Abstract
A new series of pyridazin-3-one derivatives were designed, synthesized and evaluated for their preclinical antidepressant effect on Swiss mice. Among the series, compounds 6c, 6d and 6f exhibited significant activity profile in forced swimming test. Compounds 6c and 6d were most efficacious, which at dose of 50 mg kg−1 reduced the time of immobility by 42.85 and 38.09 %, respectively, as compared to the standard drug fluoxetine which reduced the immobility time by 45.23 % at the dose of 32 mg kg−1. All the test and standard compounds were administered orally 60 min before the test. Interestingly, all active compounds did not cause any significant alteration of locomotor activity in mice as compared to control, indicating that the hybrids did not produce any motor impairment effects. The results indicate that pyridazin-3(2H)-one derivatives may have potential therapeutic value for the management of mental depression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Major depression disorder (MDD) is described as a depressive state of mind, which is associated with faulty mood, loss of interest, disruption in sleep patterns, fatigue and sometimes suicidal tendencies. It is a chronic and life-threatening mental illness, which remains hidden and untreated at most of the times (Hampton, 2012). World Health Organization (WHO) had estimated that ‘at least 350 million people live with depression and it is the leading cause of disability worldwide’ (WHO, 2012). Epidemiological studies have indicated that about 2/3 of people who commit suicide are depressed at the time of their death (Al-Habeeb et al., 2013). Exact cause of depression is not clearly known, but it is believed that imbalance of neurotransmitters in brain, genetic vulnerability, stressful life events and medical problems are the main factors leading to depression (DeWeerdt, 2013). Currently available antidepressant treatments are selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase inhibitors (MAOIs), tricyclic antidepressants (TCAs), serotonin–norepinephrine reuptake inhibitors (SNRIs) and some non-medication therapeutic options (Chancellor, 2011). Even though wide range of conventional therapies is available, nearly 15 % of depressed people are still refractory to the current existing therapies (Anderson et al., 2012). In addition, most of the people suffer from relapse and experience serious side effects after treatment with current therapies (Check, 2004; Licinio and Wong, 2005). In February 2007, the USFDA displayed ‘black box’ label on currently available antidepressants indicating that their use may increase the risk of suicidal thinking behavior in few cases of children, adolescents and adults (Friedman and Leon, 2007). Hence, there is an urgent need to develop new class of prototypes that are more effective, tolerable and safe in depressed individuals against this deadly disorder.
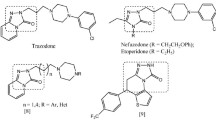
Considerable interest has been paid to derivatives containing 5-arylpyridazinone moieties, because of their effects on the central nervous system (CNS) (Bosc et al., 1990; Mokrosz et al., 1992; Perregaard et al., 1992; Sladowska, 1993). However, two pyridazine derivatives, 5-benzyl-6-methyl-2-[4-(3-trifluoromethylphenyl)piperazin-1-yl] methylpyridazin-3-one and 5-benzyl-6-methyl-2-[4-(3-chlorophenyl)piperazin-1-yl] methylpyridazin-3-one, were evaluated for their potential antidepressant effects, using classical psychopharmacological tests in mice. The intraperitoneal LD50 values of these compounds were, respectively, 1125.8 and 429.6 mg kg−1. Both compounds (5–20 mg kg−1, i.p.) reduced the duration of immobility of mice in the FST, antagonized reserpine (2.5 mg kg−1, i.p.)-induced ptosis and potentiated reserpine (2.5 mg kg−1, i.p.)-induced hypothermia (Rubat et al., 1995).
Many reports suggest that pyridazinones and their synthetic analogues can be considered as anti-inflammatory agents (Özadali et al., 2012), anticancer agents (Lattmann et al., 2003) and also possess an anti-feedant activity (Huang et al., 2003). On the other hand, Castro and col. have synthesized a series of substituted pyridazine derivatives, as new generation of triple reuptake inhibitors (TRI) and observed significant antidepressant effects (Castro et al., 1994).
Based on these observations, we were interested in evaluating the pharmacological properties of a new series of 5-(benzo[b]furan-2-ylmethyl)-6-methylpyridazin-3(2H)-one derivatives.
This report describes the chemical synthesis and biological evaluation of 5-(benzo[b]furan-2-ylmethyl)-6-methylpyridazin-3(2H)-one derivatives as antidepressant agents.
