Abstract
Purpose
Purpose of this study was to investigate outcome and toxicity of re-irradiation for recurrent primary glioblastoma (rGBM). We evaluated a group of patients with rGBM and identical primary treatment comprising adjuvant radiotherapy (30 × 2 Gy) with concurrent temozolomide (TMZ).
Methods
In this retrospective study of 46 patients, all received adjuvant or definitive normofractionated radiotherapy to a pretreated area, some with concurrent chemotherapy. Impact of different clinical, histological, or epidemiological factors on survival and radiation toxicity was reviewed.
Results
Of 46 patients, 40 completed the intended therapy. Overall survival (OS) was 20 months (range 6–72 months). Overall survival and progression-free survival after re-irradiation (OS2 and PFS2) were 9.5 and 3.4 months (range 2–40 and 0.7–44 months). Simultaneous systemic therapy improved PFS2 and OS2 (4.3 vs. 2.0, p < 0.001 and 12 vs. 4 months, p = 0.13, respectively). Therapy with TMZ or bevacizumab improved PFS2 vs. nitrosureas (6.6 vs. 2.9, p = 0.03 and 5.1 vs. 2.9 months, p = 0.035, respectively). TMZ also improved PFS2 and OS2 vs. all other systemic therapies (6.6 vs. 4, p < 0.001 and 17 vs. 10 months, p = 0.1). In a subgroup analysis for patients with methylation of the MGMT promoter, doses of >36 Gy as well as TMZ vs. no systemic therapy improved PFS2 (p = 0.045 and p = 0.03, respectively). 27.5% of all patients had no acute toxicity. Three patients with acute and four patients with late grade 3 toxicities were reported.
Conclusion
Normofractionated radiotherapy is a feasible option for rGBM with a good toxicity profile. Simultaneously applied systemic therapy was associated with improved outcome. For MGMT promoter-methylated histology, higher radiation doses improved survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma multiforme (GBM) accounts for 15.8% of all primary intracerebral tumors and despite many improvements in therapy, the 5‑year overall survival (OS) is only 5% [1].
State of the art is multimodal treatment consisting of surgical resection, radiotherapy using modern local radiation techniques, and chemotherapy. The standard radiation dose is 60 Gy in fractions of 2 Gy. However, higher dosage or accelerated radiation protocols have shown no improvement in OS or progression-free survival (PFS) [2, 3]. The combination of 60 Gy radiation with simultaneously applied temozolomide (TMZ) is regarded as standard therapy for patients up to 70 years of age and has shown benefit for even older patients [4,5,6].
Despite these aggressive treatment protocols, tumor recurrence is very high and prognosis is poor, with a median OS of 12–15 months [7].
In case of recurrence, repeated surgical resection is feasible, as long as the patient’s performance status is appropriate [8]. Even though most tumors recur within the primary tumor field, a second course of radiotherapy is possible, e.g., with 18 × 2 Gy [9]. Target volume is assessed by MRI and, if possible, amino acid PET imaging. Additional chemotherapy prolongs PFS and OS [10]. Agents containing nitrourea (e.g., lomustine) showed limited effects [11]. Because a combined therapy using radiation and TMZ is widely accepted as first-line treatment, the benefit of another cycle of TMZ after tumor recurrence is a point of discussion [12]. In this scenario, the methylation of the MGMT promoter seems to play an important role as a prognostic factor [13]. Other prognostic factors mentioned in the literature include age, performance status, tumor size, extent of initial surgery, and surgery in the recurrent situation [14]. Regarding radiation therapy of tumor recurrence, there are few data concerning the optimal radiation dose, fraction size, or application of simultaneous chemotherapy. At the moment, treatment protocols rely mainly on data from retrospective studies. Limitations in most of these studies were the differences in tumor histology and the heterogeneous application of primary and secondary therapies. To this end, we evaluated a group of patients with recurrent primary GBM and identical primary treatment (adjuvant radiochemotherapy comprising 30 × 2 Gy with concurrent TMZ).
Methods
46 patients treated in our institution for recurrent primary glioblastoma (rGBM) between 2013 and 2016 were included in the study. They had all completed adjuvant radiation therapy composed of 60 Gy in 2 Gy daily fractions with concurrent TMZ therapy and at least one subsequent adjuvant TMZ cycle for primary disease. All patients showed uni- or multifocal disease recurrence, including the primary high-dose irradiation field. All patients had primary GBM.
