Abstract
Purpose
It has been reported that the extent of intravascular thrombi and the quality of collateral filling in computed tomography (CT) angiography are predictive for the clinical outcome in patients with acute stroke. We hypothesized that multi-phase four-dimensional CTA (4D-CTA) allows better assessment of clot burden and collateral flow compared with arterial single-phase CTA (CTA).
Methods
In 49 patients (33 female; age: 77 ± 12 years) with acute anterior circulation stroke, CTA and 4D-CTA reconstructed from dynamic perfusion CT data were analyzed for absolute thrombus length (TL), clot burden score (CBS), and collateral score (CS). The length of the filling defect was also defined on thin-slice nonenhanced CT as corresponding hyperdense middle cerebral artery sign (HMCAS) when present.
Results
There was good correlation (r = 0.62, p < 0.01) between the length of HMCAS (1.29 ± 0.62 cm) and TL in 4D-CTA (1.22 ± 0.51 cm). 4D-CTA and CTA significantly varied (p < 0.01) in TL (1.42 ± 0.73 cm (CTA) versus 1.11 ± 0.62 cm (4D-CTA)), CBS (median: 5, interquartile range: 4–7 (CTA) versus median: 6, interquartile range: 5–8 (4D-CTA); p < 0.001), and CS (median: 2, interquartile range: 1–2 (CTA) versus median: 3, interquartile range: 2–3 (4D-CTA); p < 0.001). Accordingly, CTA significantly overrated clot burden and underestimated collateral flow.
Conclusions
4D-CTA more closely defines clot burden and collateral supply in anterior circulation stroke than CTA, implicating an additional diagnostic benefit.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Computed tomography (CT) is the most widely used imaging method in patients with acute stroke. Multi-modal stroke CT protocol (MMCT) typically includes nonenhanced CT (NECT), arterial single-phase CT angiography (CTA), and perfusion CT [1]. Besides the pure detection of the site of cerebral artery occlusion, cerebral hemodynamics gains more and more attention for both, patient selection for acute stroke therapy and early prognosis [1, 2].
The use of new-generation multi-section CT scanner enables the coverage of whole brain with volume perfusion CT (VPCT). Besides different perfusion parameters like cerebral blood flow, cerebral blood volume, and mean transit time, a multi-phase four-dimensional CT angiography (4D-CTA) can be reconstructed from the VPCT data [3]. On the basis of CTA [4] and NECT [5, 6], it was found that inferences about the response to thrombolytic or interventional stroke therapy and the consecutive clinical outcome can be drawn from absolute thrombus length (TL) and hyperdense middle cerebral artery sign (HMCAS) [4–6]. Also, the collateral vessel status has been detected to be a predictive outcome parameter [7, 8]. The use of semiquantitative clot burden score (CBS) and collateral score (CS) in acute ischemic stroke patients based on CTA gave similar results [9–12]. However, recently published studies have shown that absolute TL as well as quality and direction of collateral flow can be defined more closely in multi-phase 4D-CTA reconstructed from VPCT data than in CTA [2, 3, 13–15]. Providing a broad temporal resolution, 4D-CTA images demonstrate intracranial vessel contrast from nonenhanced through arterial and venous phase [16]. This new time-resolved multi-frame technique raises the question about the impact on the results of CBS and CS in comparison with CTA. We hypothesize that 4D-CTA shows significantly different results in the assessment of absolute TL, CBS, and CS compared with CTA in patients with acute anterior circulation ischemic stroke.