Materials and methods
Chemistry
The synthesis of 5-(benzo[b]furan-2-ylmethyl)-6-methylpyridazin-3(2H)-one derivatives is outlined in Scheme 1. Salicylaldehydes 2a-g, which are not commercially available, were synthesized via Reimer-Thieman (Thoer et al., 1988) formylation of the appropriate substituted phenols 1 with CHCl3 and NaOH. Aldehydes 2a–g have been previously synthesized with very low yield. Briefly, reaction of intermediates 2a–g with bromacetaldehyde diethylacetal in the presence of potassium carbonate in DMF (dimethylformamide) yielded compounds 3a–g. These compounds 3 were cyclized to Benzo[b]furan-2-ylcarboxaldehydes 4a–g by heating in concentrated acetic acid. Benzo[b]furanaldehydes 4 were prepared according to the methods described in the literature (Hirota et al., 1986). Treatment of substituted aldehydes 4 with levulinic acid in the presence of acetic acid gave products 5a–g, which were treated by hydrazine hydrate in ethanol at reflux temperature to afford the pyridazin-3(2H)-ones 6a–g (Benmoussa et al., 2012).
Antidepressant activity
Antidepressant activity was measured with fluoxetine as standard drug using the FST in Swiss mice. Swiss mice were placed in a vertical Plexiglas cylinder filled with water, maintained at 25 °C, for 15 min. Five to 6 min later immobility reached a plateau, where the mice remained immobile for approximately 80 % of the time. After 15 min in the water, the mice were removed and allowed to dry in a heated enclosure (32 °C) before being returned to their home cages. They were again placed in the cylinder for 24 h later, and the total duration of immobility was measured during a 5 min test (Table 1). After solubilizing the product in vegetable oil, the solution was administered directly into the stomach of mice via a technique called oral gavage (0.2 mL of solution/20 g of animals). The newly synthesized compounds were administered at a dose of 50 mg kg−1, orally 60 min before the test. The fluoxetine was administered at a dose of 32 mg kg−1, orally 60 min before the test. (Porsolt et al., 1977, 1978; Petit-Demouliere et al., 2005).
Results and discussion
Chemistry
Melting points were determined on a Büchi SMP 20 apparatus and are not corrected. Infrared (IR) spectra were recorded with an IR VERTEX 70 FT-IR (Bruker Optics) spectrometer. 1H Nuclear magnetic resonance (1H NMR) spectra were recorded on a Bruker Avance (400 MHz) spectrometer, using tetramethylsilane (TMS) as internal standard and CDCl3 and DMSOd6 as solvent. Mass spectra were recorded on a API 3200 LC/MS/MS mass spectrometer using electrospray ionization (ESI) in positive polarity.
General procedures for the formylation of phenols 2a–g: Method A
A solution of the substituted phenols 1 (0.5 mol) in 300 mL of 10 N NaOH (3 mol) was heated to 65 °C. Then 80 mL of CHCl3 was added in three portions over 15 min. The mixture was heated at reflux in chloroform for 2 h. After cooling, the mixture was acidified to pH 1 with 12 N HCl, the organic layer collected and the aqueous layer extracted with chloroform. The combined chloroform solution was dried and evaporated to give a crude product which was distilled or recrystallized from an appropriate solvent.
2-Hydroxybenzaldehyde 2a. This compound was obtained as yellowish oil, yield 20 %, bp 75–77 °C (0.4 mmHg); IR (KBr νmax cm−1), 1655 (C=O); 1H NMR (CDCl3, 400 MHz), δ = 7.00–8.10 (m, 4H, H3, H4, H5, H6), 9.85 (s, 1H, –CHO), 10.85 (s, 1H, exch D2O, –OH).
2-Hydroxy-5-methylbenzaldehyde 2b. This compound was obtained as white solid, yield 95 %, mp 48–50 °C (petroleum ether); IR (KBr νmax cm−1), 1650 (C=O), 2900–3000 (C–H); 1H NMR (CDCl3, 400 MHz), δ = 2.37 (s, 3H, –CH3), 6.90 (d, 1H, J = 8.60 Hz, H3), 7.25–7.50 (m, 2H, H4, H6), 9.80 (s, 1H, –CHO), 10.75 (s, 1H, exch D2O, –OH).
2-Hydroxy-5-ethylbenzaldehyde 2c. This compound was obtained as yellow oil, yield 36 %, bp 74–76 °C (0.3 mmHg); IR (KBr νmax cm−1), 1651 (C=O), 2900–3000 (C–H); 1H NMR (CDCl3, 400 MHz), δ = 1.25 (t, 3H, j = 7.41 Hz, –CH2–CH 3), 2.70 (q, 2H, j = 7.41 Hz, –CH 2–CH3), 6.83 (d, 1H, J = 9.50 Hz, H3), 7.26–7.52 (m, 2H, H4, H6), 9.87 (s, 1H, –CHO), 10.88 (s, 1H, exch D2O, –OH).
