Abstract
In this study, 19 3H-benzo[f]chromen chalcone derivatives (2a–2s) were obtained from 2-hydroxy-1-naphthaldehyde as the starting material, and their structures were confirmed by IR, 1H NMR, 13C NMR, and ESI-MS analyses. The antidepressant activities of the compounds were evaluated in mice after one 30 mg/kg dose by means of forced swimming tests, and 18 of the compounds (2a–2l, 2n–2s) showed antidepressant activity, of which three (2b, 2d, and 2n) showed strong antidepressant activity. Furthermore, all the compounds showed some anticonvulsant activity, with 11 of the compounds (2a–2g, 2k, 2m, 2n, and 2q) inhibiting convulsions in the maximal electroshock seizure (MES) test after one dose of 100 mg/kg, and the other eight inhibiting convulsions in the MES after one dose of 300 mg/kg. In the tail suspension test, all the compounds did not show neurotoxicity at the same dose. This research provides an experimental theoretical basis for finding new antidepressants with high biological activity and few side effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression is an affective disorder characterized by significant and lasting cognitive dysfunction and is one of the most disabling conditions in the world [1, 2]. Its high incidence, low cure rate, unclear pathogenesis, long treatment cycle, and repeated occurrence make research into its treatment all the more important [3]. Coppen et al. were the first to propose in 1965 that the occurrence of depression is due to the decline of 5-HT function in the central nervous system, the decrease of 5-HT release, and the decrease of synaptic cleft content [4]. Janowsky et al. proposed in 1972 that hyperactivity of Achrgic neurons and hypofunction of adrenergic neurons, the imbalance between the two lead to depression [5]. There are more and more published studies on the pathogenesis of depression, which make our understanding of its biological aspects has seen considerable progress in the past several decades [6, 7]. In these studies, it was found that the active components of anti-depressive mainly include flavonoids, alkaloids, saponins, and volatile oils [8,9,10,11,12]. Epilepsy is a brain disease characterized by recurrent seizures as well as emotional and cognitive dysfunction [13]. The clinical manifestations are characterized by repeated episodes of transient loss of consciousness, limb spasms, and convulsions. This disease affects 50 million people worldwide and is therefore one of the most common neurological diseases [14]. Antiepileptic drugs (AEDs) act on different molecular targets to selectively modify the excitability of neurons, thereby blocking the destabilizing activity without interfering with the non-epileptic activities of normal signals between neurons Excitatory discharge [15]. When considering the principle of action of AEDs, there are mainly the following mechanisms: (1) the regulation of voltage-dependent ion channels; (2) the enhancement of synaptic inhibition; (3) the inhibition of synaptic excitement [16].
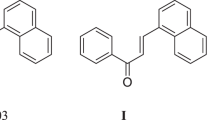
Chalcones, also known as 1,3-diaryl-2-propen-1-ones (Fig. 1), are a subclass of flavonoids that exist widely in nature, being mainly distributed in Compositae, Leguminosae, and Gramineae family plants [17]. Natural chalcones and their synthetic analogs have been demonstrated to have a wide range of biological effects, including anticancer [18], antioxidant [19], antibacterial [20], antileishmanial [21], antimalarial [22], antiangiogenesis [23], antituberculosis [24], antidepressant [25], antihistamine [26], anti-obesity [27], antispasmodic [28], and anti-inflammatory [29] activities. As a specific flavonoid, chalcone has also been shown to exhibit anticonvulsant activities [30]. Accordingly, chalcone and its derivatives are of increasing interest in academia and industry, and pure chalcone isolates from several plants have been clinically trialed for the treatment of cancer, viruses, and cardiovascular diseases [31]. Furthermore, the antidepressant activities of numerous flavonoids have been recently reported [32,33,34], with the mechanism of their antidepressant effects in mice being attributed to their effect on the gene expression of reverse monoamine neurotransmitters and receptors [35,36,37]. Although there are effective methods for the treatment of depression, about one-third of depression patients are ineffective to traditional antidepressant treatment [38], and the antidepressants that are currently on the market have certain toxic side effects. Therefore, it is still necessary to develop new antidepressants with quick effects and low side effects.
Our research group is engaged in the identification and structural optimization of flavonoid antidepressant and anticonvulsant lead compounds. We have found that benzochromene compounds have a wide range of pharmacological activities, and many studies have shown that chromene derivatives, such as monoamine oxidase, serotonin, dopamine, and acetylcholinesterase inhibitors, act on central nervous system receptors. These findings indicate that chromene compounds show promise for the treatment of depression, schizophrenia, and Alzheimer’s disease [39]. As a major biochemical enzyme, monoamine oxidase affects the levels of several major neurotransmitters in the central nervous system, such as serotonin, norepinephrine, and dopamine. It is also the main enzyme of the earliest antidepressant drugs. As we all know, coumarin (chromen-2-one) is a natural product active compound with a wide range of pharmacological effects. Chen et al. reported that coumarin derivatives have antidepressant activity [40], and Ariza et al. found that coumarin derivatives showed certain anticonvulsant activity [41]. Vergel et al. discovered the compound FCS-304 (1-propyl-3H-benzo[f]chromen-3-one) (Fig. 1), a coumarin compound, FCS-304 (25–200 mg/kg, p.o.) significantly reduces the immobility time in the forced swimming test (FST) and the tail suspension test (TST) in mice. However, no effect was shown in the rotating rod test, maximum electroshock seizure test, pentylenetetrazole seizure test, light and dark box test, barbiturate sleep time test, and cold plate test. In addition, FCS-304 can inhibit monoamine oxidase, IC50: 2.28 ± 0.15 µM. Its antidepressant activity is related to selective monoamine oxidase [42].