Materials and Methods
Study Population
Our study was based on a retrospective analysis of 49 patients with anterior circulation ischemic stroke (middle cerebral artery (MCA; M1 /M2 segment) ± internal carotid artery (ICA) or A1-segment occlusions) presenting from March 2012 to March 2013 with acute stroke symptoms. Onset of symptoms was < 4.5 h. All patients were treated with intravenous thrombolysis and/or intra-arterial mechanical thrombectomy. National Institutes of Health Stroke Scale (NIHSS) at presentation could be collected in all patients, and median modified Ranking scale (mRS) was collected at 3 months’ follow-up in 47 patients (96 %). Median NIHSS at presentation was 14 (range: 1–26, interquartile range: 7–18); 3-month mRS was 3 (range: 0–6, interquartile range: 2–6). All patients did undergo MMCT, including NECT, VPCT with 9.6-cm coverage in the z-axis, and bolus-tracked supra-aortic arterial CTA. Exclusion criteria were incomplete examinations, severe motion artifacts, insufficient coverage of the intracranial arteries, associated posterior circulation stroke, and signs of hemorrhage on the NECT. Mean patient age was 77 ± 12 years, with 33 of the 49 (67.3 %) being female.
The study was performed in compliance with the local ethics committee. Informed consent of the patient or next of kin was obtained prior to the examination according to legal requirements. An explicit consent for this specific study was not compulsory by the local ethics committee according to the retrospective nature of this study.
Scan Protocol
All examinations were performed on a 128-section CT scanner (Somatom Definition AS+; Siemens, Forchheim, Germany). Thin-section NECT (section thickness: 0.6 mm) was followed by VPCT and arterial CTA in all patients. Two contrast material injections were performed in each patient (for VPCT and for CTA). We used almost the same reconstruction parameters for VPCTA and CTA (section thickness: 0.6 mm, increment: 0.4 mm; reconstruction kernel B20f for CTA and H20f for 4D-CTA).
4D-CTA was reconstructed from VPCT (80 kV, 180 mAs, collimation: 128 × 0.6 mm, rotation time: 0.3 s) acquired with 9.6-cm coverage in the z-axis from the skull vertex. A total of 30 ml of iodinated contrast agent (Imeron 400; Bracco Imaging, Konstanz, Germany) followed by 50 ml of saline flush was injected via an 18-gauge cannula into a cubital vein at a rate of 5 ml/s using a double-piston power injector (Medtron, Saarbruecken, Germany). Pulsed full-rotation scan beginning 2 s after contrast injection with 35 scans over 60 s was used.
Single-phase arterial CTA was performed in caudocranial scan direction, with a coverage in the z-axis from the aortic arch to the cranial vertex (120 kV, 160 mAs, collimation: 128 × 0.6 mm, rotation time: 0.3 s). A total of 60 ml of iodinated contrast agent (Imeron 400; Bracco Imaging, Konstanz, Germany) followed by 50 ml of saline flush was injected via an 18-gauge cannula into a cubital vein at a rate of 5 ml/s. Monitoring started with a delay of 5 s. Bolus tracking was performed in the ascending thoracic aorta, with a fixed start delay of 4 s after exceeding 100 HU, resulting in arterial vessel enhancement. The whole scan duration was 4 s; the scan duration from the ascending thoracic aorta to the M1 segment was 2.5 s.
Image Reconstruction
Transversal, coronal, and sagittal maximum-intensity projection (MIP) reconstructions were generated from each CTA dataset (slice thickness: 25 mm). For 4D-CTA evaluation, temporal MIPs (tMIPs), which combine all 35 spiral scans and display maximal enhancement over the 60-s scan time for each voxel, were created through dynamic perfusion CT data (slice thickness: 25 mm).
Image data analysis was performed with commercial software (syngo.via CT Dynamic Angio; Siemens, Forchheim, Germany). All datasets were evaluated separately. Bolus-tracked single-phase CTA and 4D-CTA tMIP were assessed for TL by connecting straight lines between the proximal and distal clot end in axial or coronal planes (given that the distal end could be defined). HMCAS length was measured on thin-section NECT using a curved 3D-mode.
Semiquantitative extent of thromboembolic vessel occlusion was assessed with the CBS; semiquantitative pattern of collateral filling, the CS.
The CBS is a scoring scheme to define the extent of thromboembolic vessel occlusion in arterial segments of the anterior circulation using a scale from 10 to 0. Occlusions of the supraclinoid ICA as well as proximal and distal M1 segment count 2 points, whereas infraclinoid ICA-, anterior cerebral artery-, and M2-segment occlusions count 1 point. If there is thrombotic material found in one or more of these vessel segments, the corresponding points are subtracted from 10. The remaining points define the final score [11].