2-Hydroxy-5-isopropylbenzaldehyde 2d. This compound was obtained as yellow oil, yield 32 %, bp 78–80 °C (0.3 mmHg); IR (KBr νmax cm−1), 1650 (C=O), 2900–3000 (C–H); 1H NMR (CDCl3, 400 MHz), δ = 1.25 (d, 6H, j = 6.70 Hz, H 3C–CH–CH 3), 2.95 (h, 1H, j = 6.70 Hz, H3C–CH–CH3), 6.88–7.38 (m, 3H, H3,H4, H6), 9.87 (s, 1H, –CHO), 10.88 (s, 1H, exch D2O, –OH).
2-Hydroxy-5-methoxybenzaldehyde 2e, 2-Hydroxy-5-propoxybenzaldehyde 2f and 5-chloro-2-Hydroxybenzaldehyde 2g, products commercially available.
General procedures for the synthesis of 2-formylphenoxyacetadehyde diethyl acetals 3a–g: Method B
To a stirred suspension containing substituted 2-hydroxybenzaldehydes 2 (0.15 mol) and potassium carbonate (0.16 mol) in 100 mL of DMF, bromoacetaldehyde diethyl acetal (0.16 mol) was added drop wise. The mixture was refluxed for 4 h. After cooling, the precipitate was filtered off and the solvent evaporated under reduced pressure. The oily residue was distilled.
2-Formylphenoxy acetaldehyde diethylacetal 3a. This compound was obtained as yellowish oil, yield 85 %, bp 181–183 °C (5 mmHg); IR 1700 (C=O), 2900–3000 (C–H); 1H NMR (CDCl3, 400 MHz), δ = 1.23 (t, 6H, j = 6.42 Hz, (–OCH2–CH 3)2), 3.51–4.12 (m, 4H, (–OCH 2–CH3)2), 4.14 (d, 2H, j = 5.13 Hz, –CH 2–CH), 4.89 (t, 1H, j = 5.13 Hz, –CH2–CH), 6.95–7.82 (m, 4H, H3, H4, H5, H6), 10.48 (s, 1H, –CHO).
(2-Formyl-4-methylphenoxy)acetaldehyde diethylacetal 3b. This compound was obtained as yellow oil, yield 80 %, bp 192–194 °C (5 mmHg); IR (KBr νmax cm−1), 1700 (C=O), 2900–3000 (C–H); 1H NMR (CDCl3, 400 MHz), δ = 1.25 (t, 6H, j = 6.40 Hz, (–OCH2–CH 3)2), 2.30 (s, 3H, –CH3), 3.50–4.00 (m, 4H, (–OCH 2–CH3)2), 4.12 (d, 2H, j = 5.10 Hz, –CH 2–CH), 4.87 (t, 1H, j = 5.10 Hz, –CH2–CH), 6.90 (d, 1H, j = 8.20 Hz, H6), 7.40 (dd, 1H, j 1 = 2.11, j 2 = 8.20 Hz, H5), 7.62 (d, 1H, j = 2.11 Hz, H3), 10.50 (s, 1H, –CHO).
(2-Formyl-4-ethylphenoxy)acetaldehyde diethylacetal 3c. This compound was obtained as yellow oil, yield 81 %, bp 198–200 °C (5 mmHg); IR (KBr νmax cm−1), 1690 (C=O); 2900–3000(C–H); 1H NMR (CDCl3, 400 MHz), δ = 1.00–1.38 (m, 9H, (–OCH2–CH 3)2, –CH2–CH 3), 2.62 (q, 2H, j = 7.61 Hz, –CH 2–CH3), 3.50–4.00 (m, 4H, (–OCH 2–CH3)2), 4.08 (d, 2H, j = 4.70 Hz, –CH 2–CH), 4.88 (t, 1H, j = 4.70 Hz, –CH2–CH), 6.88 (d, 1H, j = 8.50 Hz, H6), 7.57 (dd, 1H, j 1 = 8.50 Hz, j 2 = 3.20 Hz, H5), 7.64 (d, 1H, j = 3.20 Hz, H3), 10.50 (s, 1H, –CHO).