Accordingly, in the present study, we adopted the rational drug design principle and synthesized 19 3H-benzo[f]chromen chalcone derivatives (Fig. 1) from β-hydroxynaphthaldehyde and acrolein via the Claisen-Schmidt reaction (Scheme 1, 2a–2s) and evaluated their antidepressant and anticonvulsant activities as well as their neurotoxicities.
Results and discussion
β-Hydroxynaphthaldehyde was subjected to aldol condensation with acrolein to produce the intermediate 3H-benzo[f]chromene-2-carbaldehyde, which was then reacted with different acetophenones through Claisen-Schmidt condensation (Scheme 1). The structures of the target compounds 2a–2s were confirmed by 1H NMR, 13C NMR, FT-IR, and ESI-MS analyses.
The 1H NMR spectrum of compound 2b features a singlet peak at 5.14 ppm, which is due to two methylene hydrogens (–CH2), and doublet peaks at 6.79 and 7.48 ppm, which are due to the two olefin hydrogens (–CH=CH–). A singlet peak appears at 7.12 ppm, which is due to the 4-methine group (=CH). Multiple peaks appear in the range 7.14–7.84 ppm due to aromatic hydrogen atoms.
The 13C NMR spectrum of compound 2d features a methylene (–CH2) carbon peak at 65.00 ppm. Compound 2b presents two peaks for the carbon–carbon double bond (–CH=CH–) at 128.81 and 143.33 ppm and peaks for the 4-methine carbon (=CH) at 124.33 ppm, and at 193.40 ppm, the carbon peak of the carbonyl group (C=O) is shown at the place.
The IR spectrum of compound 2a shows characteristic C–O and C=O peaks at 1546 and 1653 cm−1, respectively. This may be because the electronegativity of oxygen is greater than that of carbon. Therefore, the electron pair is attracted toward the oxygen atom, causing the carbon atom to carry partial positive charge, changing its dipole moment greatly.
The FST is a method that is used to induce depression in mice that was first described by Porsolt et al. [43]. In the FST, the depression of a mouse is reflected by its immobility time in the experiment, and this behavior is thought to be an adequate model of human depression. A wide range of antidepressants and chemical compounds with potential antidepressant effects have been shown to reduce immobility time [44]. Therefore, this model is the most widely used in the preclinical screening of antidepressants.
The effects of the target compounds 2a–2s and the widely known antidepressant fluoxetine hydrochloride on the duration of immobilization in the FST are given in Table 1. The data show that, at a dose of 30 mg/kg, 18 of the compounds (2a–2l, 2n–2s) show antidepressant activity, of which three (2b, 2d, and 2n) exhibit relatively high activities. Specifically, the reductions in immobility time are 85.05, 75.38, and 67.52% for 2b, 2d, and 2n, respectively. The antidepressant activity of compound 2n is slightly lower than that of fluoxetine hydrochloride; the antidepressant activity of compound 2d is similar to that of fluoxetine hydrochloride; and the antidepressant activity of compound 2b is significantly higher than that of fluoxetine hydrochloride. Indeed, the immobility-reduction rate of compound 2b is the highest of all the compounds evaluated in this study, being 11% higher than that of fluoxetine hydrochloride.
To investigate the antidepressant effects of the target chemical compounds in more detail, the percentage decrease in the duration of immobilization (% DID) was calculated using the following formula:
where X indicates the duration of immobilization (s) for the control group and Y indicates the duration of immobilization (s) for the test group (Fig. 2).
Compounds 2b, 2d, and 2n exhibit the highest antidepressant activities and were thus further evaluated for their antidepressant effects at three lower doses (5, 10, and 20 mg/kg) (Table 2). These compounds were found to decrease DID in a dose-dependent manner. Furthermore, fluoxetine has a DID value of 77.12% at 5 mg/kg per dose, which is lower than that of compound 2b (87.28%) at 20 mg/kg per dose.
The antidepressant effects of compounds 2b, 2d, and 2n were further evaluated in TSTs after three low-dose treatments (5, 10, and 20 mg/kg), and the results are shown in Table 3. Administration of compounds 2b, 2d, and 2n results in significant decreases in inactivity duration, revealing antidepressant effects in a dose-dependent manner from 10 to 20 mg/kg. Compound 2b exhibits antidepressant activity with a DID value of 71.78% at 20 mg/kg, which is superior to that of fluoxetine (56.70%) at 5 mg/kg.
