Abstract
In this study, we synthesized a series of 2′,4′,6′-trihydroxychalcone derivatives and evaluated their antidepressant activities. The results of the nine compounds showed significantly reduced times during the forced swimming test at a dose of 10 mg/kg, indicative of antidepressant activity. Among the compounds, 2-bromo-2′,4′,6′-trihydroxychalcone (3h) was found to be the most potent, and it was observed that compound 3h at dose of 10, 20, and 40 mg/kg significantly reduced the duration of immobility times in the FST and TST in mice 30 min after treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression is one of the major mental disorders. Symptoms of depression include lowered mood and reduced interest and pleasure. According to latest available data from the World Health Organization (WHO), depression is expected to become the second leading cause of disease-related disability by the year 2020 (Murray and Lopez, 1996; Rybnikova et al., 2007). It is a serious disorder with an estimate of lifetime prevalence as high as 20% and a significant number of patients (30%) do not respond to current medical treatment (Charney et al., 2002; Cryan et al., 2002). Most of these drugs, however, have undesirable side effects. Thus, there is an unmet need for new antidepressants.
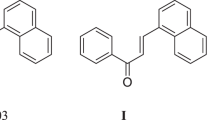
Chalcones are a group of flavonoids that exhibit a wide variety of pharmacological effects, including antibacterial, antifungal, antiviral, and analgesic properties (Bekhit et al., 2001; Lopez et al., 2001; Viana et al., 2003; Wu et al., 2003) et al. In recent years, it is reported that flavonoids possess antidepressant activity (Butterweck et al., 2000; Nakazawa et al., 2003). Wang et al. (2007) also reported that ten natural flavonoid compounds exhibited antidepressant activity. Apigenin (5,7,4′-trihydroxyflavonoid) (Fig. 1), one type of bioflavonoid widely found in citrus fruit, was reported to possess antidepressant effect (Yi et al., 2008). Analyzing the structure of apigenin, if C-ring of apigenin is opened, the chalcone compound I (4,2′,4′,6′-tetrahydroxychalcone) was obtained and its antidepressant activity was examined. The pharmacological results showed that the compound I reduced significantly the duration of immobility times at 10 mg/kg dose level when compared to the control (P < 0.05), indicative of antidepressant activity. In order to obtain compounds with better antidepressant activity, in this article, we designed and synthesized a series of 2′,4′,6′-trihydroxychalcone derivatives with ring C opened using compound I as the lead compound. The antidepressant activities of the synthesized compounds were also determined by using Porsolt’s behavioral despair (Forced Swimming Test: FST) (Porsolt et al., 1977) and Steru’s behavioral despair (Tail Suspension Test: TST) (Steru et al., 1985).The synthesized compounds were characterized by IR, 1H-NMR, and MS.
Experimental
Melting points were determined in open capillary tubes and were uncorrected. IR spectra were recorded (in KBr) on a FT-IR1730 (Bruker, Switzerland), 1H-NMR spectra were measured on an AV-300 (Bruker, Switzerland), and all chemical shifts were given in ppm relative to tetramethylsilane. Mass spectra were measured on an HP1100LC (Agilent Technologies, USA). The major chemicals were purchased from Alderich Chemical Corporation. All other chemicals were of the analytical grade.
Synthesis of 2′-hydroxy-3-fluoro-4′,6′-bis(methoxymethoxy)chalcone (2c)
To a stirred solution of KOH (2.0 g, 45.6 mmol) in water (2 ml) cooled to 0°C in an ice bath was added dropwise a solution of 2-hydroxy-4,6-bis (methoxymethoxy) acetophenone (1.0 mmol), and 3-fluorobenzaldehyde (2.0 mmol) in ethanol under nitrogen (Nishida and Kawabata, 2006). The reaction mixture was kept at room for 24 h. The mixture was poured into ice-water, adjusted to pH 2–3 with 1 M HCl, and then extracted with ethyl acetate. The organic layer was washed with water and saturated brine, dried over anhydrous MgSO4. After removing solvents, products were purified by silica gel column chromatography (petroleum ether:ethyl acetate = 6:1). The yellow solid was obtained.