Of 46 patients, 40 (87%) completed the planned radiation therapy. Only these patients have been included into further analyses (Table 1). 24 of 40 patients received re-surgery and 30 of 40 received simultaneous systemic therapy.
The impact of age, gender, planning target volume (PTV), time to the onset of the second radiation therapy, MGMT promoter and IDH mutation status, Eastern Cooperative Oncology Group performance index (ECOG), extent of surgery, radiation dose, and type of chemotherapy were evaluated. All cases were discussed in an interdisciplinary tumor board and all patients gave informed consent.
Radiation planning was based on CT and MRI scans, defining the gross tumor volume (GTV) as the contrast-enhancing lesion in the T1-weighted MRI sequence, the clinical target volume (CTV) as GTV plus a 0.5–1 cm margin, and PTV as CTV plus a 1–2 mm margin. Treatment planning was performed with respect to prior received doses to organs at risk. Individual face masks were used to ensure accurate treatment.
Fractionated radiotherapy was delivered in 5 fractions weekly once a day. Fraction size was 1.8–2 Gy using tomotherapy or a linear accelerator with IMRT. The median of the applied dose was 36 Gy (range 9–50.4 Gy) for all patients and 39.6 Gy (30–50.4 Gy) for the patients who completed their radiation therapy. A total dosage of 36 or 39.6 Gy was most common. Maximum dosage in primary irradiated areas was 39.6 Gy. Median PTV volume was 120.5 cm3 (range 25–580 cm3). Some patients received concomitant systemic therapy. The characteristics of all patients who completed the planned radiation therapy can be found in Table 1.
Patients had follow-up visits 6–8 weeks after completion of radiotherapy and then every 3–5 months at our institution. Follow-up MRI scans were conducted at outpatient clinics. Late toxicities were defined as side effects occurring more than 90 days after the end of radiotherapy.
Progression-free survival (PFS) was defined as the time of first radiation until radiological or histological proof of recurrence. PFS2 was defined as survival after the second radiation treatment to clinical or radiological proof of progression or patient’s death or was censored at the end of follow-up.
Overall survival (OS) was defined as the time from the first radiation treatment to death or the end of follow-up. Overall survival 2 (OS2) was defined as the time of re-irradiation after objective GBM recurrence to death or was censored at the end of follow-up.
Statistical analysis was performed using IBM SPSS25 statistics (SPSS Inc., Chicago, IL, USA). Correlations between categorical data were tested using chi2 tests. Survival data was analyzed using the Kaplan–Meier method and the log-rank test. Differences were considered statistically significant at a p-value <0.05. Independent variables were first analyzed with univariate analysis. Variables shown by univariate analysis to be associated with better local control or survival were further evaluated via multivariate analysis.
Results
40 patients (18 female, 22 male) completed radiotherapy for rGBM and met the inclusion criteria. Median age for the first diagnosis was 56 years (range 33–78 years). Median age at recurrence was 57 years (34–79 years). 26 patients were 51 years or older (65%). 26 patients showed an ECOG performance status of 0–1 (65%).
Median time since first radiotherapy was 10 months (3–54 months), median radiation dose was 39.6 Gy (30–50.4 Gy). 6 patients (15%) were retreated after less than 6 months, of whom 4 had histologically proven progress and 2 had radiological as well as clinical progress. For this particular group, the median PTV volume was smaller than for the whole cohort (76 cm3 vs. 120.5 cm3).
Radiation planning with 95% isodoses for primary (30 × 2 Gy, a) and recurrent disease (20 × 2 Gy, b) of a patient with GBM involving the right parietal and occipital lobe. c, d show the sum plan in axial and sagittal imaging, illustrating doses of 50 Gy or higher. The orange structure indicates the PTV, the purple structure the brainstem. GBM glioblastoma, PTV planning target volume
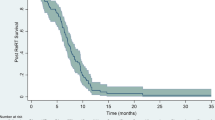
Median follow-up was 8 months (1–39 months). 6 patients (15%) had a follow-up time of 3 months or less, of whom all died during that period. Median OS2 was 9.5 months (2–40 months), median PFS2 was 3.4 months (0.7–44 months). Median OS since first radiation for GBM (OS) was 20 months (12–72 months).
