Abstract
Objective
Regeneration of autologous bone stock and formation of a stable implant bed by impaction of morselized bone allograft.
Indications
Bone loss after septic and aseptic loosening or tumour resection.
Contraindications
Persistent infection, one-stage septic revision, poor therapeutic compliance, extensive uncontained metaphyseal defects with cortical thinning of the diaphysis.
Surgical technique
Whilst the surgeon removes the loose prosthesis, the assistant prepares the graft. The medullary canal is sealed with a cement restrictor. Graft particles of different sizes are densely impacted around a trial stem. The highest level of stability is achieved by using large particles interspersed with small filler particles. Low-viscosity cement facilitates cement penetration and ensures strong interdigitation with the impacted graft mass after implantation of the prosthesis. Uncontained metaphyseal defects are treated with prosthetic augments.
Postoperative management
Gait training, physiotherapy with isometric quadriceps exercises, partial weight-bearing for 6 weeks, resistance training begins 8 weeks postoperatively.
Results
Between 2010 and 2012, 28 patients with large bone defects [Anderson Orthopaedic Research Institute (AORI) grade: 21 × F3, 3 × F2, 13 × T3, 8 × T2] underwent total knee revision with impaction bone grafting. The mean follow-up was 27.7 months (range 21–47 months). On average, patients had undergone 2.5 previous revisions. Implant survival was 82.0 % (95 % CI = 62.5 %–92.1 %) for any reason of revision as the endpoint and 93.1 % (95 % CI = 74.5–98.4 %) for aseptic revision as the endpoint. The mean postoperative Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score was 35.4 (range 3.3–101.6, SD ± 26.2). The mean KSS was 70.6 (range 20–100, SD ± 26.8).
Zusammenfassung
Operationsziel
Regeneration autologer Knochensubstanz und Schaffung eines stabilen Implantatlagers durch Impaktion allogener Knochenchips.
Indikationen
Knochensubstanzverlust nach aseptischer und septischer Lockerung oder Tumorresektion.
Kontraindikationen
Persistierende Infektsituation, einzeitiger septischer Wechsel, fehlende Patientencompliance, ausgedehnte offene metaphysäre Defekte und kortikale Ausdünnung der Diaphyse.
Operationstechnik
Während der Entfernung der einliegenden Prothese wird das Transplantat bearbeitet und für die Implantation vorbereitet. Der Markkanal wird mit einem Zementstopper versiegelt. Transplantatpartikel unterschiedlicher Größe werden möglichst dicht gepackt um einen Probestiel eingebracht. Die höchste Stabilität gewährleisten hierbei möglichst große Partikel, die mit kleinen Füllerpartikeln durchsetzt sind. Während der Prothesenimplantation führt die Verwendung von Knochenzement mit geringer Viskosität zu optimaler Interdigitation mit den Transplantatpartikeln. Implantataugmentate dienen der Füllung von in sich nicht geschlossenen metaphysären Defekten.
Weiterbehandlung
Gangschule, Physiotherapie mit isometrischer und funktioneller Beübung des M. Quadriceps, Teilbelastung der operierten Extremität für 6 Wochen, Kräftigungsübungen gegen Wiederstand nach frühestens 8 Wochen.
Ergebnisse
Zwischen 2010 und 2012 wurde bei 28 Patienten mit periartikulären ausgedehnten Knochendefekten [AORI-Grad (Anderson Orthopaedic Research Institute): 21 × F3, 3 × F2, 13 × T3, 8 × T2] ein Knieprothesenwechsel durchgeführt und eine Defektauffüllung durch Impaktierung von Spenderknochen vorgenommen. Das durchschnittliche Follow-up betrug 27,7 Monate (21–47 Monate). Im Durchschnitt waren pro Patient 2,5 vorausgehende Wechseloperationen erfolgt. Das Implantatüberleben betrug 82,0 % (95 %-KI = 62,5–92,1 %) für den Endpunkt jegliche Revision und 93,1 % (95 %-KI = 74,5–98,4 %) für den Endpunkt aseptischer Wechseleingriff. Der mittlere postoperative WOMAC-Score (Western Ontario and McMaster Universities Osteoarthritis Index) lag bei 35,4 (3,3–101,6; Standardabweichung, SD: ± 26,2), der mittlere KSS (Knee Society Score) bei 70,6 (20–100; SD: ± 26,8).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introductory remarks
Between 2008 and 2012, the number of revision total knee arthroplasties performed in Germany increased by 61 % [1]. Similar observations have been made in the US, where the number of revision knee arthroplasties is predicted to increase by 600 % by 2030 [2]. These projections may give orthopedic specialists a foretaste of the surgical problems they will face unless more sophisticated treatment concepts can be developed.