MP 70–72°C; yield = 71%; 1H-NMR (CDCl3, 300 MHz): δ 3.49 (s, 3H, OCH3), 3.54 (s, 3H, OCH3), 5.20 (s, 2H, CH2), 5.30 (s, 2H, CH2), 6.25–7.37 (m, 6H, Ar–H). 7.73 (d, 1H, J = 15.6 Hz, Hα), 7.91 (d, 1H, J = 15.6 Hz, Hβ), 13.76 (s, H, –OH); IR (KBr) cm−1: 3312 (OH), 1636 (C=O); MS m/z 363 (M + 1).
General procedure for the preparation of compounds (3a–q)
In a round-bottomed flask, to a stirred solution of chalcones 2a–q (0.25 mmol) in methanol was added dropwise 3 M HCl, the mixture was refluxed for 30 min. The solvents were removed under reduced pressure and diluted with water, and extracted with ethyl acetate. The organic layer was washed with water and brine, dried over anhydrous Na2SO4. After concentration under reduced pressure, the resultant was recrystallized from ethanol. The yield, melting point, and spectral data of part compounds are given below.
2′,4′,6-Trihydroxychalcone (3a)
MP 124–126°C; yield = 81%; 1H-NMR (DMSO-d 6 , 300 MHz): δ 5.86–7.46 (m, 7H, Ar–H), 7.69 (d, 1H, J = 15.9 Hz, Hα), 8.13 (d, 1H, J = 15.7 Hz, Hβ), 10.48 (s, H, –OH), 12.48 (s, H, –OH); IR (KBr) cm−1: 3328(OH), 1646 (C=O); MS m/z 257 (M + 1).
2-Fluoro-2′,4′,6′-trihydroxychalcone (3b)
MP 172–174°C; yield = 62%; 1H-NMR (DMSO-d 6 , 300 MHz): δ 5.87–7.79 (m, 6H, Ar–H), 7.73 (d, 1H, J = 15.9 Hz, Hα), 8.24 (d, 1H, J = 15.9 Hz, Hβ), 10.54 (s, H, –OH), 12.47 (s, H, –OH); IR (KBr) cm−1: 3334 (OH), 1646 (C=O); MS m/z 275 (M + 1).
3-Fluoro-2′,4′,6′-trihydroxychalcone (3c)
MP 178–180°C; yield = 66%; 1H-NMR (DMSO-d 6 , 300 MHz): δ 5.86–7.53 (m, 6H, Ar–H), 7.66 (d, 1H, J = 15.7 Hz, Hα), 8.12 (d, 1H, J = 15.7 Hz, Hβ), 10.52 (s, H, –OH), 12.44 (s, H, –OH); IR (KBr) cm−1: 3328 (OH), 1642 (C=O); MS m/z 275 (M + 1).
4-Fluoro-2′,4′,6′-trihydroxychalcone (3d)
MP 168–170°C; yield = 70%; 1H-NMR (DMSO-d 6 , 300 MHz): δ 5.85–7.53 (m, 6H, Ar–H), 7.68 (d, 1H, J = 16.2 Hz, Hα), 8.12 (d, 1H, J = 16.2 Hz, Hβ), 10.48 (s, H, –OH), 12.47 (s, H, –OH); IR (KBr) cm−1: 3330 (OH), 1644 (C=O); MS m/z 275 (M + 1).
2-Chloro-2′,4′,6′-trihydroxychalcone (3e)
MP 160–162°C; yield = 63%; 1H-NMR (DMSO-d 6 , 300 MHz): δ 5.88–7.84 (m, 6H, Ar–H), 7.94 (d, 1H, J = 15.6 Hz, Hα), 8.16 (d, 1H, J = 15.6 Hz, Hβ), 10.57 (s, H, –OH), 12.45 (s, H, –OH); IR (KBr) cm−1: 3322 (OH), 1644 (C=O); MS m/z 291 (M + 1).