Impact of epidemiological factors, MGMT promoter methylation, and IDH mutation status
Patients with a methylated MGMT promoter (n = 14, 35%) had a significantly longer PFS (12.4 vs. 9.8 months, p = 0.01) and time to second radiation (12.5 vs. 9 months p = 0.03). A longer PFS2 (3.5 vs. 3 months, p = 0.9) as well as OS (43.5 vs. 19.2 months, p = 0.07) and OS2 (19 vs. 9 months, p = 0.2) were estimated, but failed to show significance.
IDH mutation status was available for 25 patients (62.5%) of whom 22 (55%) had no mutation (wildtype) and 3 (7.5%) showed an IDH mutation. The patients with IDH mutation showed a better but not significantly improved PFS2 (5.2 vs. 2.9 months, p = 0.2), PFS (15.5. vs. 8.4 months, p = 0.3), OS2 (16 vs. 10 months, p = 0.4), and OS (67.7 vs. 20.8 months, p = 0.3).
Patients ≤50 years (n = 14, 35%) showed slightly longer PFS (9.6 vs. 9.1 months, p = 0.5) as well as longer OS and OS2 (25.6 vs. 20.8 months, p = 0.5, and 12 vs. 10 months, p = 0.5, respectively).
Patients with a time to second radiation over 12 months (n = 15, 37.5%) showed a significantly longer OS (43.5 vs. 16.8 months, p = 0.03) as well as a longer PFS2 (4.8 vs. 2.9 months, p = 0.25) and OS2 (16 vs. 7 months, p = 0.19).
ECOG status of less than 2 at the beginning of second radiotherapy showed a trend towards better OS2 (16 vs. 7 months, p = 0.06) and PFS2 (4 vs. 2.7 months, p = 0.065).
Impact of surgical intervention
Information about surgical intervention was available for 37 patients, of whom 12 (30%) had undergone gross tumor resection and 9 (22.5%) at least tumor debulking. PFS2 and OS2 after gross resection vs. debulking was 3.3 months vs. 4.8 months (p = 0.19) and 15 vs. 10 months (p = 0.4), respectively. Patients without surgery or biopsy only showed a median PFS2 of 2.6 months and median OS2 of 9 months. No significant differences concerning PFS2 and OS2 in comparison to tumor debulking (2.6 vs. 4.8 months, p = 0.9 and 9 vs. 10 months, p = 0.8, respectively) were seen. A trend towards worse PFS2 and slightly worse OS2 vs. gross resection was observed (2.6 vs. 3.3 months p = 0.06 and 9 vs. 15 months, p = 0.8, respectively).
Impact of chemotherapy
28 patients showed progression during primary TMZ therapy (70%). The median OS for these was 20.8 vs. 21 months for patients with progressive disease not during primary TMZ therapy (p = 0.35). OS2 was 10 vs. 9 months (p = 0.6) and PFS2 3.5 vs. 2.8 months (p = 0.8).
30 of 40 patients (75%) received systemic therapy simultaneously with radiation. Nitrosoureas were most common (n = 14, 35%), 6 patients received TMZ (15%), 5 patients received bevacizumab (12.5%), and 5 patients were treated with other substances or combination therapies (12.5%). Of the 6 patients receiving TMZ, 3 showed a methylated MGMT promoter.
PFS2 and OS2 were improved in all patients receiving a systemic therapy vs. no systemic therapy (4.3 vs. 2.0 months, p < 0.001 and 12 vs. 4 months, p = 0.13, respectively). TMZ improved OS2 and PFS2 vs. bevacizumab (17 vs. 12 months, p = 0.2 and 6.6 vs. 5.1 months, p = 0.6, respectively). TMZ improved PFS2 and OS2 vs. nitrosoureas (6.6 vs. 2.9 months, p = 0.03 and 17 vs. 8 months, p = 0.07, respectively). Bevacizumab improved PFS2 and OS2 vs. nitrosoureas (5.1 vs. 2.9 months, p = 0.035 and 15 vs. 7 months, p = 0.4, respectively).
TMZ also improved PFS2 and OS2 vs. all other systemic therapies (6.6 vs. 4 months, p < 0.001 and 17 vs. 10 months, p = 0.1, respectively; Fig. 2).