Given the increasing rates of total knee revision, it is not unusual that patients present with a history of multiple revisions. Thus the treatment of infections, arthrofibrosis, soft tissue defects and bone loss has become routine in specialized departments. According to data obtained by the AQUA institute, more than 20 % of all knee revisions performed in Germany are associated with radiographically proven femoral or tibial bone loss [1]. However, these numbers are undoubtedly underestimated, since many defects only become apparent intraoperatively after removal of the prosthetic components and thorough debridement of necrotic soft tissue [3]. In North America and Central Europe, long-stemmed prostheses have traditionally been used in combination with metal augments to treat larger bone defects in revision knee arthroplasty. However, this treatment concept focuses on the replacement of damaged bone with permanent implants rather than on the repair and reconstitution of tissue structures and function [4]. Particularly in younger patients where multiple revisions can be expected, this technique may create a vicious cycle of bone loss due to stress shielding, instability, osteolysis, and/or infection [5]. Bone impaction grafting, however, might be an alternative therapeutic strategy to minimize further bone loss and additionally restore endogenous bone stock. This technique was first reported in 1975 by Hastings and Parker for the treatment of acetabular defects [6], but has been refined and studied most extensively by Slooff et al. [7, 8]. It was adopted in 1984 for the treatment of proximal femur defects by Gie et al. [9] and its use for the treatment of bone defects around the knee was first reported by Ullmark and Hovelius in 1996 [10]. Since then, several authors have reported good clinical results using the technique described in the following article [10, 11, 12, 13, 14, 15, 16, 17].
The biology of the impacted graft mass needs to be appreciated from an understanding of the processes of osteoconduction and osteoinduction. The grafted bone particles serve as a porous scaffold that facilitates the ingrowth of host blood vessels and tissue, while the morselization procedure releases growth factors from the fracture surface of the graft [18]. This leads to a recruitment of host-derived osteoprogenitors and mononuclear cells whose progeny are the key cellular players in the process of “creeping substitution” in which the grafted material is slowly replaced by newly formed host bone [19]. While prima facie it seems desirable to achieve fast and full incorporation and remodeling of the impacted morselized bone, we know today from a plethora of preclinical and clinical studies that this is not a conditio sine qua non to achieve good clinical outcomes [18]. In fact, it has been shown that some parts of the graft material do not get remodeled even after prolonged periods of implantation, especially when they are not mechanically loaded [20]. However, this unremodeled composite material consisting of densely packed bone particles, fibrous tissue and interdigitating cement which is not in direct contact with osteogenic host elements, is usually able to provide sufficient stability to ensure permanent and stable fixation of the implant [21, 22].
For optimal clinical outcomes, a thorough understanding of the biomechanical principles and surgical technique of impaction bone grafting is required. While there are biological and patient-inherent factors, such as vascularisation and osteogenic capacity of the remaining bone stock that hardly can be influenced by the surgeon, he can still take measures to control the rate of bone remodeling and the stability of the composite construct. One important factor is the size of the graft particles used. Larger particles offer higher mechanical stability and shear resistance and provide larger voids for better vascularisation and cement penetration than smaller particles [23, 24]. The biomechanical performance of the graft can be even further enhanced when small filler particles are used in between the large particles. The use of very small slurry grafts should be avoided as this is associated with a high risk for stem subsidence [25, 26]. Another important factor to consider is the energy used to impact the graft. Higher and consistent impaction energy improves initial stability of the construct but decreases re-vascularisation capacity and bony ingrowth [21]. Therefore, over-impaction leading to fracture of the particles should be avoided. On the other hand, it has been shown that loose impaction results in fast resorption of the graft, increasing the risk for subsidence of the prosthesis [21].