3-Chloro-2′,4′,6′-trihydroxychalcone (3f)
MP 178–180°C; yield = 72%; 1H-NMR (DMSO-d 6 , 300 MHz): δ 5.92–7.74 (m, 6H, Ar–H), 7.63 (d, 1H, J = 15.7 Hz, Hα), 8.13 (d, 1H, J = 15.7 Hz, Hβ), 10.53 (s, H, –OH), 12.45 (s, H, –OH); IR (KBr) cm−1: 3327 (OH), 1647 (C=O); MS m/z 291 (M + 1).
4-Chloro-2′,4′,6′-trihydroxychalcone (3g)
MP 175–177°C; yield = 78%; 1H-NMR (DMSO-d 6 , 300 MHz): δ 5.86–7.71 (m, 6H, Ar–H), 7.67 (d, 1H, J = 15.1 Hz, Hα), 8.11 (d, 1H, J = 15.7 Hz, Hβ), 10.51 (s, H, –OH), 12.46 (s, H, –OH); IR (KBr) cm−1: 3328 (OH), 1649 (C=O); MS m/z 291 (M + 1).
2-Bromo-2′,4′,6′-trihydroxychalcone (3h)
MP 186–188°C; yield = 53%; 1H-NMR (DMSO-d 6 , 300 MHz): δ 5.87–7.82 (m, 6H, Ar–H), 7.90 (d, 1H, J = 15.5 Hz, Hα), 8.10 (d, 1H, J = 15.5 Hz, Hβ), 10.55 (s, H, –OH), 12.43 (s, H, –OH); IR (KBr) cm−1: 3328 (OH), 1640 (C=O); MS m/z 334 (M + 1).
3-Bromo-2′,4′,6′-trihydroxychalcone (3i)
MP 182–184°C; yield = 65%; 1H-NMR (DMSO-d 6 , 300 MHz): δ 5.86–7.88 (m, 6H, Ar–H), 7.63 (d, 1H, J = 15.7 Hz, Hα), 8.12 (d, 1H, J = 15.7 Hz, Hβ), 10.52 (s, H, –OH), 12.45 (s, H, –OH); IR (KBr) cm−1: 3335 (OH), 1651 (C=O); MS m/z 334 (M + 1).
4-Bromo-2′,4′,6′-trihydroxychalcone (3j)
MP 192–194°C; yield = 69%; 1H-NMR (DMSO-d 6 , 300 MHz): δ 5.86–7.65 (m, 6H, Ar–H), 7.65 (d, 1H, J = 15.7 Hz, Hα), 8.12 (d, 1H, J = 15.7 Hz, Hβ), 10.51 (s, H, –OH), 12.46 (s, H, –OH); IR (KBr) cm−1: 3322 (OH), 1647 (C=O); MS m/z 334 (M + 1).
2,4-Dichloro-2′,4′,6′-trihydroxychalcone (3k)
MP 179–181°C; yield = 71%; 1H-NMR (DMSO-d 6 , 300 MHz): δ 5.88–7.76 (m, 5H, Ar–H), 7.86 (d, 1H, J = 15.7 Hz, Hα), 8.14 (d, 1H, J = 15.6 Hz, Hβ), 10.57 (s, H, –OH), 12.41 (s, H, –OH); IR (KBr) cm−1: 3328 (OH), 1649 (C=O); MS m/z 325 (M + 1).