(2-Formyl-4-isopropylphenoxy)acetaldehyde diethylacetal 3d. This compound was obtained as yellow oil, yield 80 %, bp 160–162 °C (0.3 mmHg); IR (KBr νmax cm−1), 1700 (C=O), 2900–3000 (C–H); 1H NMR (CDCl3, 400 MHz), δ = 1.00–1.30 (m, 12Η, (–OCH2–CH 3)2, H 3C–CH–CH 3), 2.92 (h, 1H, j = 6.41 Hz, H3C–CH–CH3), 3.50–3.90 (m, 4H, (–OCH 2–CH3)2), 4.10 (d, 2H, j = 5.30 Hz, –CH 2–CH), 4.88 (t, 1H, j = 5.30 Hz, –CH2–CH), 6.90 (d, 1H, j = 8.51 Hz, H6), 7.40 (dd, 1H, j 1 = 2.70, j 2 = 8.50 Hz, H5), 7.70 (d, 1H, j = 2.70 Hz, H3), 10.50 (s, 1H, –CHO).
(2-Formyl-4-methoxyphenoxy)acetaldehyde diethylacetal 3e. This compound was obtained as yellow oil, yield 68 %, bp 154–156 °C (0.3 mmHg); IR (KBr νmax cm−1), 1700 (C=O), 2900–3000 (C–H); 1H NMR (CDCl3, 400 MHz), δ = 1.25 (t, 6H, j = 6.40 Hz, (–OCH2–CH 3)2), 2.38 (s, 3H, –OCH3), 3.45–4.30 (m, 4H, (–OCH 2–CH3)2), 4.42 (d, 2H, j = 5.10 Hz, –CH 2–CH), 4.97 (t, 1H, j = 5.10 Hz, –CH2–CH), 6.95 (d, 1H, j = 8.21 Hz, H6), 7.45 (dd, 1H, j 1 = 2.11, j 2 = 8.21 Hz, H5), 7.65 (d, 1H, j = 2.11 Hz, H3), 10.56 (s, 1H, –CHO).
(2-Formyl-4-propoxyphenoxy)acetaldehyde diethylacetal 3f. This compound was obtained as yellow oil, yield 67 %, bp 180–183 °C (0.5 mmHg); IR (KBr νmax cm−1), 1700 (C=O), 2900–3000 (C–H); 1H NMR (CDCl3, 400 MHz), δ = 1.01 (t, 3H, j = 7.21 Hz, –OCH2–CH2–CH 3), 1.24 (t, 6H, j = 6.91 Hz, (–OCH2–CH 3)2), 1.78 (m, 2H, –OCH2–CH 2–CH3), 3.69 (m, 4H, (–OCH 2–CH3)2), 3.90 (t, 2H, j = 6.21 Hz, –OCH 2–CH2–CH3), 4.07 (d, 2H, j = 5.11 Hz, –CH 2–CH), 4.83 (t, 1H, j = 5.11 Hz, –CH2–CH), 6.76–7.31 (m, 3H, H3, H5, H6), 10.46 (s, 1H, –CHO).
(4-Chloro-2-formylphenoxy)acetaldehyde diethylacetal 3g. This compound was obtained as yellow oil, yield 90 %, bp 140–143 °C (0.3 mmHg); IR (KBr νmax cm−1), 1690 (C=O), 2900–3000 (C–H); 1H NMR (CDCl3, 400 MHz), δ = 1.26 (t, 6H, j = 7.60 Hz, (–OCH2–CH 3)2), 3.50–4.00 (m, 4H, (–OCH 2–CH3)2), 4.10 (d, 2H, j = 5.71 Hz, –CH 2–CH), 4.87 (t, 1H, j = 5.71 Hz, –CH2–CH), 6.95 (d, 1H, j = 8.50 Hz, H6), 7.48 (dd, 1H, j 1 = 2.81, j 2 = 8.50 Hz, H5), 7.79 (d, 1H, j = 2.81 Hz, H3), 10.43 (s, 1H, –CHO).
General procedures for the synthesis of substituted Benzo[b]furan-2-yl carboxaldehydes 4a–g: Method C
A stirred solution of compounds 3 (0.1 mol) in 35 mL of concentrated acetic acid was refluxed for 24 h. After cooling, the solution was evaporated to dryness. The crude product was distilled or recrystallized from an appropriate solvent.
2-Formylbenzo[b]furan 4a. This compound was obtained as yellow oil, yield 75 %, bp 121–123 °C (3 mmHg); IR (KBr νmax cm−1), 1680 (C=O); 1H NMR (CDCl3, 400 MHz), δ = 7.26–7.74 (m, 5Η, H3′, H4′, H5′, H6′, H7′), 9.84 (s, 1H, –CHO).