Surgical principle and objective
Tibial and femoral cavitary defects are filled with impacted morselized bone allograft. A neo-medullary canal is created by using trial implants and bone tamps. Modular revision stems are cemented into the densely packed graft bed to create a stable composite construct consisting of graft particles and interdigitating cement on one side and the implant and its surrounding cement mantle on the other. Graft preparation and impaction are key technical steps determining the rate of bone remodeling and the stability of the composite construct. Non-contained defects are treated with additional metal augments or meshes. Native joint line and posterior condylar offset are restored in order to achieve normal knee kinematics.
Advantages
-
Reduction of further bone loss
-
Restoration of host bone stock by incorporation of the graft material—particularly advantageous in younger patients where future revisions are anticipated
-
The technique provides the possibility to fill irregular defects. No need for further removal of vital bone for the fixation of the implant
-
May be used in posttraumatic or congenital femoral or tibial deformities where the treatment of bone defects by large cavitary-filling stems or sleeves is difficult
-
The technique can be combined with almost every modular knee revision system and can be used in a wide variety of clinical situations
Disadvantages
-
Technically highly demanding procedure
-
Prolonged operative time
-
Potential immunological reaction or transmission of infectious diseases
-
Access to femoral heads from a bone bank is required
Contraindications
-
Poor therapeutic compliance
-
Persistent infection
-
One-stage septic revision
-
Extensive uncontained and open metaphyseal defects combined with severe cortical thinning or perforation of the diaphysis
Patient information
-
Potential risk of infectious disease transmission associated with the use of donor bone
-
General risks of surgery (thrombosis, embolism, vascular or neural lesions, haematoma, infection, wound complications)
-
Intraoperative fracture, iatrogenic tendon or ligament lesions
-
Arthrofibrosis, malalignment or instability
-
Aseptic or septic loosening
-
In case of persistent infection: debridement, irrigation and antibiotic-impregnated cement spacer implantation instead of bone impaction grafting and re-implantation of the prosthesis
-
Postoperative management concept: partial weight-bearing for 6 weeks
Preoperative work-up
-
Standard work-up for revision knee arthroplasty in consultation with an anaesthetist.
-
Management of anticoagulation therapy.
-
Metabolic diseases should be appropriately treated.
-
Crossmatching and blood tests (including inflammatory blood laboratory markers such as CRP, WBC, ERC).
-
Intraoperative cell-saver therapy or autologous blood donation.
-
Joint aspiration (with gram stain, cell count, differential and culture for at least 13 days [29]) is in our view mandatory in the case of previous prosthetic infection, elevated levels of inflammatory blood markers or clinical signs of infection; for the diagnosis of periprosthetic joint infection we follow the AAOS guidelines given by the Workgroup of the Musculoskeletal Infection Society [30, 31].
-
Antibiotic-free interval of at least 14 days prior to joint aspiration or revision arthroplasty.
-
Radiographs of the knee (anteroposterior with the patient standing, true lateral with the knee flexed at 30° and tangential view of the patella represent the minimum evaluation; we recommend an additional 52-inch cassette three-joint view for the assessment of overall alignment).
-
Digital planning with assessment of the implant’s type, size, location, level of constraint and need for stems or augments. The common assumption that the joint line is located 15 mm above the most proximal point of the fibula or 25–30 mm distal from the medial femoral epicondylus is an approximate one as there are large inter-individual differences [32]. Ideally, the exact position of the joint line and the length of the posterior condylar offset are determined on initial radiographs without any prosthesis implanted or alternatively on the contralateral side.
Instruments and implants
-
Standard modular knee revision system
-
Implant extraction instruments including straight and curved chisels, thin saw blades and a slap hammer (e.g. RENOVATION, Implant Removal System, Smith & Nephew, Marl, Germany); Jet lavage
-
Straight and curved bone tamps, mallet
-
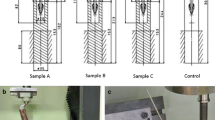
Bone mill (e.g. Noviomagus Bone Mill, Spierings Orthopaedics B.V., Nijmegen, Netherlands; distributed in Germany by Peter Brehm GmbH) with varying drum sizes (Fig. 1) and large rongeurs to create different morsel sizes
-
Femoral head fixation device to remove cartilage remnants (e.g. Noviomagus Bone Vice, Reamer Set)
-
Low viscosity cement (e.g. PALACOS LV + Gentamycin, Zimmer Medical Systems, Neu-Ulm, Germany or Simplex P + Tobramycin, Stryker, Duisburg, Germany), delivery system with long nozzle, cement plugs
Commercially available bone mill with five different drum sizes. The particles shown have cross sections from 1 × 1 to 8 × 8 mm and mean lengths from 1 to 15 mm [33])
Anesthesia and positioning
-
General or spinal anesthesia.