The CS indicates the collateral supply of the occluded territory scored from 0 to 3 (CS = 0: complete absence of collateral vessels, CS = 1: collateral supply ≤ 50 % and > 0 %, CS = 2: collateral supply > 50 % and < 100 %, CS = 3: collateral supply 100 %) [11].
Referring to the studies of Smit et al. [2], collateral blood supply was scored according to the anatomical extent of the collateral pathways in the territory of the MCA compared with the contralateral hemisphere at the time point of peak attenuation of these vessels. 4D-CTA and single-phase arterial CTA were also assessed with respect to timing issues. The time point of peak vessel opacification in the Sylvian fissure indicating best collateralization over the 60-s scan time was visually identified on 4D-CTA and noted.
This time point was compared with the predetermined delay in single-phase arterial CTA.
Statistical Analysis
For statistical data analysis, commercial software (SPSS 20; IBM, Chicago, IL) was used. The data evaluation was performed by two raters in consensus, one with 15 and one with 3 years of experience in acute stroke imaging. Results are described as mean ± standard deviation or as median with interquartile range. To assess the differences in absolute values of TL and delay measurements, the paired two-sample t-test was performed. The Pearson correlation was used to investigate the correlation of HMCAS on NECT and the filling defect in 4D-CTA. For the correlation statistics of ordinal parameters, the nonparametric Spearman’s rank correlation coefficient was applied. Mean differences are indicated with standard deviation, p-value, and confidence interval (CI). A p-value of < 0.05 was considered statistically significant. CI was set at 95 %. For the comparison of ordinal values as described by CBS and CS, the Wilcoxon signed-rank test as a nonparametric statistical test was used. Differences in TL and delay measurements in CTA compared with 4D-CTA were visualized with a Bland–Altman plot.
Results
Thrombus Length
TL could be defined with single-phase CTA in 24 of 49 patients (49.0 %) and with 4D-CTA in 43 of 49 patients (87.8 %). In 24 of 49 subjects (49.0 %), TL could be determined in both CTA methods. Mean TL was 1.11 ± 0.62 cm on tMIP and 1.42 ± 0.73 cm on CTA. TL was, on average, 0.31 ± 0.33 cm (p < 0.001, 95 % CI: 0.2–0.5) larger in CTA compared with tMIP. In five patients of this subgroup (5 of 24, 20.8 %), TL was exactly the same on CTA and tMIP. The results are visualized in Fig. 1.
In 19 of 49 patients (38.8 %), TL could be defined on 4D-CTA, but not on CTA. In these cases, arterial-phase CTA failed to depict the distal end of the thromboembolic occlusion.
In 6 of 49 patients (12.2 %), the exact extent of vessel occlusion could be defined neither with CTA nor with 4D-CTA.
On thin-slice NECT, 30 of 49 patients (61.2 %) showed an HMCAS. In 26 of these cases, the distal clot end could be defined on tMIP, with a mean TL of 1.22 ± 0.51 cm. Corresponding length of HMCAS of these 26 patients was 1.29 ± 0.62 cm. With p > 0.1, there was no significant difference, but good correlation (n = 26, r = 0.6, p < 0.01) between HMCAS length and TL on tMIP.
Clot Burden Score
Similarly to TL, measurement of semiquantitative CBS also varied depending on the CTA method used. Median CBS was 6 (range: 0–9, interquartile range: 5–8) on tMIP versus 5 (range: 0–9, interquartile range: 4–7) on CTA. In 24 patients (49.0 %), CBS was lower on CTA than on tMIP; in 25 patients (51.0 %), CBS was identical on CTA and tMIP. On average, CBS was 1 point (0.98 ± 1.30, p < 0.001) higher on tMIP compared with CTA. Largest difference was 5 points (1 point on CTA versus 6 points on tMIP). CBS was never lower on tMIP compared with CTA.
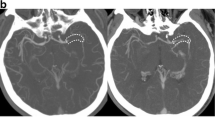
Figure 2 shows a patient (marked by a black square in Fig. 3) with considerably different results in TL and CBS for CTA and tMIP.