4-Methyl-2′,4′,6′-trihydroxychalcone (3l)
MP 117–119°C; yield = 60%; 1H-NMR (DMSO-d 6 , 300 MHz): δ 2.35 (s, 3H, CH3), 5.90–7.44 (m, 6H, Ar–H), 7.67 (d, 1H, J = 15.7 Hz, Hα), 8.09 (d, 1H, J = 15.7 Hz, Hβ), 10.46 (s, H, –OH), 12.50 (s, H, –OH); IR (KBr) cm−1: 3330 (OH), 1646 (C=O); MS m/z 271 (M + 1).
3,4-Dimethyl-2′,4′,6′-trihydroxychalcone (3m)
MP 156–158°C; yield = 68%; 1H-NMR (DMSO-d 6 , 300 MHz): δ 2.23 (s, 6H, (CH3)2), 5.85–7.44 (m, 5H, Ar–H), 7.64 (d, 1H, J = 15.7 Hz, Hα), 8.09 (d, 1H, J = 15.7 Hz, Hβ), 10.44 (s, H, –OH), 12.50 (s, H, –OH); IR (KBr) cm−1: 3329 (OH), 1645 (C=O); MS m/z 285 (M + 1).
4-Methoxyl-2′,4′,6′-trihydroxychalcone (3n)
MP 107–109°C; yield = 68%; 1H-NMR (DMSO-d 6 , 300 MHz):δ 3.82 (s, 3H, OCH3), 5.85–7.65 (m, 6H, Ar–H), 7.69 (d, 1H, J = 15.8 Hz, Hα), 8.03 (d, 1H, J = 15.6 Hz, Hβ), 10.42 (s, H, –OH), 12.52 (s, H, –OH); IR (KBr) cm−1: 3327 (OH), 1646 (C=O); MS m/z 287 (M + 1).
3,4-Dimethoxy-2′,4′,6′-trihydroxychalcone (3o)
MP 112–114°C; yield = 68%; 1H-NMR (DMSO-d 6 , 300 MHz): δ 3.77, 3.82 (each s, 3H, OCH3-3,4), 5.90–7.65 (m, 5H, Ar–H), 7.64 (d, 1H, J = 15.7 Hz, Hα), 8.09 (d, 1H, J = 15.7 Hz, Hβ), 10.41 (s, H, –OH), 12.53 (s, H, –OH); IR (KBr) cm−1: 3327 (OH), 1646 (C=O); MS m/z 317 (M + 1).
3,4,2′,4′,6′-Pentahydroxychalcone (3p)
MP 202–204°C; yield = 68%; 1H-NMR (DMSO-d 6 , 300 MHz): δ 5.89–7.41 (m, 5H, Ar–H), 7.65 (d, 1H, J = 15.6 Hz, Hα), 8.06 (d, 1H, J = 15.7 Hz, Hβ), 9.67 (s, H, –OH), 10.37 (s, H, –OH), 12.12 (s, H, –OH), 12.51 (s, H, –OH); IR (KBr) cm−1: 3328 (OH), 1647 (C=O); MS m/z 289 (M + 1).
3-Methoxy-4,2′,4′,6′-tetrahydroxychalcone (3q)
MP 188–190°C; yield = 68%; 1H-NMR (DMSO-d 6 , 300 MHz): δ 3.83 (s, 3H, OCH3), 5.89–7.27 (m, 5H, Ar–H), 7.65 (d, 1H, J = 15.5 Hz, Hα), 7.99 (d, 1H, J = 15.7 Hz, Hβ), 10.38 (s, H, –OH), 10.78 (s, H, –OH); 12.16 (s, H, –OH), 12.54 (s, H, –OH); IR (KBr) cm−1: 3329 (OH), 1645 (C=O); MS m/z 303 (M + 1).