Examining the structure-activity relationship for the antidepressant effects of compounds 2a–2s reveals that compounds with with donor and acceptor group substituents on the benzene ring demonstrate good antidepressant activities. Furthermore, for the seven compounds with electron-donating substituents, i.e., –CH3, –CH2CH3, –(CH3)2, –OCH3, –(OCH3)2, –NH2, and –N(CH3)2 groups, the antidepressant activities decrease in the order 2n > 2o > 2r > 2s > 2q > 2p > 2m. Compound 2n, which has a –OCH3 substituent on the benzene ring, significantly decreases the immobility time in the FST and thus has high antidepressant activity.
In addition, the nine compounds with electron-withdrawing substituents on the benzene ring, namely F atoms (2a, 2b, and 2c), Cl atoms (2d, 2e, and 2f), and Br atoms (2h, 2i, and 2j), show extremely good antidepressant effects. Furthermore, the substitution pattern significantly affects their antidepressant activities. For the compounds with an F atom on the benzene ring, their activities increase in the order o-F < p-F < m-F, while the corresponding order for Cl substitution is p-Cl < m-Cl < o-Cl, and that for Br substitution is p-Br < o-Br < m-Br. However, compound 2g, which has 2,4-dichloro substitution on the benzine ring, exhibits no antidepressant activity. Compounds 2k and 2l, which have trifluoromethyl (–CF3) and nitro (–NO2) substituents, respectively, show significant antidepressant effects and decrease the fixed duration of mice by 56.19% and 41.84%, respectively. Therefore, it may be important to consider the relationship between the structure and activity and the substituent groups attached to the benzene ring.
We used the maximal electroshock seizure (MES) test to study the anticonvulsant activities of the compounds, and the neurotoxicities of the compounds were evaluated by the rotating rod experiment. The test results are shown in Table 4. Of compounds 2a–2s, 11 of the compounds (2a, 2b, 2c, 2d, 2e, 2f, 2g, 2k, 2m, 2n, and 2q) inhibit convulsion at a dose of 100 mg/kg, while eight (2h, 2i, 2j, 2l, 2o, 2p, 2r, and 2s) inhibit convulsion at a dose of 300 mg/kg.
In general, the anticonvulsant activities of the 3H-benzo[f]chromen chalcone derivatives with electron-withdrawing groups on the benzene ring are stronger than those with corresponding electron-donating groups. Additionally, the F-substituted and Cl-substituted derivatives show anticonvulsant activity at 100 mg/kg, which is lower than the dose required for the Br-substituted derivatives. For the compounds with electron-donating groups on the benzene ring, the p-OCH3, p-CH2CH3, and p-CH3 derivatives exhibit higher anticonvulsant activities than the p-N(CH3)2, p-NH2, 2,4-(OCH3)2, and 2,4-(CH3)2 derivatives.
Rotating rod experiments were performed to determine whether the 3H-benzo[f]chromen chalcone derivatives cause motor dysfunction. As shown in Table 4, none of the compounds except 2g and 2m show any neurotoxicity at 0.5 or 4 h after administration at either 100 or 300 mg/kg.
Conclusion
In this study, we synthesized 19 3H-benzo[f]chromen chalcone derivatives and evaluated their antidepressant and anticonvulsant activities as well as their neurotoxicities. Compounds 2a–2s were shown to decrease immobility time in FST at a dose of 30 mg/kg, reflecting their antidepressant effects. Compounds 2b, 2d, and 2n were shown to have antidepressant effects better than or comparable to that of fluoxetine hydrochloride. In addition, all the chemical compounds were demonstrated to exhibit anticonvulsant activities. In general, the anticonvulsant activities of the derivatives with electron-withdrawing groups on the benzene ring were found to be stronger than those with electron-donating groups on the benzene ring, and all the F– and Cl-substituted derivatives were found to be more active than the Br-substituted derivatives. In neurotoxicity experiments, the results showed that all 3H-benzo[f]chromen chalcone derivatives did not show any neurotoxicity within 0.5–4 h after administration at the same dose level.
Material and methods
Materials
Fluoxetine hydrochloride (purity >99%) was purchased from Sigma-Aldrich (Saint Louis, MO, USA). Melting points were recorded using a digital melting point measuring instrument (WRS-1B; Shanghai Precision Scientific Instruments & Equipment, China, Shanghai). An HP1100LC/MS system (CA, USA, Santa Clara, Agilent Technologies) was used to record the mass spectra. A Fourier transform-infrared (FT-IR) system (1730, Billerica, Bruker, MA, USA) was used to record IR using KBr disks. An AV-300 system (Bruker) was used to measure the nuclear magnetic resonance (1H-NMR and 13C-NMR) of the compounds. All reagents were of analytical grade. The compounds 2a–2s were synthesized through Claisen-Schmidt condensation with commercial 2-hydroxy-1-naphthaldehyde and substituted acetophenone [45, 46].