-
Spinal or epidural analgesia during and after the operation via an infusion pump, which can be postoperatively controlled by the patient themselves (patient-controlled anaesthesia, PCA). Alternatively, a femoral nerve block may be performed, which can also be applied in a patient-controlled manner.
-
Supine positioning. A pneumatic tourniquet is applied at the patient’s proximal thigh after cotton padding. Scrubbing of the leg from tourniquet to toe, marking of the scar and drawing of transverse lines before draping the leg and covering the knee with iodophor-impregnated incise drapes. The pneumatic tourniquet is filled during the cementation procedure or in case of extensive bleeding.
-
Intravenous antibiotic prophylaxis (2nd or 3rd generation cephalosporin, e.g. Cefazolin 1.0 g i.v.) after obtaining samples for histological and microbiological analyses.
Surgical technique
(Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6)
Graft preparation: Whilst the surgeon is removing the prosthesis and cleaning the implant bed of cement remnants or necrotic tissue debris, the assistant is preparing the graft. Fresh frozen femoral heads are reconstituted in 37 °C saline for 10 min. Cartilage is carefully removed using a large rongeur or concave femoral head reamer as described by Petheram and Howell [34]. This is an important step as cartilage can have adverse effects on stability and incorporation of the graft particles [35]. To fill the defect, we use both milled graft particles and particles created with a large rongeur to achieve a mixture of particles with a graded size distribution. Such an aggregate of different particle sizes (mostly large with some filler particles) acts as a porous scaffold, which has been demonstrated to be most resistant to shear stress and to allow fluid escape [23, 26]. A sterile gauze sponge is unfolded and the graft particles are placed in its centre to form a little bag which serves as a sieve. Then the particles are repeatedly rinsed using jet lavage to remove bone marrow and fat. This washing step has been shown to improve shear strength of the impacted graft material and to stimulate ingrowth of host bone [36, 37]. Finally, the graft particles are dried by compressing them between two gauze sponges to decrease lubrication and increase frictional resistance [26]
Positioning of the medullary canal plug: The medullary canal is sealed with a stable cement restrictor to prevent dissemination of the graft particles beyond the diaphyseal isthmus. However, in cases with large cavitary defects and widened medullary canal, this can be difficult as most commercially available cement restrictors do not have dimensions large enough to plug the deficient isthmus. For this reason we use a custom made restrictor formed by sewing the edges of three commercially available cement restrictors (ETHISORB absorbable femur plugs, ETHICON, Johnson&Johnson) together using 3-0 Vicryl sutures [38]
Graft placement and impaction: The tibia is prepared first. Later it provides the reference point from which to balance the flexion and extension gaps provided that the collateral ligaments are structurally intact. Uncontained proximal defects are either converted into contained defects using metal meshes or replaced by tibial wedges. We prefer the second technique. Debris and fluid are completely removed before grafting. Aspenberg and colleagues demonstrated that mechanical loading of the graft increases the rate of graft remodeling [20], whereas strong impaction of the particles decreases remodeling [21]. Total and fast remodeling results in fast resorption of the graft, which might weaken the construct and lead to subsidence of prosthetic components. Therefore, an equilibrium is desired between graft resorption and apposition of new bone. This is best achieved by strong impaction of large particles interspersed with small filler particles of different sizes [18, 26]. The dimension of the bone defect and the size of the medullary canal determine the particle size of the graft particles that can be used. Diaphyseal defects with a flute-like eroded medullary canal do not allow the use of large particles. In this situation, the impaction process is started by filling loosely impacted small particles (2 mm) into the distal defect close to the cement restrictor. Then a trial stem one size larger in diameter than the planned final stem is positioned in the centre of the widened medullary canal by pushing it into the distally located loosely packed particles. This keeps the trial stem in a fixed central position to allow strong impaction around it from distally to proximally. The particle size that can be used increases from the tip of the stem to the metaphysis. For large cavitary defects of the metaphysis (AORI grade 2 and 3), we usually prepare 7- to 15-mm particles using a large rongeur and mix them with 2- to 5-mm milled particles at a ratio of approximately 5:1 (a). Curved bone tamps of different sizes are used in combination with a small mallet to ensure a consistently impacted graft mass (b)