Kaplan–Meier curves showing PFS2 (a) and OS2 (b) for different systemic therapy groups. Curves indicate no systemic therapy (blue), TMZ (red), bevacizumab (green), nitrosoureas (orange), and other substances (yellow); + indicates a censored case. Log-rank tests were performed to compare groups, see text for p-values
Impact of radiation dose on survival parameters
Univariate regression analyses showed no significant impact of radiation dose on OS2 (p = 0.7) or PFS2 (p = 0.6). Median radiation dose was 39.6 Gy and doses of 36 Gy (n = 11, 27.5%) and 39.6 Gy (n = 14, 35%) were most common. Two groups were retrospectively analyzed, one with radiation doses up to 36 Gy and one with higher doses. Patients who received doses ≤36 Gy (n = 18, 45%) showed a median PFS2 of 2.6 months and OS2 of 10 months vs. 3.9 (p = 0.13) and 10 months, respectively, in the group receiving higher doses. No correlation between PTV volume and survival was seen.
After performing multivariate analyses for independent factors concerning PFS2, simultaneous therapy with TMZ vs. other or no therapies remained a significant factor (Table 2).
Subgroup analysis according to MGMT status
In a subgroup analysis of patients with methylated MGMT promoter (n = 14, 35%), we found higher PFS2 and OS2 in the TMZ group vs. no systemic therapy (6.2 vs. 2.7 months, p = 0.03 and 17 vs. 8 months, p = 0.2, respectively). OS2 (15 months for <36 Gy, median survival not reached at end of analysis in the group >36 Gy, p = 0.09) and PFS2 (4 vs. 2.6 months, p = 0.045) were improved through doses of >36 Gy, see Fig. 3.
Impact of different factors on toxicity
Table 3 shows the incidence of acute and late toxicities. Local alopecia (n = 15), fatigue (n = 7), or focal seizures (n = 4) were the most common acute toxicities. 11 patients (27.5%) showed no acute toxicity, 18 showed grade 1, 8 showed grade 2, and 3 showed grade 3 acute toxicity (one with urinary tract infection, two with decrease of general state).
Information about late side effects was available for 28 patients. 6 showed grade 1, 13 patients showed grade 2, and 4 patients showed grade 3 toxicities. Persisting fatigue was the most common side effect (n = 11). Other common late effects were focal seizures grade 1–2 (n = 4) and ataxia or dysphasia grade 1–2 (n = 6). For 4 patients, grade 3 adverse events have been reported (one case of decreased platelet count after adjuvant chemotherapy, one case of wound infection after post-radiation insertion of thermotherapy particles, one case of impaired wound healing after a third tumor resection, and one case of wound infection of the initial resection cavity).
Of 3 patients with acute toxicities grade 3, 2 had not received systemic therapy and all had received less than 36 Gy at the onset of the toxicity. No correlation was seen between incidence or grading of toxicities and simultaneously given systemic therapy or radiation dose. Also, no significant impact of patients’ age or ECOG status was estimated. Brain necrosis was assumed in two cases after radiation. Unfortunately, histology revealed tumor progression instead for these two patients.
Discussion
In cases of rGBM, the optimal treatment approach still remains elusive. In this study, the outcome of a group of patients receiving the same primary treatment (60 Gy in 2 Gy doses along with TMZ) with different treatments for disease recurrence was followed.
Mean OS2 in this study was 9.5 months, comparing well to the literature [15] for fractionated therapies with different single and total doses (2–5 Gy and 20–40 Gy, respectively). Also, the time between first and second radiotherapy is similar to other researchers’ descriptions [9].
Our data show a significant impact of MGMT promoter methylation status and time to second treatment (12 months or less) as well as choice of systemic therapy on survival parameters. Patients aged <51 years and patients with an ECOG of less than 2 showed a trend towards better survival. Tumor size and extent of surgery did not show a significant impact on outcome. In our cohort, also patients with an ECOG of 3 were re-irradiated. In these cases, taking into account the expected impact of therapy on quality of life and survival benefit according to factors such as age, histology, etc. is an important task for radiation therapists.
Combs and colleagues published a prognostic score to predict outcome for recurrent brain tumor patients. In this score, age, ECOG score, and histology were identified as the most important prognostic factors. Our findings are concordant with this scoring tool, even though we evaluated a histologically homogenous group of patients [14].
As published in the DIRECTOR study, the patients in this study had a difference in OS if wider surgical resection was used [16].