Chemistry
Synthesis of 3H-benzo[f]chromene-2-carbaldehyde (1)
β-hydroxynaphthaldehyde (0.02 mol, 3.444 g) and potassium carbonate (0.023 mol, 3.179 g) were added to a round-bottom flask and placed in a magnetic stirrer. An appropriate amount of 1, 4- dioxane solvent was added to the flask and heated to 101 °C. The mixture was refluxed, stirred, and activated for 1 h. Then, acrolein (0.03 mol, 2.002 mL) was added to the system, and the mixture was heated to reflux. Next, 50 mL of 1 M NaOH solution was added to the system and extracted with ether (30 mL × 2) when the reaction was complete. The washed organic layer was dried overnight with an appropriate amount of anhydrous sodium sulfate. Subsequently, the dried sample was filtered to remove sodium sulfate, and the solvent was evaporated using rotary evaporation apparatus to obtain the intermediate solid 3H-benzo[f]chromene-2-carbaldehyde.
Synthesis of 3H-benzo[f]chromen chalcone derivatives (2a–2s)
Compound 1 (3 mmol, 0.63 g) and 3.9 mmol of differently substituted acetophenone was placed into a round-bottom flask. Consequently, 20 mL absolute ethanol and 6 mL 3.5 M NaOH solution were added to the flask, and the system was placed in an ice-water bath and stirred overnight. The completely reacted solution was diluted with water and filtered to obtain a yellow or orange precipitate. The precipitate was dissolved using ethyl acetate and recrystallized to obtain a series of 3H-benzo[f]chromen chalcone derivative crystals (2a–2s).
(E)-3-(3H-benzo[f]chromen-2-yl)-1-(2-fluorophenyl) propyl-2-en-1-one (2a)
Yield: 43.5%, m.p.150.5–151.7 °C. 1H-N-MR (300 MHz, CDCl3): δ 5.14 (s, 2H, –CH2), 6.79 (d, 15.57 Hz, 1H, = CH), 7.12 (s, 1H, = CH), 7.14–7.33 (m, 2H, Ar–H), 7.37–7.46 (m, 1H, Ar–H), 7.55 (d, J = 15.57 Hz, 1H, = CH), 7.49–7.62 (m, 3H, Ar–H), 7.63–7.68 (m, 1H, Ar–H), 7.72–7.80 (m, 2H, Ar–H), 7.84 (t, J = 1.86, 7.51 Hz, 1H, Ar–H).13C-NMR (75 MHz, CDCl3): 65.1, 115.2, 116.4, 116.7, 117.4, 121.2, 124.0, 124.3, 124.6, 127.4, 127.5, 128.8, 129.0, 129.5, 130.2, 131.0, 131.8, 133.9, 142.1, 153.9, 162.8, 188.51. IR (KBr) cm−1: 1653 (C=O), 1546 (C–O). HRMS m/z: calcd for C22H15FO2 330.11, found 330.10 (M + H+).
(E)-3-(3H-benzo[f]chromen-2-yl)-1-(3-fluorophenyl) propyl-2-en-1-one (2b)
Yield: 44.3%, m.p. 173.5–176.1 °C. 1H NMR (300 MHz, CDCl3): δ 5.14 (s, 2H, –CH2), 6.79 (d, J = 15.57 Hz, 1H, = CH), 7.12 (s, 1H, = CH), 7.14–7.24 (m, 1H, Ar–H), 7.25–7.33 (m, 1H, Ar–H), 7.37–7.46 (m, 1H, Ar–H), 7.48 (d, J = 15.57 Hz, 1H, = CH), 7.49–7.62 (m, 3H, Ar–H), 7.63–7.80 (m, 3H, Ar–H), 7.84 (m, J = 1.86, 7.51 Hz, 1H, Ar–H).13C-NMR (75 MHz, CDCl3): 65.1, 115.1, 115.4, 117.4, 119.6, 119.9, 121.2, 124.0, 124.4, 127.2, 127.5, 128.8, 129.3, 129.5, 130.3, 131.9, 140.4, 142.8, 153.9, 161.3, 164.6, 188.7. IR (KBr) cm−1: 1654 (C=O), 1548 (C–O). HRMS m/z: calcd for C22H15FO2 330.11, found 330.18 (M + H+).