Cementing technique and reconstruction of the tibial plateau: The preoperatively determined position of the native joint line serves as a reference for the anatomical reconstruction of the tibial plateau. Insufficient height of the reconstructed plateau will inevitably result in difficulties balancing the knee during the femoral preparation. After impaction, the trial stem is removed, leaving a bed of strongly impacted particles behind that form a neo-medullary canal. This canal allows an extra 2 mm cement mantle to be used with the definitive implant (a). A cement gun with a long nozzle is used for cement pressurization and retrograde filling of the canal (b). Low-viscosity cement facilitates cement penetration and ensures strong interdigitation with the impacted graft material after implantation of the prosthesis (c). Rotational alignment of the tibial component is referenced at the lateral border of the medial third of the tibial tubercle and/or the tibial margo anterior (boarder between proximal and middle third of the total tibial length)
Femur preparation: In cases of severe cortical thinning of the metaphyseal bone, cerclage wiring is recommended before impaction in order to prevent periprosthetic fractures [39]. The implant bed of the femur is prepared in the same way as described for the tibia. Grafting of particles into uncontained defects is unacceptable. Loose particles might impinge during motion and lead to wear of the components. Posterior condyle defects need to be replaced by a modular prosthesis if it is not possible to contain the graft material. Bone loss or scar tissue might make it difficult to reference the femoral component rotation by means of the transepicondylar or trochlear anteroposterior axis. In these cases we reference the femoral component rotation parallel to the resection of the tibia in a 90° tensioned flexion gap [40]. If this is also not possible due to ligament insufficiency, the preoperatively measured femoral α-angle can be used to determine the trochlear anteroposterior axis in relation to the femoral anatomical axis. The latter can be determined using an intramedullary guide. The femoral component is then positioned perpendicular to the trochlear anteroposterior axis. Its size is chosen next but must not be a sole function of the remaining bone as this might lead to implantation of an undersized femoral component [41, 42]. The preoperatively planned posterior femoral offset can be reconstructed using a large femur shield or posterior modular augments. This should lead to stable conditions in particular in flexion. The extension gap can then be modified by the use of distal augments or, if not otherwise possible, by the use of a higher tibial insert [42]. However, the latter will inevitably result in an elevation of the joint line. If one of the two gaps cannot be stabilized, a constrained implant with stem extensions needs to be used. Before cementation of the definitive implant into the neo-medullary canal, we control patella tracking and intraoperative range of motion with the trial implants in place
Postoperative management
-
Sterile wound dressing
-
Anticoagulation therapy starting with PTT adjusted unfractionated heparin (150 IU/kg body weight/24 h i.v.) for the first 24 h postoperatively. Then administration of low-molecular weight heparins until full weight-bearing is achieved (under regular monitoring of anti-Xa activity and thrombocyte numbers)
-
Pain management according to WHO guidelines
-
Suction drains are removed after 24–48 h depending on the blood volume collected (should be < 100 ml/24 h).
-
Continuous passive motion therapy
-
Mobilization starting on the first postoperative day, gait training on even surfaces, partial weight-bearing for 6 weeks
-
Physiotherapy with strength training focusing on isometric and functional quadriceps control, resistive exercises are initiated after 8 weeks
Errors, hazards and complications
-
In cases of severe cortical thinning of the femoral condyles, there is a high risk of fracture during impaction which can be prevented by using cerclage wires; if the surgeon decides against prophylactic wiring, prolonged partial weight-bearing should be recommended.
-
Insufficient sealing of the medullary canal leads to dissemination of the graft particles beyond the diaphyseal isthmus. During cementation this might lead to cement filling of the diaphysis and prevent proper pressurization. As a result there will be inadequate coherence of the distal part of the graft-cement composite around the implant, which affects the stability of the prosthesis.
-
Periprosthetic infection: Explantation of the prosthesis and graft material is mandatory. Arthrodesis or amputation should be discussed with the patient.
-
Graft subsidence and loosening of the prosthesis: Revision arthroplasty and re-grafting is possible provided that infection is ruled out.