2-Formyl-5-methylbenzo[b]furan 4b. This compound was obtained as yellow solid, yield 66 %, mp 26–27 °C (ehanol/water 9/1); IR (KBr νmax cm−1), 1680 (C=O), 2900–3000 (C–H); 1H NMR (CDCl3, 400 MHz), δ = 2.38 (s, 3H, –CH3), 7.40–7.77 (m, 4Η, H3′, H4′, H6′,H7′), 9.87 (s, 1H, –CHO).
5-Ethyl-2-formylbenzo[b]furan 4c. This compound was obtained as yellowish oil, yield 80 %, bp 130–132 °C (3 mmHg); IR (KBr νmax cm−1), 1680 (C=O), 2900–3000 (C–H); 1H NMR (CDCl3, 400 MHz), δ = 1.30 (t, 3H, j = 7.90 Hz, –CH2–CH 3), 2.76 (q, 2H, j = 7.90 Hz, –CH 2–CH3), 7.25–7.70 (m, 4Η, H3′, H4′, H6′, H7′), 9.86 (s, 1H, –CHO).
2-Formyl-5-isopropylbenzo[b]furan 4d. This compound was obtained as yellowish oil, yield 56 %, bp 131–133 °C (3 mmHg); IR (KBr νmax cm−1), 1700 (C=O), 2900–3000 (C–H); 1H NMR (CDCl3, 400 MHz), δ = 1.28 (d, 6H, j = 6.30 Hz, H 3C–CH–CH 3), 3.00 (h, 1H, j = 6.30 Hz, H3C–CH–CH3), 7.48–7.63 (m, 4H, H3′, H4′, H6′, H7′), 9.85 (s, 1H, –CHO).
2-Formyl-5-methoxybenzo[b]furan 4e. This compound was obtained as yellow solid, yield 70 %, mp 82–84 (diisopropyl ether); IR (KBr νmax cm−1), 1700 (C=O), 2900–3000 (C–H); 1H NMR (CDCl3, 400 MHz), δ = 2.41 (s, 3H, –OCH3), 7.43–7.80 (m, 4H, H3′, H4′, H6′, H7′), 9.89 (s, 1H, –CHO).
2-Formyl-5-propoxybenzo[b]furan 4f. This compound was obtained as yellow solid, yield 85 %, mp 65–67 °C (diisopropyl ether); IR (KBr νmax cm−1), 1700 (C=O), 2900–3000 (C–H); 1H NMR (CDCl3, 400 MHz), δ = 0.97 (t, 3H, j = 7.20 Hz, –OCH2–CH2–CH 3), 1.73 (m, 2H, –OCH2–CH 2–CH3), 3.94 (t, 2H, j = 6.60, –OCH 2–CH2–CH3), 7.15 (dd, 1H, j 1 = 9.10 Hz, j 2 = 2.70 Hz, H6′), 7.31 (d, 1H, j = 2.70 Hz, H4′), 7.61 (d, 1H, j = 9.10 Hz, H7′), 7.86 (s, 1H, H3′), 9.79 (s, 1H, –CHO).
5-Chloro-2-formylbenzo[b]furan 4g. This compound was obtained as yellow solid, yield 60 %, mp 126–127 °C (ethyl acetate); IR (KBr νmax cm−1), 1670 (C=O); 1H NMR (CDCl3, 400 MHz), δ = 7.25–7.75 (m, 4Η, H3′, H4′, H6′, H7′), 9.86 (s, 1H, CHO).
General procedures for the synthesis of substituted 3-benzo[b]furan-2-ylmethylene-levulinic acids 5a–g: Method D
A stirred solution of compounds 4 (0.1 mol) in 35 mL of concentrated acetic acid was refluxed for 24 h. After cooling, the solution was evaporated to dryness. The obtained residue was used crude for the continuation.
General procedures for the synthesis of substituted 5-(benzo[b]furan-2-ylmethyl)-6-methylpyridazin-3(2H)-ones 6a–g: Method E
The mixture of acids 5 and hydrazine hydrate solution in ethanol was refluxed for 2 h; the precipitate formed is filtered and recrystallized from an appropriate solvent.
5-(Benzo[b]furan-2-ylmethyl)-6-methylpyridazin-3(2H)-one 6a. This compound was obtained as yellow solid, yield 69 %, mp 181–183 °C (ethanol); IR (KBr νmax cm−1), 1604 (C=N), 1662 (C=O), 2900–3000 (C–H); 1H NMR (DMSOd6, 400 MHz), δ = 2.22 (s, 3H, –N=C–CH3), 4.08 (s, 2H, –CH2–), 6.97 (s, 1H, H4), 6.71 (s, 1H, H3′), 7.19–7.58 (m, 4H, H4′, H5′, H6′, H7′), 12.71 (ls, 1H, NH). MS m/z; 241.2 [M + H]+, 238.9 [M + H]−, 263.3 [M + Na]+.