Additionally, statistical analyses showed a significant impact of radiation dose on survival after diagnosis of recurrent disease in MGMT promoter-methylated patients. All patients receiving doses of more than 36 Gy showed a trend towards better PFS after recurrence. Caution has to be paid, as treatment dose has to maintain a delicate balance between efficacy and toxicity.
If re-irradiation is supported by the interdisciplinary board, all patients should receive at least 30 Gy, as many authors described this dose as safe and lower doses are less effective [17, 18]. In smaller radiation fields, even stereotactic radiosurgery might be applied and showed similar survival rates in comparison to fractionated radiotherapy [15]. Anyhow, putative increased toxicity from single doses higher than 5 Gy and/or total doses higher than 40 Gy were reported [17]. A necrosis rate of brain tissue of about 10% might be expected after BED doses of 90 Gy or more [19], so most physicians are cautious with higher doses after a first radiotherapy course of 60 Gy. Brain necrosis can occur 6–24 months after radiation [20]. In our patient group, the median survival after the second radiation treatment was 10 months, so taking early and late toxicities into account is inevitable.
Anyhow, the risk for brain necrosis may be overestimated, as even with the time interval between the first and the second treatment being less than 6 months and some patients receiving more than 90 Gy in sum, no brain necroses were reported in this study. Our findings correspond with the review of Mayer and colleagues, who did not find an increasing incidence of necrosis with increasing doses in small radiation volumes as they are used with modern radiation techniques or with smaller time intervals between therapies [20].
As 3 patients developed grade 3 symptoms during the radiation course, coherence between radiation and symptoms cannot be excluded. As no impact of the total radiation dosage or simultaneously applied systemic therapy was seen, it is unclear whether these symptoms were caused by radiation or the proceeding disease.
The evidence of our findings concerning late toxicities might be limited, as several patients conducted their late follow-up MRIs with external practices and reports were not continuously brought to the radiation oncology follow-ups. To differentiate between tumor progression and pseudoprogression remains a difficult task with usual MRI techniques [21], as up to 30% of patients show increased gadolinium uptake in the irradiated region, mostly during the first 12 weeks after treatment. To evaluate the response to treatment, the updated response criteria of the Neuro-Oncology Working Group should be consulted [22].
Deeper insight into the role of gross resection and data on re-irradiation dose concepts are awaited from ongoing studies, such as the dose escalation study Dose Escalation Trial of Re-irradiation in Good Prognosis Recurrent Glioblastoma (NCT02709226) or the ongoing GlioCave study, where observation after resection is compared to resection with adjuvant fractionated radiotherapy with 46 or 39 Gy [23].
The combination of re-radiation and systemic therapies was described as safe and effective by several groups [24, 25]. Yet, it is a point of discussion whether it would be advantageous to apply another cycle of TMZ. In our findings, patients seemed to profit from systemic therapies, especially from TMZ, despite receiving it during the primary disease situation. These data are similar to the findings of Scholtyssek et al., who found a significant advantage through concurrent TMZ in contrast to radiation only or radiation and carboplatin/etoposide [26]. Similar findings are described by Grosu et al. [10], whereas other groups could not estimate an improvement trough TMZ or through CTX in general [27].
Combs and colleagues did not find a significant impact of prior TMZ application on survival parameters in a similar group of GBM patients, which underlines our suggestion to consider a second combination of radiation and TMZ for recurrent disease [24].
The majority of patients in our analysis received nitrosoureas in combination with radiation, which is considered effective and safe [28]. Yet we could not observe a significant advantage of this therapy versus radiotherapy alone or versus combination with other systemic therapies.
Even though the VEGF antagonist bevacizumab showed no advantage for primary GBM, it is commonly used in clinical practice for recurrent disease [29]. As several authors described a positive impact on PFS with application of bevacizumab for recurrent disease, we could show a significant improvement PFS2 for this systemic drug vs. use of nitrosoureas during re-irradiation [30].
In general, it must be kept in mind that most data on concurrent CTX are retrospective. To interpret the findings of previous publications, it must be considered that the study populations were often small, with heterogeneous tumor entities and different therapeutic approaches.
One limitation of this study is the small study population, which might be caused by the small number of patients being sent to radiotherapy with this respective diagnosis. Another weakness is the variety in chemotherapy schemes used for the described patients and the limited information about adjuvant chemotherapy dose and number of cycles. As we saw a strong impact of chemotherapy on survival parameters, with a significance concerning PFS2, the very low number of patients, especially in the group receiving TMZ, must be taken into account.
