Wing R, Leister D, Schulze-Lefert P, Wise RP (1999) The Mla (Powdery Mildew) resistance cluster is associated with three NBS-LRR gene families and suppressed recombination within a 240 kb DNA interval on chromosome 5S (1HS) of barley. Genetics 153:1929–1948
Wei F, Wing RA, Wise RP (2002) Genome dynamics and evolution of the Mla (Powdery Mildew) resistance locus in barley. Plant Cell 14:1903–1917
Yahiaoui N, Srichumpa P, Dudler R, Keller B (2004) Genome analysis at different ploidy levels allows cloning of the powdery mildew resistance gene Pm3b from hexaploid wheat. Plant J 37:528–538
Yan L, Loukoianov A, Tranquilli G, Helguera M, Fahima T, Dubcovsky J (2003) Positional cloning of the wheat vernalization gene VRN1. Proc Natl Acad Sci 100:6263–6268
Zeller FJ (1973) 1B/1R wheat-rye chromosome substitutions and translocations. In: Sears ER, Sears LMS (eds) Proceedings of the 4th International Wheat Genet Symposium, Columbia, Missouri, USA, pp 209–221
Acknowledgements
We thank Dr. K.W. Shepherd, Waite Campus, University of Adelaide, Australia for providing the ‘King II’ derivative line. We acknowledge excellent technical assistance provided by Luch Hac, Pat Atkinson, Xiaodi Xia, Kim Newell and Cassie Wesley. This project (CSP0017) is supported by financial assistance from the Grains Research and Development Corporation and was undertaken as part of The Australian Cereal Rust Control Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B. Keller
Rights and permissions
About this article
Cite this article
Mago, R., Miah, H., Lawrence, G. et al. High-resolution mapping and mutation analysis separate the rust resistance genes Sr31, Lr26 and Yr9 on the short arm of rye chromosome 1. Theor Appl Genet 112, 41–50 (2005). https://doi.org/10.1007/s00122-005-0098-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-005-0098-9