5-(5-Methylbenzo[b]furan-2-ylmethyl)-6-methylpyridazin-3(2H)-one 6b. This compound was obtained as yellow solid, yield 80 %, mp 183–184 °C (ethanol); IR (KBr νmax cm−1), 1603 (C=N), 1666 (C=O), 2900–3000 (C–H); 1H NMR (DMSOd6, 400 MHz), δ = 2.23 (s, 3H, –N=C–CH3), 2.37 (s, 3H, Ar–CH3), 4.07 (s, 2H, –CH2–), 6.58 (s, 1H, H4), 6.64 (s, 1H, H3′), 7.07 (dd, 1H, j 1 = 8.41 Hz, j 2 = 1.21 Hz, H6′), 7.36 (d, 1H, j = 1.21 Hz, H4′), 7.40 (d, 1H, j = 8.41 Hz, H7′), 12.74 (ls, 1H, NH). MS m/z; 255.1 [M + H]+, 253.3 [M + H]−, 276.9 [M + Na]+.
5-(5-Ethylbenzo[b]furan-2-ylmethyl)-6-methylpyridazin-3(2H)-one 6c. This compound was obtained as brown solid, yield 73 %, mp 180–181 °C (ethanol); IR (KBr νmax cm−1), 1605 (C=N), 1661 (C=O), 2900–3000 (C–H); 1H NMR (DMSOd6, 400 MHz), δ = 1.18 (t, 3H, j = 7.50 Hz, –CH2–CH 3), 2.21 (s, 3H, –N=C–CH3), 2.65 (q, 2H, j = 7.50 Hz, –CH 2–CH3), 4.05 (s, 2H, –CH2–), 6.56 (s, 1H, H4), 6.64 (s, 1H, H3′), 7.08 (dd, 1H, j 1 = 8.40 Hz, j 2 = 1.80 Hz, H6′), 7.37 (d, 1H, j = 1.80 Hz, H4′), 7.41 (d, 1H, j = 8.40 Hz, H7′), 12.76 (ls, 1H, NH). MS m/z; 269.5 [M + H]+, 267.1 [M + H]−, 291.3 [M + Na]+.
5-(5-Isopropylbenzo[b]furan-2-ylmethyl)-6-methylpyridazin-3(2H)-one 6d. This compound was obtained as brown solid, yield 82 %, mp 185–186 °C (ethanol); IR (KBr νmax cm−1), 1605 (C=N), 1682 (C=O), 2900–3000 (C–H); 1H NMR (DMSOd6, 400 MHz), δ = 1.24 (d, 6H, j = 6.90 Hz, H 3C–CH–CH 3), 2.10 (s, 3H, –N=C–CH3), 2.99 (h, 1H, j = 6.90 Hz, H3C–CH–CH3), 3.90 (s, 2H, –CH2–), 7.30–7.76 (m, 5H, H4, H3′, H4′, H6′, H7′), 12.32 (ls, 1H, NH). MS m/z; 283.4 [M + H]+, 281.2 [M + H]−, 337.4 [M + Na]+.
5-(5-Methoxybenzo[b]furan-2-ylmethyl)-6-methylpyridazin-3(2H)-one 6e. This compound was obtained as yellow solid, yield 78 %, mp 190–192 °C (ethanol); IR (KBr νmax cm−1), 1650 (C=O), 2950–3000 (C–H); 1H NMR (DMSOd6, 400 MHz), δ = 2.25 (s, 3H, –N=C–CH3), 3.74 (s, 3H, –OCH3), 4.04 (s, 2H, –CH2–), 6.55 (s, 1H, H4), 6.64 (s, 1H, H3′), 6.82 (dd, 1H, j = 8.70 and 2.71 Hz, H6′), 7.08 (d, 1H, j = 2.71 Hz, H4′), 7.40 (d, 1H, j = 8.70 Hz, H7′), 12.75 (ls, 1H, NH). MS m/z; 299.4 [M + H]+, 297.1 [M + H]−, 321.4 [M + Na]+.