Comparison of single-phase arterial computed tomography angiography (CTA) and temporal maximum-intensity projection (tMIP) for clot burden score (CBS). CTA with peak arterial enhancement (a) shows undefined length of occlusion in CTA CBS, with a resulting CBS of 1 point. In contrast, tMIP with opacification of collateral filling (b) determined a thrombus length of 1.2 cm, with a resulting CBS of 5 points
Delay Measurements
Mean delay of visualization of the M1 segment after initiation of contrast injection was 23.6 ± 5.2 s for arterial CTA. Delay of optimum vessel contrast for assessment of collateral pattern on 4D-CTA was 30.0 ± 7.4 s. Hence, the delay of visualization of the M1 segment on CTA was, in mean, 6.4 ± 5.5 s shorter than the visually chosen delay of peak collateral filling on 4D-CTA (p < 0.001, 95 % CI: 4.8–7.9). In five patients (10.2 %), the chosen delay on 4D-CTA was earlier compared with CTA. The results are visualized in Fig. 3.
Collateral Score
Median CS was 3 (range: 0–3, interquartile range: 2–3; p < 0.001) for 4D-CTA and 2 (range: 0–3, interquartile range: 1–2; p < 0.001) for CTA. CS was higher on tMIP in 32 patients (65.3 %), with the largest difference of 3 points (0 points on CTA versus 3 points on tMIP). Mean difference was 1 point (0.9 ± 0.8, p < 0.001). CS was never lower on 4D-CTA compared with CTA.
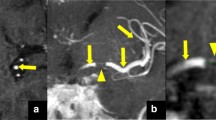
Figure 4 demonstrates an example of a patient with a significant difference in the collateral supply shown in arterial-phase CTA versus tMIP (CS: 0 versus 2 points).
Comparison of single-phase arterial computed tomography angiography (CTA) and temporal maximum-intensity projection (tMIP) for collateral score (CS). CTA with peak arterial enhancement (a) shows absent collateral supply in the occluded middle cerebral artery territory, with a resulting CS of 0 points. In contrast, tMIP (b) detects a collateral supply of nearly 100 %, with a resulting CS of 2 points
Correlation Analysis Between CBS/CS and Clinical Outcome
Generally, we could observe a weak correlation between CBS and 3-month mRS, and it was almost identical for CTA (r = − 0.31, p < 0.05) and 4D-CTA (r = − 0.35, p < 0.05). Correlation between CS and 3-month mRS was − 0.22 (p = 0.12) for CTA and − 0.34 (p < 0.05) for 4D-CTA.
Discussion
Thrombus Length
CTA reconstructions of peak arterial phase of 4D-CTA have been detected to be a feasible alternative to conventional CTA for the detection of an intracranial arterial occlusion [1, 17].
Though, similar to the studies by Baxa et al. [13] and Frölich et al. [14], we could assert that arterial single-phase CTA significantly overrates TL. In more than one-third of the examined patients, TL could not even be defined on CTA, while it could be measured on 4D-CTA tMIP due to visualization of the distal end of thrombus by opacification of collateral filling. Through analysis of thin-slice NECT, it was found that a TL of more than 8 mm in acute MCA stroke minimizes the chance of successful recanalization by intravenous thrombolysis [4]. As previously described, the filling defect on 4D-CTA—in contrast to arterial single-phase CTA—shows strong correlation with the length of the HMCAS on thin-slice NECT [14]. This conforms to our results and may affirm that 4D-CTA can give a more precise statement on TL than single-phase arterial CTA. Knowledge of exact clot extent could help to identify patients, in whom a systemic therapy or rather an intra-arterial approach might be successful and could also facilitate the estimation of the efficiency and assumable complication rate in thrombectomy [18]. Recanalization success decreases significantly with simultaneously increasing complication rates in occlusions exceeding 10 mm [19]. Baxa et al. [13] recognized a TL of 12 mm as threshold for favorable recanalization.