(E)-3-(3H-benzo[f]chromen-2-yl)-1-(4-fluorophenyl) propyl-2-en-1-one (2c)
Yield: 45.6%, m.p. 175.4–177.1 °C. 1H NMR (300 MHz, CDCl3): δ 5.17 (s, 2H, –CH2), 6.85 (d, J = 2.41, 15.57 Hz, 1H, = CH), 7.11 (s, 1H, = CH), 7.14–7.25 (m, 1H, Ar–H), 7.25–7.33 (m, 1H, Ar–H), 7.38–7.46 (m, 1H, Ar–H), 7.49–7.65 (m, 4H, Ar–H), 7.68 (d, J = 15.57 Hz, 1H, = CH), 7.72–7.80 (m, 2H, Ar–H), 7.84 (t, J = 1.86, 7.51 Hz, 1H, Ar–H).13C-NMR (75 MHz, CDCl3): 65.1, 115.2, 115.6, 115.9, 117.4, 119.7, 121.2, 124.3, 127.2, 127.5, 128.7, 128.8, 128.9, 129.5, 130.2, 130.9, 131.0, 131.8, 134.6, 142.3, 153.8, 188.4. IR(KBr) cm−1: 1654 (C=O), 1547 (C–O). HRMS m/z: calcd for C22H15FO2 330.11, found 330.06 (M + H+).
(E)-3-(3H-benzo[f]chromen-2-yl)-1-(2-chlorophenyl) propyl-2-en-1-one (2d)
Yield: 46.8%, m.p. 164.5–165.9 °C. 1H NMR (300 MHz, CDCl3): δ 5.13 (s, 2H, –CH2), 6.53 (d, J = 15.57 Hz, 1H, = CH), 7.11 (s, 1H, = CH), 7.34–7.42 (m, 2H, Ar–H), 7.50 (d, J = 15.57 Hz, 1H, = CH), 7.53 (m, 1H, Ar–H), 7.67 (s, 1H, Ar–H), 7.72–7.82 (m, 3H, Ar–H), 7.91–8.02 (m, 3H, Ar–H). 13C-NMR (75 MHz, CDCl3): 65.0, 115.1, 117.4, 121.1, 124.3, 124.6, 126.9, 127.1,127.5, 128.8, 129.2, 129.4, 129.5, 130.2, 130.3, 131.3, 131.4, 132.0, 139.1, 143.3, 153.9, 193.4. IR (KBr) cm−1: 1655 (C=O), 1547 (C–O). HRMS m/z: calcd for C22H15ClO2 346.08, found 346.11 (M + H+).
(E)-3-(3H-benzo[f]chromen-2-yl)-1-(3-chlorophenyl) propyl-2-en-1-one (2e)
Yield: 41.8%, m.p. 161.9–164.0 °C. 1H NMR (300 MHz, CDCl3): δ 5.16 (s, 2H, –CH2), 6.82 (d, J = 15.57 Hz, 1H, = CH), 7.12 (s, 1H, = CH), 7.38–7.45 (m, 1H, Ar–H), 7.49 (d, J = 15.57 Hz, 1H, = CH), 7.53–7.64 (m, 2H, Ar–H), 7.69 (m, 1H, Ar–H), 7.72–7.82 (m, 3H, Ar–H), 7.91–8.02 (m, 3H, Ar–H). 13C-NMR (75 MHz, CDCl3): 65.1, 115.1, 117.4, 119.5, 121.2, 124.4, 126.4, 127.2,127.6, 128.5, 128.8, 129.4, 129.5, 130.0, 130.2, 132.0, 132.6, 135.0, 139.9, 142.9, 153.9, 188.6. IR (KBr) cm−1: 1653 (C=O), 1545 (C–O). HRMS m/z: calcd for C22H15ClO2 346.08, found 346.15 (M + H+).
(E)-3-(3H-benzo[f]chromen-2-yl)-1-(4-chlorophenyl) propyl-2-en-1-one (2f)
Yield: 45.4%, m.p. 191.0–193.4 °C. 1H NMR (300 MHz, CDCl3): δ 5.16 (s, 2H, –CH2), 6.84 (d, J = 15.57 Hz, 1H, = CH), 7.12 (s, 1H, = CH), 7.37–7.45 (m, 1H, Ar–H), 7.49 (d, J = 15.57 Hz, 1H, = CH), 7.50–7.64 (m, 2H, Ar–H), 7.67 (m, 1H, Ar–H),7.72–7.82 (m, 3H, Ar–H), 7.91–8.02 (m, 3H, Ar–H). 13C-NMR (75 MHz, CDCl3): 65.1, 115.1, 117.4, 119.6, 121.2, 124.3, 127.2, 127.5,128.8, 129.0, 129.1, 129.5, 129.8, 130.2, 130.9, 131.9, 134.6, 136.6, 139.2, 142.6, 153.9, 188.7. IR (KBr) cm−1: 1654 (C=O), 1546 (C–O). HRMS m/z: calcd for C22H15ClO2 346.08, found 346.04 (M + H+).