Results
Between April 2010 and June 2012, 28 consecutive patients (13 female, 15 male) underwent total knee revision with impaction bone grafting. The mean follow-up period was 27.7 months (range 21–47 months). Mean patient age at the time of surgery was 66.5 years (range 47–86 years). Bone defects were graded according to the AORI classification (21 × F3, 3 × F2, 13 × T3, 8 × T2). On average, patients had undergone 2.5 previous revisions. In all, 10 patients had a history of previous infection or were treated with a two-stage procedure due to infection. All operations were performed at the Department of Orthopaedics, Koenig-Ludwig Haus of the University of Wuerzburg. A rotating hinge prosthetic system with stem extensions was used in 25 patients (22 × RHK system, Biomet; 2 × MRH, Stryker; 1 × LINK Endo-Model). A semi-constrained prosthesis with stem extension was implanted in three patients (Sigma-TC3, DePuySynthes). The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) [43] and the Knee Society Score (KSS) [44] were used to measure the efficacy of the surgical procedure. Values were recorded as mean, range and standard deviation (SD). SigmaStat 3.5® software was used for statistical analyses. The Saphiro-Wilk test revealed that the data was normally distributed. Therefore, the student’s t-test was applied to test for statistically significant differences between the pre- and postoperative status. Implant survival was evaluated by Kaplan-Meier analysis.
Re-revisions were necessary in five patients. Distal femoral amputation was necessary in two patients with clinical and serological signs of a deep infection. These patients had a history of three and eight previous septic revisions, respectively. Another patient developed a new infection. He was treated by removal of the prosthesis and arthrodesis. Two cases were revised due to aseptic loosening of the tibial or femoral component. There were no other implant-related complications. Implant survival at 2 years of follow-up was 82.0 % (95 % CI = 62.5–92.1 %) (Fig. 7) for any reason for revision as the endpoint and 93.1 % (95 % CI = 74.5–98.4 %) for aseptic revision as the endpoint. The mean WOMAC score was 79.8 (range 9–117, SD ± 26.7) preoperatively and 35.4 (range 3.3–101.6, SD ± 26.2) postoperatively (P < 0.001). The mean KSS was 32.5 (range 0–90, SD ± 26.3) preoperatively and 70.6 (range 20–100, SD ± 26.8) postoperatively (P < 0.001).
This study demonstrates that impaction bone grafting can lead to good mid-term clinical outcomes in revision knee arthroplasty and is suitable to treat even large bone defects around the knee (Fig. 8).
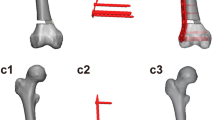
A 57 year old male patient with a history of a distal femur fracture during childhood and two-stage revision surgery due to infection with Propionibacterium acnes at the age of 56 years. At the index revision procedure, a hinged knee prosthesis (LINK) was implanted. The preoperative radiographs demonstrated radiolucencies at the cement–bone interface and extensive femoral and tibial bone loss (AORI F3T3) (a). Preoperative joint aspiration revealed once again an infection with Propionibacterium acnes. The prosthesis was removed, a custom-made antibiotic-impregnated cement spacer was implanted and i.v. antibiotic therapy was initiated (b). Joint aspiration after a 14-day antibiotic-free interval was negative. The large cavitary bone defects were filled with impacted morselized grafts obtained from three femoral heads and a rotating hinge knee prosthesis (RHK system, Biomet) was implanted. Control radiographs 1 year postoperatively showed a stable prosthesis and a partially remodeled graft mass (c). The preoperative KSS increased from 20 preoperatively to 90 postoperatively
References
Institut A (2013) 17/7—Knieendoprothesenwechsel und -Komponentenwechsel; Bundesauswertung zum Erfassungsjahr 2012. https://www.sqg.de/downloads/Bundesauswertungen/2012/bu_Gesamt_17N7-KNIE-WECH_2012.pdf. Accessed 03 Mar 2014