Checkpoint inhibitors have recently gained attention with groundbreaking improvements in survival rates for several tumors, and they are also target of multiple studies on GBM. As promising as these new drugs are, interactions between them and radiation need to be investigated carefully, as, for example, in the study Hypofractionated Stereotactic Irradiation (HFSRT) with Pembrolizumab and Bevacizumab for Recurrent High-Grade Gliomas (NCT02313272), which analyses the toxicity of bevacizumab, pembrolizumab, and re-radiation as a phase I study.
Re-irradiation for recurrent GBM seems safe and effective. MGMT promoter methylation status, time to re-irradiation, and ECOG status are strong predictors for a patient’s outcome after therapy. Simultaneously applied chemotherapy, especially TMZ, seems highly effective in the recurrent situation with or without proven MGMT methylation.
Our findings suggest that normofractionated radiotherapy leads to good outcomes for patients with recurrent GBM after primary therapy with adjuvant radiation and TMZ. Doses of more than 36 Gy should be applied, especially for MGMT promoter-methylated histology, whereas the ideal dose is still to be defined.
Future research is expected to reveal new insights into the effects of higher radiation doses and the interplay between systemic substances, with special interest in the new checkpoint inhibitors.
References
Dolecek TA, Propp JM, Stroup NE, Kruchko C (2012) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol 14(5):v1–49. https://doi.org/10.1093/neuonc/nos218
Laperriere N, Zuraw L, Cairncross G, Cancer Care Ontario Practice Guidelines Initiative Neuro-Oncology Disease Site Group (2002) Radiotherapy for newly diagnosed malignant glioma in adults: a systematic review. Radiother Oncol 64:259–273
Nieder C, Andratschke N, Wiedenmann N et al (2004) Radiotherapy for high-grade gliomas. Does altered fractionation improve the outcome? Strahlenther Onkol 180:401–407. https://doi.org/10.1007/s00066-004-1220-7
Hart MG, Garside R, Rogers G et al (2013) Temozolomide for high grade glioma. Cochrane Database Syst Rev 4:CD7415. https://doi.org/10.1002/14651858.CD007415.pub2
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. https://doi.org/10.1056/NEJMoa043330
Perry JR, Laperriere N, O’Callaghan CJ et al (2017) Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med 376:1027–1037. https://doi.org/10.1056/NEJMoa1611977
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5‑year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466. https://doi.org/10.1016/S1470-2045(09)70025-7
Park C‑K, Kim JH, Nam D‑H et al (2013) A practical scoring system to determine whether to proceed with surgical resection in recurrent glioblastoma. Neuro Oncol 15:1096–1101. https://doi.org/10.1093/neuonc/not069
Combs SE, Thilmann C, Edler L et al (2005) Efficacy of fractionated stereotactic reirradiation in recurrent gliomas: long-term results in 172 patients treated in a single institution. J Clin Oncol 23:8863–8869. https://doi.org/10.1200/JCO.2005.03.4157
Grosu AL, Weber WA, Franz M et al (2005) Reirradiation of recurrent high-grade gliomas using amino acid PET (SPECT)/CT/MRI image fusion to determine gross tumor volume for stereotactic fractionated radiotherapy. Int J Radiat Oncol Biol Phys 63:511–519. https://doi.org/10.1016/j.ijrobp.2005.01.056
Wick W, Puduvalli VK, Chamberlain MC et al (2010) Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol 28:1168–1174. https://doi.org/10.1200/JCO.2009.23.2595
Wick A, Pascher C, Wick W et al (2009) Rechallenge with temozolomide in patients with recurrent gliomas. J Neurol 256:734–741. https://doi.org/10.1007/s00415-009-5006-9
Weller M, Tabatabai G, Kästner B et al (2015) MGMT promoter methylation is a strong prognostic biomarker for benefit from dose-intensified temozolomide rechallenge in progressive glioblastoma: the DIRECTOR trial. Clin Cancer Res 21:2057–2064. https://doi.org/10.1158/1078-0432.CCR-14-2737