(E)-3-(3H-benzo[f]chromen-2-yl)-1-(2,4-dichlorophenyl) propyl-2-en-1-one (2g)
Yield: 44.4%, m.p. 191.8–193.9 °C. 1H-NMR (300 MHz, CDCl3): δ 5.12 (s, 2H, –CH2), 6.50 (d, J = 16.00 Hz, 1H, = CH), 7.11 (s, 1H, = CH), 7.31–7.46 (m, 2H, Ar–H), 7.50 (d, J = 16.00 Hz, 1H, = CH), 7.59 (m, 1H, Ar–H), 7.67 (m, 1H, Ar–H), 7.73–7.77 (m, 3H, Ar–H), 7.91–8.02 (m, 3H, Ar–H). 13C-NMR (75 MHz, CDCl3): 64.9, 115.0, 117.4, 121.2, 124.2, 124.4, 127.0, 127.4,127.6, 128.8, 129.4, 129.6, 130.1, 130.2, 130.4, 132.0, 132.1, 136.6, 137.4, 143.8, 153.9, 192.0. IR (KBr) cm−1: 1655 (C=O), 1547 (C–O). HRMS m/z: calcd for C22H14Cl2O2 380.04, found 380.13 (M + H+).
(E)-3-(3H-benzo[f]chromen-2-yl)-1-(2-bromophenyl) propyl-2-en-1-one (2h)
Yield: 40.3%, m.p. 157.6–160.6 °C. 1H-NMR (300 MHz, CDCl3): δ 5.12 (s, 2H, –CH2), 6.50 (d, J = 16.00 Hz, 1H, = CH), 7.11 (s, 1H, = CH), 7.33 (m, J = 8.55 Hz, 2H, Ar–H), 7.45 (d, 1H, = CH), 7.57 (s, 1H, Ar–H), 7.61–7.77 (m, 4H, Ar–H), 7.79–7.95 (m, 3H, Ar–H). 13C-NMR (75 MHz, CDCl3): 65.0, 115.0, 117.4, 119.5, 121.1, 124.3, 124.5, 127.1, 127.4,127.5, 128.8, 129.2, 129.3, 129.5, 130.2, 131.4, 132.0, 133.5, 141.2, 143.7, 154.0, 194.3. IR (KBr) cm−1: 1656 (C=O), 1548 (C–O). HRMS m/z: calcd for C22H15BrO2 390.03, found 390.11 (M + H+).
(E)-3-(3H-benzo[f]chromen-2-yl)-1-(3-bromophenyl) propyl-2-en-1-one (2i)
Yield: 43.3%, m.p. 161.7–164.3 °C. 1H-NMR (300 MHz, CDCl3): δ 5.13 (s, 2H, –CH2), 6.91 (d, J = 15.57 Hz, 1H, = CH), 7.03 (s, 1H, = CH), 7.33 (m, J = 8.55 Hz, 2H, Ar–H), 7.47 (d, 1H, J = 15.57 Hz, =CH), 7.57 (m, 1H, Ar–H), 7.61–7.77 (m, 4H, Ar–H), 7.93–8.08 (m, 3H, Ar–H). 13C-NMR (75 MHz, CDCl3): 65.1, 115.2, 117.4, 119.9, 121.4, 122.9, 124.4, 127.1, 127.5,127.6, 128.8, 129.1, 129.4, 130.2, 130.4, 131.3, 131.8, 135.5, 140.0, 142.6, 153.9, 188.1. IR (KBr) cm−1: 1655 (C=O), 1547 (C–O). HRMS m/z: calcd for C22H15BrO2 390.03, found 390.07 (M + H+).
(E)-3-(3H-benzo[f]chromen-2-yl)-1-(4-bromophenyl) propyl-2-en-1-one (2j)
Yield: 42.5%, m.p. 193.8–194.8 °C. 1H-NMR (300 MHz, CDCl3): δ 5.17 (s, 2H, –CH2), 6.84 (d, J = 15.57 Hz, 1H, = CH), 7.13 (s, 1H, = CH), 7.37–7.58 (m, 3H, Ar–H), 7.64–7.70 (m, 2H, Ar–H), 7.73(d, J = 15.57 Hz, 1H, = CH), 7.76–7.82 (m, 2H, Ar–H), 7.87–8.00 (m, J = 8.55 Hz, 3H, Ar–H). 13C-NMR (75 MHz, CDCl3): 65.1, 115.2, 117.4, 119.9, 121.4, 122.9, 124.4, 127.1, 127.5,127.6, 128.8, 129.1, 129.4, 130.2, 130.4, 131.3, 131.8, 135.5, 140.0, 142.6, 153.9, 188.1. IR (KBr) cm−1: 1654 (C=O), 1547 (C–O). HRMS m/z: calcd for C22H15BrO2 390.03, found 390.01 (M + H+).