5-(5-Propoxybenzo[b]furan-2-ylmethyl)-6-methylpyridazin-3(2H)-one 6f. This compound was obtained as brown solid, yield 87 %, mp 194–196 °C (ethanol); IR (KBr νmax cm−1), 1652(C=O), 2940–3000(C–H); 1H NMR (DMSOd6, 400 MHz), δ = 0.96 (t, 3H, j = 6.90 Hz, –OCH2–CH2–CH 3), 1.71 (m, 2H, –OCH2–CH 2–CH3), 2.21 (s, 3H, –N=C–CH3), 3.90 (t, 2H, j = 6.90 Hz, –OCH 2–CH2–CH3), 4.04 (s, 2H, –CH2–), 6.55 (s, 1H, H4), 6.62 (s, 1H, H3′), 6.81 (dd, 1H, j = 9.00 and 2.71 Hz, H6′), 7.06 (d, 1H, j = 2.71 Hz, H4′), 7.38 (d, 1H, j = 9.00 Hz, H7′), 12.74 (ls, 1H, NH).
5-[(5-Chlorobenzo[b]furan-2-yl)methyl]-6-methylpyridazin-3(2H)-one 6g. This compound was obtained as yellow solid, yield 70 %, mp 198–200 °C (ethanol); IR (KBr νmax cm−1), 1649(C=O); 1H NMR (DMSOd6, 400 MHz), δ = 2.23 (s, 3H, –N=C–CH3), 4.11 (s, 2H, –CH2–), 6.60 (s, 1H, H4), 6.74 (s, 1H, H3′), 7.29 (dd, 1H, j = 8.71 and 2.16 Hz, H6′), 7.58 (d, 1H, j = 8.71 Hz, H4′), 7.66 (d, 1H, j = 2.16 Hz, H7′), 12.76 (ls, 1H, NH). MS m/z; 275.0 [M + H]+, 272.7 [M + H]−, 297.0 [M + Na]+.
Antidepressant activity
To evaluate the structure–activity relationship, the effects of the substituents on C5 of the benzofuran ring were considered. As shown in Table 1, we noticed that the increase in the aliphatic chain size in the C5 of benzofuran reduced the immobility time of the mice. Among all the pyridazinone compounds tested at a dose of 50 mg kg−1 orally in adult mice in FST (Hirota et al., 1986), compounds 6c and 6d were found to be the most active structures, compared to fluoxetine tested at a dose of 32 mg kg−1.
In conclusion, through our drug design and discovery program, we were able to develop a new series of pyridazinone derivatives as potential antidepressant agents. Our ongoing studies are directed toward the detailed mechanistic and pharmacokinetic studies on compound 6d so as to advance this molecule into a therapeutic option. Interestingly, all active compounds did not cause any significant alteration of locomotor activity in mice as compared to control, indicating that the hybrids did not produce any motor impairment effects. The results indicate that pyridazin-3(2H)-one derivatives may have potential therapeutic value for the management of mental depression.
Abbreviations
- 1H NMR:
-
1H nuclear magnetic resonance
- CDCl3 :
-
Deuterated chloroform
- CHCl3 :
-
Chloroform
- CNS:
-
Central nervous system
- DMF:
-
Dimethylformamide
- DMSOd6 :
-
Deuterated dimethylsulfoxide
- ESI:
-
Electrospray ionization
- FST:
-
Forced swimming test
- HCl:
-
Chlohydric acid
- IR:
-
Infrared
- KBr:
-
Potassium bromide
- LD:
-
Lethal dose
- MAOIs:
-
Monoamine oxidase inhibitors
- MDD:
-
Major depression disorder
- NaOH:
-
Potassium hydroxide
- SNRIs:
-
Serotonin–norepinephrine reuptake inhibitors
- SSRIs:
-
Selective serotonin reuptake inhibitors
- TCAs:
-
Tricyclic antidepressants
- TMS:
-
Tetramethylsilane
- TRI:
-
Triple reuptake inhibitors
- USFDA:
-
United States Food and Drug Administration
- WHO:
-
World Health Organization
References
Al-Habeeb AA, Sherra KS, Al-Sharqi AM, Qureshi NA (2013) Assessment of suicidal and self-injurious behaviours among patients with depression. East Mediterr Health J 19:248–254
Anderson HD, Pace WD, Libby AM, West DR, Valuck RJ (2012) Rates of 5 common antidepressant side effects among new adult and adolescent cases of depression: a retrospective US claims study. Clin Ther 34(1):113–123
Benmoussa A, El harti J, Ansar M, Bouchrik M, Zahidi A, Cherrah Y, Bouklouze A, Taoufik J (2012) Synthesis and antimicrobial properties of some pyridazin-3-thiones derivatives. Int J Pharm Tech Res 4:1591–1594
Bosc JJ, Jarry C, Carpy A, Panconi E, Descas P (1990) Synthesis and antidepressant activity of 5-(1-aryl-4-piperazino)methyl-2-amino-2-oxazollines. Eur J Med Chem 27:437–442