Combs SE, Edler L, Rausch R et al (2013) Generation and validation of a prognostic score to predict outcome after re-irradiation of recurrent glioma. Acta Oncol 52:147–152. https://doi.org/10.3109/0284186X.2012.692882
Barney C, Shukla G, Bhamidipati D, Palmer JD (2017) Re-irradiation for recurrent glioblastoma multiforme. Chin Clin Oncol 6:36. https://doi.org/10.21037/cco.2017.06.18
Suchorska B, Weller M, Tabatabai G et al (2016) Complete resection of contrast-enhancing tumor volume is associated with improved survival in recurrent glioblastoma-results from the DIRECTOR trial. Neuro Oncol 18:549–556. https://doi.org/10.1093/neuonc/nov326
Ryu S, Buatti JM, Morris A et al (2014) The role of radiotherapy in the management of progressive glioblastoma: a systematic review and evidence-based clinical practice guideline. J Neurooncol 118:489–499. https://doi.org/10.1007/s11060-013-1337-6
Hudes RS, Corn BW, Werner-Wasik M et al (1999) A phase I dose escalation study of hypofractionated stereotactic radiotherapy as salvage therapy for persistent or recurrent malignant glioma. Int J Radiat Oncol Biol Phys 43:293–298. https://doi.org/10.1016/S0360-3016(98)00416-7
Lawrence YR, Li XA, el Naqa I et al (2010) Radiation dose—volume effects in the brain. Int J Radiat Oncol Biol Phys 76:S20–S27. https://doi.org/10.1016/j.ijrobp.2009.02.091
Mayer R, Sminia P (2008) Reirradiation tolerance of the human brain. Int J Radiat Oncol Biol Phys 70:1350–1360. https://doi.org/10.1016/j.ijrobp.2007.08.015
Siepmann DB, Siegel A, Lewis PJ (2005) Tl-201 SPECT and F‑18 FDG PET for assessment of glioma recurrence versus radiation necrosis. Clin Nucl Med 30:199–200
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972. https://doi.org/10.1200/JCO.2009.26.3541
Straube C, Scherb H, Gempt J et al (2018) Adjuvant stereotactic fractionated radiotherapy to the resection cavity in recurrent glioblastoma—the GlioCave study (NOA 17 - ARO 2016/3 - DKTK ROG trial). BMC Cancer 18:15. https://doi.org/10.1186/s12885-017-3928-7
Combs SE, Bischof M, Welzel T et al (2008) Radiochemotherapy with temozolomide as re-irradiation using high precision fractionated stereotactic radiotherapy (FSRT) in patients with recurrent gliomas. J Neurooncol 89:205–210. https://doi.org/10.1007/s11060-008-9607-4
Osman MAM (2014) Phase II trial of temozolomide and reirradiation using conformal 3D-radiotherapy in recurrent brain gliomas. Ann Transl Med 2:44. https://doi.org/10.3978/j.issn.2305-5839.2014.05.06
Scholtyssek F, Zwiener I, Schlamann A et al (2013) Reirradiation in progressive high-grade gliomas: outcome, role of concurrent chemotherapy, prognostic factors and validation of a new prognostic score with an independent patient cohort. Radiat Oncol 8:161. https://doi.org/10.1186/1748-717X-8-161
Fogh SE, Andrews DW, Glass J et al (2010) Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol 28:3048–3053. https://doi.org/10.1200/JCO.2009.25.6941
Arcicasa M, Roncadin M, Bidoli E et al (1999) Reirradiation and lomustine in patients with relapsed high-grade gliomas. Int J Radiat Oncol Biol Phys 43:789–793
Winkler F, Osswald M, Wick W (2018) Anti-angiogenics: their role in the treatment of glioblastoma. Oncol Res Treat 41:181–186. https://doi.org/10.1159/000488258
Khasraw M, Ameratunga MS, Grant R et al (2014) Antiangiogenic therapy for high-grade glioma. Cochrane Database Syst Rev 9:CD8218. https://doi.org/10.1002/14651858.CD008218.pub3
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A. Baehr, D. Trog, M. Oertel, S. Welsch, K. Kröger, O. Grauer, U. Haverkamp, and H.T. Eich declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants or on human tissue were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards and modifications and in accordance with good clinical practice guidelines. Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Baehr, A., Trog, D., Oertel, M. et al. Re-irradiation for recurrent glioblastoma multiforme: a critical comparison of different concepts. Strahlenther Onkol 196, 457–464 (2020). https://doi.org/10.1007/s00066-020-01585-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-020-01585-0