Kurtz S, Ong K, Lau E et al (2007) Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 89(4):780–785. doi:10.2106/JBJS.F.00222
Reish TG, Clarke HD, Scuderi GR et al (2006) Use of multi-detector computed tomography for the detection of periprosthetic osteolysis in total knee arthroplasty. J Knee Surg 19(4):259–264
Holzapfel BM, Reichert JC, Schantz JT et al (2013) How smart do biomaterials need to be? A translational science and clinical point of view. Adv Drug Deliv Rev 65(4):581–603. doi:10.1016/j.addr.2012.07.009
Toms AD, Barker RL, Jones RS, Kuiper JH (2004) Impaction bone-grafting in revision joint replacement surgery. J Bone Joint Surg Am 86-A(9):2050–2060
Hastings DE, Parker SM (1975) Protrusio acetabuli in rheumatoid arthritis. Clin Orthop Relat Res 108:76–83
Slooff TJ, Huiskes R, Horn J van, Lemmens AJ (1984) Bone grafting in total hip replacement for acetabular protrusion. Acta Orthop Scand 55(6):593–596
Schreurs BW, Slooff TJ, Buma P, Verdonschot N (2001) Basic science of bone impaction grafting. Instr Course Lect 50:211–220
Gie GA, Linder L, Ling RS et al (1993) Impacted cancellous allografts and cement for revision total hip arthroplasty. J Bone Joint Surg Br 75(1):14–21
Ullmark G, Hovelius L (1996) Impacted morsellized allograft and cement for revision total knee arthroplasty: a preliminary report of 3 cases. Acta Orthop Scand 67(1):10–12
Benjamin J, Engh G, Parsley B et al (2001) Morselized bone grafting of defects in revision total knee arthroplasty. Clin Orthop Relat Res 392:62–67
Bradley GW (2000) Revision total knee arthroplasty by impaction bone grafting. Clin Orthop Relat Res 371:113–118
Heyligers IC, Haaren EH van, Wuisman P (2001) Revision knee arthroplasty using impaction grafting and primary implants. J Arthroplasty 16(4):533–537. doi:10.1054/arth.2001.22391
Lonner JH, Lotke PA, Kim J, Nelson C (2002) Impaction grafting and wire mesh for uncontained defects in revision knee arthroplasty. Clin Orthop Relat Res 404:145–151
Lotke PA, Carolan GF, Puri N (2006) Technique for impaction bone grafting of large bone defects in revision total knee arthroplasty. J Arthroplasty 21(4 Suppl 1):57–60. doi:10.1016/j.arth.2006.01.019
Steens W, Loehr JF, Wodtke J, Katzer A (2008) Morselized bone grafting in revision arthroplasty of the knee: a retrospective analysis of 34 reconstructions after 2–9 years. Acta Orthop 79(5):683–688. doi:10.1080/17453670810016713
Loon CJ van, Wijers MM, Waal Malefijt MC de et al (1999) Femoral bone grafting in primary and revision total knee arthroplasty. Acta Orthop Belg 65(3):357–363
Tagil M, Aspenberg P (2004) Impaction grafting: how does it work? In: Delloye C, Bannister G (eds) Impaction bone grafting in revision arthroplasty, 1st edn. Marcel Dekker, Inc., New York, pp 205–224
Phemister DB (2008) The classic: repair of bone in the presence of aseptic necrosis resulting from fractures, transplantations, and vascular obstruction. Clin Orthop Relat Res 466(5):1021–1033. doi:10.1007/s11999-008-0206-7
Wang JS, Tagil M, Aspenberg P (2000) Load-bearing increases new bone formation in impacted and morselized allografts. Clin Orthop Relat Res 378:274–281
Tagil M, Aspenberg P (1998) Impaction of cancellous bone grafts impairs osteoconduction in titanium chambers. Clin Orthop Relat Res 352:231–238
Tagil M, Aspenberg P (2001) Fibrous tissue armoring increases the mechanical strength of an impacted bone graft. Acta Orthop Scand 72(1):78–82. doi:10.1080/000164701753606743
Cornu O, Schubert T, Libouton X et al (2009) Particle size influence in an impaction bone grafting model. Comparison of fresh-frozen and freeze-dried allografts. J Biomech 42(14):2238–2242. doi:10.1016/j.jbiomech.2009.06.045
Xu ZJ, Chen LY, Zhong C et al (2011) Mechanical properties of 7–10 mm bone grafts and small slurry grafts in impaction bone grafting. J Orthop Res 29(10):1491–1495. doi:10.1002/jor.21357
Brewster NT, Gillespie WJ, Howie CR et al (1999) Mechanical considerations in impaction bone grafting. J Bone Joint Surg Br 81(1):118–124