(E)-3-(3H-benzo[f]chromen-2-yl)-1-(4-(trifluoromethyl) phenyl)-propyl-2-en-1-one (2k)
Yield: 39.6%, m.p. 187.5–189.1 °C. 1H-NMR (300 MHz, CDCl3): δ 5.21 (s, 2H, –CH2), 6.88 (d, J = 15.63 Hz, 1H, = CH), 7.16 (s, 1H, = CH), 7.42–7.48 (m, 1H, Ar–H), 7.56(d, J = 15.63 Hz, 1H, = CH), 7.57–7.65 (m, 2H, Ar–H), 7.73(m, 1H, Ar–H), 7.75–7.85 (m, 4H, Ar–H), 7.97–8.11 (d, J = 8.07 Hz, 2H, Ar–H). 13C-NMR (75 MHz, CDCl3): 65.1, 115.7, 116.4, 117.8, 119.3, 121.0, 122.2, 123.7, 124.8, 126.1, 127.1, 127.6, 128.0, 128.7, 129.1, 129.5, 129.6, 129.8, 132.2, 142.2, 143.1, 153.9, 188.8. IR (KBr) cm−1: 1656 (C=O), 1548 (C–O). HRMS m/z: calcd for C23H15F3O2 380.10, found 380.19 (M + H+).
(E)-3-(3H-benzo[f]chromen-2-yl)-1-(4-nitrophenyl) propyl-2-en-1-one (2l)
Yield: 37.6%, m.p. 223.7–225.8 °C. 1H-NMR (300 MHz, CDCl3): δ 5.25 (s, 2H, –CH2), 7.13 (s, 1H, = CH), 7.26 (d, J = 3.51, 15.35 Hz, 1H, = CH), 7.34–7.43 (m, 1H, Ar–H), 7.54 (t, J = 7.67 Hz, 1H, Ar–H), 7.72(d, J = 15.57 Hz, 1H, = CH), 7.81 (m, J = 6.58 Hz,3H, Ar–H), 7.94 (m, 1H, Ar–H), 8.11–8.23 (m,4H, Ar–H). 13C-NMR (75 MHz, CDCl3):65.1, 115.3, 116.2, 117.7, 119.2, 120.7, 122.0, 123.6, 124.1, 124.6, 126.1, 127.8, 128.0, 128.4, 128.8, 129.1, 129.5, 130.1, 132.2, 143.4, 153.7, 188.2. IR (KBr) cm−1: 1655(C=O), 1548 (C–O). HRMS m/z: calcd for C22H15NO4 357.10, found 357.06 (M + H+).
(E)-3-(3H-benzo[f]chromen-2-yl)-1-(4-methylphenyl) propyl-2-en-1-one (2m)
Yield: 44.4%, m.p. 174.5–176.4 °C. 1H-NMR (300 MHz, CDCl3): δ 3.90–3.97 (m, 3H, –CH3),5.21 (s, 2H, –CH2), 6.96 (d, J = 15.63 Hz, 1H, = CH), 7.03 (d, J = 8.74 Hz, 2H, Ar–H), 7.15(s, 1H, = CH), 7.40–7.47 (m, 1H, Ar–H), 7.55–7.62(m, 1H, Ar–H), 7.65 (d,J = 15.63 Hz,1H, = CH), 7.68–7.84 (m, 3H, Ar–H), 8.01–8.09 (m,3H, Ar–H). 13C-NMR (75 MHz, CDCl3):21.7, 65.2, 115.2, 117.4, 120.3, 121.2, 124.3, 125.0, 127.4, 127.5, 128.3, 128.6, 128.8, 129.4, 129.5, 130.2, 131.6, 132.3, 135.7, 141.7, 143.6, 153.7, 189.6. IR (KBr) cm−1: 1653 (C=O), 1546 (C–O). HRMS m/z: calcd for C23H18O2 326.13, found 326.70 (M + H+).
(E)-3-(3H-benzo[f]chromen-2-yl)-1-(4-methoxyphenyl) propyl-2-en-1-one (2n)
Yield: 37.7%, m.p. 159.1–160.6 °C. 1H-NMR (300 MHz, CDCl3): δ 3.94 (m, 3H, –CH3),5.21 (s, 2H, –CH2), 6.96 (d, J = 15.63 Hz, 1H, = CH), 7.03 (d, J = 8.74 Hz, 2H, Ar–H), 7.15 (s, 1H, = CH), 7.40–7.47 (m, 1H, Ar–H), 7.56–7.60(m, 2H, Ar–H), 7.68 (d, J = 15.63 Hz, 1H, = CH), 7.73–7.84 (m, 3H, Ar–H), 8.01–8.09 (m, 2H, Ar–H). 13C-NMR (75 MHz, CDCl3):55.6, 65.3, 113.9, 115.3, 117.4, 120.1, 121.3, 124.3, 125.2, 127.5, 127.6, 128.1, 128.6, 128.8, 129.4, 129.5, 130.2, 131.2, 131.6, 141.4, 153.7, 163.5, 188.3. IR (KBr) cm−1: 1655 (C=O), 1547 (C–O). HRMS m/z: calcd for C23H18O3 342.13, found 342.20 (M + H+).