Pharmacology
Forced swimming test (FST)
The synthesized compounds were screened for their antidepressant activity using Porsolt’s behavioral despair (forced swimming) test (Porsolt et al., 1977). Local breed, male Kunming mice (20–24 g) were used in the forced swimming test under standard conditions with free access to food and water. They were housed in groups of six. On the test day, mice were dropped one at a time into a plexiglass cylinder (height 25 cm, diameter 10 cm3) containing 10 cm of water at 23–25°C. On this day, mice were assigned into different groups (n = 10 for each group). The synthesized compounds (10 mg/kg) and Fluoxetine as a reference antidepressant drug (10 mg/kg) were suspended in a 0.5% aqueous solution of methylcellulose injected intraperitoneally (ip) in a standard volume of 0.05 ml/20 g body weight, 30 min prior to the test. Control animal received 0.5% aqueous solution of methylcellulose. Then, the mice were dropped individually into the plexiglass cylinder and left in the water for 6 min. After the first 2 min of the initial vigorous struggling, the animals were immobile. A mouse was judged immobile if it floated in the water in an upright position and made only slight movements in order to prevent sinking. The duration of immobility was recorded during the last 4 min of the 6 min test.
Tail suspension test (TST)
The tail suspension test was based on the method of Steru et al. (1985). Briefly, each mouse was individually suspended by its tail using a clamp (2 cm from the end) for 6 min in a box (25 × 25 × 30 cm) with the head 5 cm from the bottom. Testing was carried out in a darkened room with minimal background noise. On this day, mice were assigned into different groups (n = 10 for each group). The synthesized compounds (10 mg/kg) and Fluoxetine as a reference antidepressant drug (10 mg/kg) were suspended in a 0.5% aqueous solution of methylcellulose injected intraperitoneally (ip) in a standard volume of 0.05 ml/20 g body weight, 30 min prior to the test. Control animal received 0.5% aqueous solution of methylcellulose. After the first 2 min of the initial vigorous struggling, the animals were immobile. The duration of immobility was recorded during the last 4 min of the 6 min test.
Statistical analysis
Results are expressed as mean ± SEM; n represents the number of animals. Data obtained from pharmacological experiments were analyzed with one-way analysis of variance (ANOVA) followed by Dunnet’s post hoc test, using Pharmacologic Calculation System Version 4.1. (Microcomputer Specialists). A P-value of less than 0.05 was considered statistically significant.
Results and discussion
The target compounds 3a–q was synthesized according to Scheme 1. Compound 1 (1-(2-hydroxy-4,6-bismethoxymethoxy-phenyl)-ethanone was prepared as reported previously (Raiendra Prasad et al., 2005). Intermediates 2a–q was prepared by Claisen–Schmidt condensation of 1 with appropriate aromatic aldehydes or hydroxyaromatic aldehydes, protected as chloromethyl methyl ether (Raiendra Prasad et al., 2005; Zhao et al., 2005; Nishida et al., 2007). Then intermediates 2a–q were treated with 3 M HCl in methanol to yield the hydroxychalcones 3a–q in good yield.
First, the forced swimming test is a behavioral test used to predict the efficacy of antidepressant treatments (Porsolt et al., 1977). It is used effectively in predicting the activity of a wide variety of antidepressants such as MAO inhibitors and atypical antidepressants (Porsolt, 1981). It has good predictive value for antidepressant potency in humans (Willner and Mitchell, 2002). The obtained data on the antidepressant activity of the compounds and reference drug are given in Table 1. In our study, all of the compounds except 3a, 3c, 3d, 3e, 3j, 3l, 3m, 3n, and 3o significantly reduced the duration of immobility time at 10 mg/kg compared to control (P < 0.05, P < 0.01, or P < 0.001, Table 1). Among the compounds, 3h (2-bromo-2′,4′,6′-trihydroxychalcone) was the most antidepressant activity and significantly reduced the duration of immobility times at 10 mg/kg dose level when compared to the control (P < 0.001).