Dunlop D (2004) Impaction bone grafting: a mechanical appraisal with reference to soil engineering. In: Delloye C, Bannister G (eds) Impaction bone grafting in revision arthroplasty. Marcel Dekker, Inc., New York, pp 57–74
Engh GA (1997) Bone defect classification. In: Engh GA, Rorabeck CH (eds) Revision total knee arthroplasty. Lippincott Williams & Wilkins, Baltimore, pp 63–120
Engh GA, Ammeen DJ (1998) Classification and preoperative radiographic evaluation: knee. Orthop Clin North Am 29(2):205–217
Schafer P, Fink B, Sandow D et al (2008) Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis 47(11):1403–1409. doi:10.1086/592973
Parvizi J, Zmistowski B, Berbari EF et al (2011) New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res 469(11):2992–2994. doi:10.1007/s11999-011-2102-9
Della Valle C, Parvizi J, Bauer TW et al (2011) American Academy of Orthopaedic Surgeons clinical practice guideline on: the diagnosis of periprosthetic joint infections of the hip and knee. J Bone Joint Surg Am 93(14):1355–1357. doi:10.2106/JBJS.9314ebo
Servien E, Viskontas D, Giuffre BM et al (2008) Reliability of bony landmarks for restoration of the joint line in revision knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 16(3):263–269. doi:10.1007/s00167-007-0449-y
Spierings Orthopaedics: Noviomagus Bone Mill. http://www.spierings.biz/index.php/de/products/noviomagus-bone-mill. Accessed 02 Jun 2014
Petheram TG, Howell JR (2014) The Exeter method-acetabular impaction grafting with cemented reimplantation. Oper Orthop Traumatol 26(2):114–125. doi:10.1007/s00064-013-0273-0
Donk S van der, Buma P, Slooff TJ et al (2002) Incorporation of morselized bone grafts: a study of 24 acetabular biopsy specimens. Clin Orthop Relat Res 396:131–141
Dunlop DG, Brewster NT, Madabhushi SP et al (2003) Techniques to improve the shear strength of impacted bone graft: the effect of particle size and washing of the graft. J Bone Joint Surg Am 85-A(4):639–646
Donk S van der, Weernink T, Buma P et al (2003) Rinsing morselized allografts improves bone and tissue ingrowth. Clin Orthop Relat Res 408:302–310
Holzapfel BM, Noth U, Rudert M (2014) A simple technique to seal the medullary canal in cemented revision arthroplasty. Eur J Orthop Surg Traumatol. doi:10.1007/s00590-014-1530-0
Holzapfel BM, Prodinger PM, Hoberg M et al (2010) Periprosthetic fractures after total hip arthroplasty: classification, diagnosis and therapy strategies. Orthopade 39(5):519–535. doi:10.1007/s00132-010-1612-6
Stiehl JB, Cherveny PM (1996) Femoral rotational alignment using the tibial shaft axis in total knee arthroplasty. Clin Orthop Relat Res 331:47–55
Vince KG, Droll KP, Chivas D (2007) Your next revision total knee arthroplasty: why start in flexion? Orthopedics 30(9):791–792
Hube R, Mayr HO, Kalteis T, Matziolis G (2011) Extension first technique for TKA implantation. Oper Orthop Traumatol 23(3):241–248. doi:10.1007/s00064-011-0036-8
Bellamy N, Buchanan WW, Goldsmith CH et al (1988) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15(12):1833–1840
Insall JN, Dorr LD, Scott RD, Scott WN (1989) Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res 248:13–14
Compliance with ethical guidelines
Conflict of interest. M. Rudert, B. M. Holzapfel, E. von Rottkay, D. E. Holzapfel and U. Noeth state that there are no conflicts of interest.
All studies on humans described in the present manuscript were carried out with the approval of the responsible ethics committee and in accordance with national law and the Helsinki Declaration of 1975 (in its current, revised form). Informed consent was obtained from all patients included in studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Maximilian Rudert and Boris Michael Holzapfel contributed equally to this publication.
Rights and permissions
About this article
Cite this article
Rudert, M., Holzapfel, B., von Rottkay, E. et al. Impaction bone grafting for the reconstruction of large bone defects in revision knee arthroplasty. Oper Orthop Traumatol 27, 35–46 (2015). https://doi.org/10.1007/s00064-014-0330-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00064-014-0330-3