Clot Burden Score
Similarly to absolute TL, we found that semiquantitative clot burden is overrated in single-phase arterial CTA described by lower scores in CBS compared with multi-phase 4D-CTA. Differences between CTA and 4D-CTA reached up to 5 points. Nevertheless, the overall difference in CBS between arterial single-phase CTA and 4D-CTA was only 1 point, as slightly more than half of the examined patients showed the same scores in CBS. Aside from other factors such as clot composition, site of clot impaction, thrombolytic therapy, or collateral supply, CBS was found to be an independent predictor of clinical and radiologic outcomes in patients with acute anterior circulation ischemic stroke [11]. CBS > 6 on CTA predicts good clinical outcome and significantly higher recanalization rates with rtPA than CBS ≤ 6 [11]. On the basis of autotriggered arterial-phase CTA, Puetz et al. [10] observed that the chance of an independent functional outcome increases with higher scores in CBS and that these patients are less likely at risk of fatal outcome. This conforms to our results, as we could assert a negative correlation between CBS and mRS at 3 months for both, CTA and 4D-CTA. However, the results of Puetz et al. rely on arterial single-phase CTA, and there is no distinction between low scores in CBS caused by extended clot length and late collateral filling, e.g., in consequence of a low ejection fraction of the heart. Time-resolved vessel imaging reveals the dependency of CBS on hemodynamics and the striking interaction of CBS and CS.
Collateral Score and Delay to Scanning
Peak enhancement of retrograde collateral flow, in mean, appeared 6 s after early arterial phase. Accordingly, single-phase CTA with mainly arterial contrast fails to depict the timing point of peak collateral supply reliably in the majority of cases reflected in significant lower scores in CS compared with 4D-CTA. 4D-CTA, on the contrary, can provide a wide temporal resolution with intracranial vessel contrast from nonenhanced through arterial and venous phase visualizing the retrograde filling of pial arteries and even allowing a noninvasive distinction between antegrade flow across incomplete vessel occlusions and retrograde collateral flow [1, 15].
Referring to single-phase CTA with a fixed delay of 25 s, Maas et al. [7] found that in-hospital worsening nearly quadrupled in patients with proximal MCA occlusions and poor collateral filling. They also suggested that CTA-based assessment of collaterals could help to cull patients likely to benefit from intra-arterial therapies or treatments directed at improving cerebral blood flow such as induced hypertension.
However, these results only refer to a single time frame. According to our results, we advise a standardized inclusion of time-resolved CTA in the MMCT, allowing the consideration of delayed collateral filling with precise information on retrograde filling time, size, and extent of collateral filling. As we could observe a broad temporal variability of peak opacification of collaterals, we would not recommend to generally adjust single-phase protocols by adding 6 s of delay. The temporal variability of backfilling of pial arteries might also serve as an indicator of the functional status of leptomeningeal collaterals or their autoregulatory capacity.
We could observe a significant negative correlation between CS on 4D-CTA and mRS at 3 months, but there was no significant correlation between CS on CTA and mRS at 3 months, which could point to a tendency that CS on 4D-CTA has a higher predictive value for outcome estimations than CS on CTA. However, as we did not consider the effect of recanalization and the grade of reperfusion as the most important outcome factors, our data can only be preliminary.