Analyzing the antidepressant activities of synthesized compounds 3a–q, the following SAR was gained. From compounds 3b–j, as halogen substituted on B ring of chalcone compounds, alternating variations of activity were observed. The atom Br gave more contribution to the antidepressant activity than atoms F and Cl, the rank of activity order of halogen substituted derivatives was Br > Cl > F. Among the compounds, 3h (2-bromo-2′,4′,6′-trihydroxychalcone) showed the most antidepressant activity, which reduced immobility time by 50.04% at 10 mg/kg compared with the same dose of Fluoxetine (58.68%), and compared with the leading compound I exhibited enhanced antidepressant activity. In addition, the position of halogen substituted on the phenyl ring greatly influenced the antidepressant activity, compared with the derivatives with different F-substituted positions on phenyl ring, their rank of activity order was 2-F > 3-F > 4-F. The rank of activity order of the Cl-substituted derivatives was 3-Cl > 2,4-Cl2 > 4-Cl > 2-Cl. The rank of activity order of the Br-substituted derivatives was 2-Br > 3-Br > 4-Br.
Next, the influence of the electron-donor group with antidepressant activity was compared (Table 1). Their contribution order was 3,4-(OH)2 > 4-OH (I) > 3-OCH3-4-OH > 3,4-(OCH3)2 > 4-CH3 > H > 3,4-(CH3)2 > 4-OCH3. Comparing with the compound 3a, compounds 3p, 3q, and I had potent antidepressant effects, and it seemed that the increase of hydroxyl group on chalcone B ring could influence activity. The similar result had been reported that the number of hydroxyl group influenced activity (Husan et al., 1987).
Finally, as shown in Fig. 2, immobility in the FST was significantly reduced after treatment with all three doses of compound 3h, similar to the positive Fluoxetine, indicating a significant antidepressant-like effect. The decrease in immobility time in the TST showed similarity to that seen in the FST, See Fig. 3. 20 mg/kg showed most significant activity among three doses of 3h in both tests. Both forced swimming and tail suspension tests are the accepted stress models of depression. Immobility has been shown to reflect a state of ‘behavioral despair and variants’ or ‘failure to adapt to stress’ (Borsini et al., 1986; Willner, 1991). Immobility displayed in both of these behavioral despair models has been hypothesized to reflect behavioral despair which in turn may reflect depressive disorders in humans. There was a significant correlation between clinical potency and the potency of antidepressant in both models. The compound 3h produced significant antidepressant-like activity in both the FST and TST in mice, which indicates that compound 3h has some antidepressant effects.
Conclusion
In conclusion, a series 2′,4′,6′-trihydroxychalcone derivatives were synthesized and their antidepressant activities was evaluated. The results showed that compound 3h (2-bromo-2′,4′,6′-trihydroxy-chalcone) was the most promising compound and significantly reduced the duration of immobility times at 10 mg/kg dose level when compared to the control (P < 0.001) in the FST and the TST. Further studies should be initiated to reveal the mechanism of the antidepressant-like effect of the compound 3h.