Castro ME, Rosa E, Osuna JA, Ferreiro TG, Loza M, Cadavid MI, Fontenla JA, Masaguer CF, Cid J, Raviña E, Mera GG, Rodriguez J, Ceballos ML (1994) Pyridazine derivatives XII. Synthesis and antipsychotic-antidepressant activity of some butyrophenone derivatives of 6-phenylpyridazine. Eur J Med Chem 29(11):831–839
Chancellor D (2011) The depression market. Nat Rev Drug Discov 10(11):809–810
Check E (2004) Antidepressants: bitter pills. Nature 431:122–124
DeWeerdt S (2013) Mood disorders: the dark night. Nature 497:14–15
Friedman RA, Leon ACN (2007) Expanding the black box-depression, antidepressants, and the risk of suicide. Engl J Med 356(23):2343–2346
Hampton T (2012) Chronic stress and depression. JAMA 308(5):444. World Health Organisation. http://www.who.int/entity/mental_health/management/depression/flyer_depression_2012.Pdf. Accessed 23 July 2013
Hirota T, Sasaki K, Namba T (1986) A novel synthesis of Benzofuran and related compounds III. The vilsmeier reaction of phenoxyacetaldehyde diethyl acetals. Het Chem 23:1715–1716
Huang Q, Qian X, Song G, Cao S (2003) The toxic and anti-feedant activity of 2H-pyridazin-3-one-substituted 1,3,4-oxadiazoles against the armyworm Pseudaletia separata (Walker) and other insects and mites. Pest Manag Sci 59(8):933–939
Lattmann E, Ayuko WO, Kinchinaton D, Langley CA, Singh H, Karimi L, Tisdale MJJ (2003) Synthesis and evaluation of 5-arylated 2(5H)-furanones and 2-arylated pyridazin-3(2H)-ones as anti-cancer agents. Pharm Pharmacol 55(9):1259–1265
Licinio J, Wong ML (2005) Depression, antidepressants and suicidality: a critical appraisal. Nat Rev Drug Discov 4(2):165–171
Mokrosz JL, Pietrasiewicz M, Duszyńska B, Cegła MT (1992) Structure-activity relationship studies of central nervous system agents. Effect of the hydrocarbon chain on the affinity of 4-substituted 1-(3-chlorophenyl)piperazines for 5-HT1A receptor site. J Med Chem 35(13):2369–2374
Özadalı K, Özkanlı F, Jain S, Rao PP, Velázquez-Martínez CA (2012) Synthesis and biological evaluation of isoxazolo[4,5-d]pyridazin-4-(5H)-one analogues as potent anti-inflammatory agents. Bioorg Med Chem 20(9):2912–2922
Perregaard J, Arnt J, Bøgesø KP, Hyttel J, Sánchez C (1992) Non cataleptogenic, centrally acting dopamine D-2 and serotonin 5-HT2 antagonists within a series of 3-substituted 1-(4-fluorophenyl)-1H-indoles. J Med Chem 35(6):1092–1101
Petit-Demouliere B, Chenu F, Bourin M (2005) Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology 177(3):245–255
Porsolt RD, Bertin A, Jalfre M (1977) Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn 229(2):327–336
Porsolt RD, Anton G, Blavet N, Jalfre M (1978) Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharm 47(4):379–391
Rubat C, Coudert P, Bastide P, Tronche P (1995) Behavioural profile of two potential antidepressant pyridazine derivatives including arylpiperazinyl moieties in their structure, in mice. J Pharm Pharmacol 47(2):162–170
Sladowska H (1993) Investigations on the synthesis and properties of some N-arylpiperazinylalkyl derivatives of imides of 3,4-pyridinedicarboxylic acids. Farmaco 48(1):85–94
Thoer A, Denis G, Delmas M, Gaset A (1988) The Reimer–Tiemann reaction in slightly hydrated solid-liquid medium: a new method for the synthesis of formyl and diformylphenols. Synth Commun 18(16–17):2095–2101
World Health Organisation (2012). http://www.who.int/entity/mental_health/management/depression/flyer_depression_2012.Pdf. Accessed 23 July 2013
Acknowledgments
The authors are grateful to the University Mohammed V and Laboratory of control of drugs, Rabat-Morocco, for, IR, RMN and MASS spectra and the studies of antidepressant activity.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boukharsa, Y., Meddah, B., Tiendrebeogo, R.Y. et al. Synthesis and antidepressant activity of 5-(benzo[b]furan-2-ylmethyl)-6-methylpyridazin-3(2H)-one derivatives. Med Chem Res 25, 494–500 (2016). https://doi.org/10.1007/s00044-015-1490-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-015-1490-x