Study Limitations
Our study has limitations. Firstly, the retrospective design of the study bears a risk of selection bias. Secondly, the consensus reading obviates inter-rater reliability assessment. Thirdly, the study lacks a reliable gold standard for the assessment of TL. Although specificity of HMCAS seems to be comparable to the filling defect in 4D-CTA, sensitivity is much worse (61.2 versus 87.8 %), which limits the applicability of HMCAS as a true gold standard for quantifying clot extent. In five cases, especially in patients with long clots (> 2 cm), HMCAS was measured to be longer than the corresponding filling defect on tMIP, which might be due to the curved 3D-analysis of thin-slice NECT. TL could be defined on CTA in only approximately 50 % of cases. On the one hand, this might be regarded as a limitation of the study, as a comparison of absolute values of TL on 4D-CTA and CTA only succeeded in a subset of study patients. On the other hand, it illustrates that conventional single-frame CTA fails to depict the collaterals, which allow the definition of the distal thrombus end in a considerable amount of cases. Fourthly, the radiation exposure has not been investigated in detail in this study. The effective dose is higher for 4D-CTA than for CTA (4.2 versus 3.3 mSv) [17]. However, as VPCT is an inherent part of the MMCT, reconstruction of 4D-CTA from VPCT data causes no additional radiation dose. A general use of 4D-CTA as a substitute of CTA in the stroke setting is not advisable, as conventional CTA can provide valuable information about the extracranial vessel status of the supra-aortic arteries, including the carotid bifurcation, while 4D-CTA has a limited coverage in the z-axis. A reasonable consideration could be the use of 4D-CTA in exchange of the intracranial part of CTA, which would reduce the effective dose of MMCT by approximately 0.5 mSV (2.8 mSV is allotted to the cervical part of CTA). Fifthly, our study is hypothesis generating. As the outcome depends on various parameters, such as patient age, duration of symptoms or treatment, mRS before admission, site and composition of the clot, and especially, the effectiveness of recanalization, we recognize the need for a future larger prognostic study with a more homogenous study population and an elimination of potential confounding factors to make significant outcome predictions on the basis of CS and CBS [10].
Conclusion
Compared with arterial single-phase CTA, the postprocessing of 4D-CTA allows a better assessment of thrombus extent and collateral supply in patients presenting with acute ischemic stroke. 4D-CTA shows significantly higher scores in semiquantitative CBS and CS, two elaborated grading systems used for individualized therapy decisions and estimation of clinical outcome in stroke patients.
References
Frölich AMJ, Psychogios MN, Klotz E, Schramm R, Knauth M, Schramm P. Angiographic reconstructions from whole-brain perfusion CT for the Detection of large vessel occlusion in acute stroke. Stroke. 2012;43:97–102.
Smit EJ, Vonken EJ, van Seeters T, Dankbaar JW, van der Schaaf IC, Kappelle LJ, van Ginneken B, Velthuis BK, Prokop M. Timing-invariant imaging of collateral vessels in acute ischemic stroke. Stroke. 2013;44(8):2194–9. doi:10.1161/STROKEAHA.111.000675.
Menon BK, O’Brien B, Bivard A, Spratt NJ, Demchuk AM, Miteff F, Lu X, Levi C, Parsons MW. Assessment of leptomeningeal collaterals using dynamic CT angiography in patients with acute ischemic stroke. J Cereb Blood Flow Metab. 2013;33:365–71.
Riedel CH, Zimmermann P, Jensen-Kondering U, Stingele R, Deuschl G, Jansen O. The importance of size: successful recanalization by intravenous thrombolysis in acute anterior stroke depends on thrombus length. Stroke. 2011;42:1775–7.
Shobha N, Bal S, Boyko M, Kroshus E, Menon BK, Bhatia R, Sohn SI, Kumarpillai G, Kosior J, Hill MD, Demchuk AM. Measurement of length of hyperdense MCA sign in acute ischemic stroke predicts disappearance after IV tPA. J Neuroimaging. 2014;24(1):7–10. doi:10.1111/j.1552-6569.2012.00761.x.
Paliwal PR, Ahmed A, Shen L, Yeo LLL, Loh PK, Ng KWP, Chong VF, Ong BKC, Venketasubramanian N, Sinha AK, Teoh HL, Bathla G, Chan BPL, Sharma VK. Persistence of hyperdense middle cerebral artery sign on follow-up CT scan after intravenous thrombolysis is associated with poor outcome. Cerebrovasc Dis. 2012;33:446–52.
Maas MB, Lev MH, Ay H, Singhal AB, Greer DM, Smith WS, Harris GJ, Halpern E, Kemmling A, Koroshetz WJ, Furie KL. Collateral vessels on CT angiography predict outcome in acute ischemic stroke. Stroke. 2009;40:3001–5.