References
Bekhit AA, Habib NS, Din A, Bekhit A (2001) Synthesis and antimicrobial evaluation of chalcone and syndrome derivatives of 4(3H)-quinazolinone. Boll Chim Farm 140:297–301
Borsini F, Voltera G, Meli A (1986) Dose the behavioral ‘despair’ test measure ‘despair’. Physiol Behav 38:385–389
Butterweck V, Jürgenliemk G, Nahrstedt A, Winterhoff H (2000) Flavonoids from Hypericum perforatum show antidepressant activity in the forced swimming test. Planta Med 66:3–6
Charney DS, Crothe DR, Smith SL, Brady KT, Kaltsounis-Pucktt J, Wright CW, Lsitd LK, Rush AJ (2002) Overview of psychiatric disorder and the role of newer antidepressants. J Clin Psychiatry 63:3–9
Cryan JF, Markou A, Luck L (2002) Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci 23:238–245
Husan SR, Cliard J, Cillard P (1987) Hydroxyl radical scavenging activity of flavoids. Phytochemistry 26:2487–2491
Lopez SN, Castelli MV, Zacchino SA, Dominguez JC, Lobo G, Chrris-Charriss J, Cortes JC, Ribas JC, Devia C, Rodriguez AM, Enrizz RD (2001) In vitro antifungal evaluation and structure–activity relationships of a new series of chalcone derivatives and synthetic analogues, with inhibitory properties against polymers of the fungal cell wall. Bioorg Med Chem 9:1999–2013
Murray CJL, Lopez AD (1996) The global burden of disease. Harvard Univ Press, Cambridge, p 21
Nakazawa T, Yasuda T, Ueda J, Ohsawa K (2003) Antidepressant-like effects of apigenin and 2,4,5-trimethoxycinnamic acid from Perilla frutescents in the forced swimming test. Biol Pharm Bull 26:474–480
Nishida J, Kawabata J (2006) DPPH radical scavenging reaction of hydroxy- and methoxychalcones. Biosci Biotechnol Biochem 70:193–202
Nishida J, Gao H, Kawabata J (2007) Synthesis and evaluation of 2′,4′,6′-trihydroxychalcones as a new class of tyrosinase inhibitors. Bioorg Med Chem 15:2396–2402
Porsolt RD (1981) In: Enna SJ, Malick JB, Richelson E (eds) Antidepressants: neurochemical behavioral and clinical perspectives. Raven Press, New York, pp 129–139
Porsolt RD, Bertin A, Jalfre M (1977) Behavioural despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229:327–336
Raiendra Prasad Y, Lakshmana Rao A, Prasoona L, Murali K, Ravi Kumar P (2005) Synthesis and antidepressant activity of some 1,3,5-triphenyl-2-pyrazolines and 3-(2″-hydroxynaphthal-en-1″-yl)-1,5-diphenyl-2-pyrazolines. Bioorg Med Chem Lett 15:5030–5034
Rybnikova E, Mironova V, Pivina S, Tulkova E, Ordyan N, Vataeva L, Vershinina E, Abritalin E, Kolchev A, Nalivaeva N, Tumer AJ, Samoilov M (2007) Antidepressant-like effects of mild hypoxia preconditioning in the learned helplessness model in rats. Neurosci Lett 417:234–239
Steru L, Chermat R, Thierry B, Simon P (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology 85:367–370
Viana GS, Bandeira MA, Matos FJ (2003) Analgesic and anti-inflammatory effects of chalcones isolated from Myracrodruon urundeuva allemal. Phytomedicine 10:189–196
Wang WX, Hu XY, Liu H, Guo HZ, Guo D (2007) A studies on antidepressant activity of ten natural flavonoid compounds of hyperin. Chin Tradit Herb Drugs 38:900–902
Willner P (1991) Animal models as stimulations of depression. Trends Pharmacol Sci 12:131–138
Willner P, Mitchell PJ (2002) The validity of animal models of predisposition to depression. Behav Pharmacol 13:169–188
Wu JH, Wang XH, Yi YH, Lee KL (2003) Anti-AIDS agents 54, a potent anti-HIV chalcone and flavonoids from genus desmos. Bioorg Med Chem Lett 13:1813–1815
Yi LT, Li JM, Li YC, Pan Y, Xu Q, Kong LD (2008) Antidepressant-like behavioral and neurochemical effects of the citrus-associated chemical apigenin. Life Sci 82:741–751
Zhao LM, Jin HS, Sun LP, Piao HR, Quan ZS (2005) Synthesis and evaluation of antiplatelet activity of trihydroxychalcone derivatives. Bioorg Med Chem Lett 15:5027–5029
Acknowledgments
This is provided by Zhejiang Ocean University R&D start funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sui, X., Quan, YC., Chang, Y. et al. Synthesis and studies on antidepressant activity of 2′,4′,6′-trihydroxychalcone derivatives. Med Chem Res 21, 1290–1296 (2012). https://doi.org/10.1007/s00044-011-9640-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-011-9640-2