Grech R, Galvin PL, Power S, O’Hare A, Looby S, Brennan P, Thornton J. Outcome prediction in acute stroke patients considered for endovascular treatment: a novel tool. Interv Neuroradiol. 2014;20(3):312–24. doi:10.15274/NRJ-2014-10029.
Puetz V, Dzialowski I, Hill MD, Steffenhagen N, Coutts SB, O’Reilly C, Demchuk AM. Malignant Profile detected by CT angiographic information predicts poor prognosis despite thrombolyses within three hours from symptom onset. Cerebrovasc Dis. 2010;29:584–91.
Puetz V, Dzialowski I, Hill MD, Subramaniam S, Sylaja PN, Krol A, O’Reilly C, Hudon ME, Hu WY, Coutts SB, Barber PA, Watson T, Roy J, Demchuk AM. Intracranial thrombus extent predicts clinical outcome, final infarct size and hemorrhagic transformation in ischemic stroke: the clot burden score. Int J Stroke. 2008;3:230–6.
Tan IYL, Demchuk AM, Hopyan J, Zhang L, Gladstone D, Wong K, Martin M, Symons SP, Fox AJ, Aviv RI. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol. 2009;30:525–31.
Sillanpaa N, Saarinen JT, Rusanen H, Hakomaki J, Lahteela A, Numminen H, Elovaara I, Dastidar P, Soimakallio S. The clot burden score, the Boston Acute Stroke Imaging Scale, the cerebral blood volume ASPECTS, and two novel imaging parameters in the prediction of clinical outcome of ischemic stroke patients receiving intravenous thrombolytic therapy. Neuroradiology. 2012;54:663–72.
Baxa J, Rohan V, Tupy R, Cerna L, Flohr T, Polivka J, Ferda J. Determination of the middle cerebral artery occlusion length in acute stroke: contribution of 4D CT angiography and importance for thrombolytic efficacy prediction. Clin Neuroradiol. 2014. doi:10.1007/s00062-014-0302-x.
Frölich AMJ, Schrader D, Klotz E, Schramm R, Wasser K, Knauth M, Schramm P. 4D CT angiography more closely defines intracranial thrombus burden than single-phase ct angiography. AJNR Am J Neuroradiol. 2013. doi:10.3174/ajnr.A3533.
Frölich AMJ, Psychogios MN, Klotz E, Schramm R, Knauth M, Schramm P. Antegrade flow across incomplete vessel occlusions can be distinguished from retrograde collateral flow using 4-dimensional computed tomographic angiography. Stroke. 2012;43:2974–9.
Willems PWA, Taeshineetanakul P, Schenk B, Brouwer PA, Terbrugge KG, Krings T. The use of 4D-CTA in the diagnostic work-up of brain arteriovenous malformations. Neuroradiology. 2012;54:123–31.
Saake M, Goelitz P, Struffert T, Breuer L, Volbers B, Doerfler A, Kloska S. Comparison of conventional CTA and volume perfusion CTA in evaluation of cerebral arterial vasculature in acute stroke. AJNR Am J Neuroradiol. 2012;33:2068–73.
Zhu G, Michel P, Jovin T, Patrie JT, Xin W, Eskandari A, Zhang W, Wintermark M. Prediction of recanalization in acute stroke patients receiving intravenous and endovascular revascularization therapy. Int J Stroke. 2014. doi:10.1111/ijs.12312.
Gralla J, Burkhardt M, Schroth G, El-Koussy M, Reinert M, Nedeltchev K. Occlusion length is a crucial determinant of efficiency and complication rate in thrombectomy for acute ischemic stroke. AJNR Am J Neuroradiol. 2008;29:247–52.
Acknowledgements
None.
Conflict of Interest
The authors declare that there is no conflict of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Iris N. Kaschka and Stephan P. Kloska contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kaschka, I., Kloska, S., Struffert, T. et al. Clot Burden and Collaterals in Anterior Circulation Stroke: Differences Between Single-Phase CTA and Multi-phase 4D-CTA. Clin Neuroradiol 26, 309–315 (2016). https://doi.org/10.1007/s00062-014-0359-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-014-0359-6