(E)-3-(3H-benzo[f]chromen-2-yl)-1-(4-(dimethylamino)phenyl)prop-2-en-1-one (2o)
Yield: 30.8%, m.p. 214.5–216.3 °C. 1H-NMR (300 MHz, CDCl3): δ 3.06–3.30 (s, 6H, –CH3),5.24 (s, 2H, –CH2), 6.74 (d, J = 15.63 Hz, 1H, = CH), 7.14 (s, 1H, = CH), 7.29–7.47 (m, 2H, Ar–H), 7.50–7.59 (m, 2H, Ar–H), 7.61 (d, J = 15.63 Hz, 1H, = CH), 7.77–7.86 (m, 4H, Ar–H), 8.00 (d, J = 8.99 Hz, 1H, Ar–H), 8.18 (d, J = 8.55 Hz, 1H, Ar–H). 13C-NMR (75 MHz, CDCl3):55.6, 65.3, 78.9, 79.3, 79.6, 79.8, 111.19, 115.3, 117.4, 120.1, 122.1, 124.3, 125.77, 127.6, 128.6, 128.8, 129.5, 130.2, 131.2, 131.6, 141.4, 153.7, 163.5, 188.3. IR (KBr) cm−1: 1654 (C=O), 1547 (C–O). HRMS m/z: calcd for C24H21NO2 355.16, found 355.22 (M + H+).
(E)-3-(3H-benzo[f]chromen-2-yl)-1-(4-aminophenyl) propyl-2-en-1-one (2p)
Yield: 29.8%, m.p. 222.5–224.6 °C. 1H-NMR (300 MHz, CDCl3): δ 5.25 (s, 2H, –CH2), 6.35 (s, 2H, –NH2), 7.12 (s, 1H, = CH), 7.21 (d, J = 15.57 Hz, 1H, = CH), 7.32–7.55 (m, 2H, Ar–H), 7.68(d, J = 15.57 Hz, 1H, = CH), 7.78 (m, J = 6.58 Hz, 3H, Ar–H), 7.89–7.99 (m, 2H, Ar–H), 8.04–8.13 (m,3H, Ar–H). 13C-NMR (75 MHz, CDCl3): 65.3, 113.2, 115.8, 117.8, 120.1, 121.9, 122.1, 124.3, 125.8, 127.4, 128.2, 128.6, 129.0, 129.5, 130.3, 131.5, 136.2, 139.5, 141.4, 154.0, 163.5, 186.1. IR (KBr) cm−1: 1656 (C=O), 1548 (C–O). HRMS m/z: calcd for C22H17NO2 327.13, found 327.19 (M + H+).
(E)-3-(3H-benzo[f]chromen-2-yl)-1-(4-ethylphenyl) propyl-2-en-1-one (2q)
Yield: 38.3%, m.p. 158.7–161.1 °C. 1H-NMR (300 MHz, CDCl3): δ 1.27 (m, 3H, –CH3), 3.32 (m, 2H, –CH2),5.20 (s, 2H, –CH2), 7.05 (d, J = 15.63 Hz, 1H, = CH), 7.11 (s, 1H, = CH), 7.33–7.38 (m, 2H, Ar–H), 7.52–7.65 (m, 2H, Ar–H), 7.75 (d, J = 15.63 Hz,1H, = CH), 7.76–7.95 (m, 3H, Ar–H), 7.97–8.06 (m, 3H, Ar–H). 13C-NMR (75 MHz, CDCl3):15.3, 29.0, 65.2, 115.2, 117.4, 120.3, 121.2, 124.3, 126.2, 127.5, 128.2, 128.7, 128.8, 129.4, 130.2, 131.6, 132.3, 135.9, 137.4, 141.7, 143.6, 149.8, 153.7, 189.6. IR (KBr) cm−1: 1655 (C=O), 1548 (C–O). HRMS m/z: calcd for C24H20O2 340.15, found 340.08 (M + H+).
(E)-3-(3H-benzo[f]chromen-2-yl)-1-(2,4-dimethylphenyl) propyl-2-en-1-one (2r)
Yield: 37.8%, m.p. 117.8–119.7 °C. 1H-NMR (300 MHz, CDCl3): δ 2.41 (s, 3H, -CH3), 3.47 (s, 3H, –CH3),5.13 (s, 2H, –CH2), 6.56 (d, J = 16.00 Hz, 1H, = CH), 7.11 (s, 1H, = CH), 7.35–7.47 (m, 4H, Ar–H), 7.54 (d, J = 16.00 Hz, 1H, = CH), 7.57–7.76 (m, 2H, Ar–H), 7.76–7.98 (m, 3H, Ar–H). 13C-NMR (75 MHz, CDCl3):20.4, 21.4, 65.1, 115.1, 117.4, 121.2, 124.3, 125.0, 126.2, 127.3, 127.4, 128.2, 128.5, 128.8, 129.5, 130.2, 131.7, 132.3, 136.2, 137.4, 141.0, 142.5, 153.7, 195.5. IR (KBr) cm−1: 1656 (C=O), 1549(C–O). HRMS m/z: calcd for C24H20O2 340.15, found 340.23 (M + H+).
(E)-3-(3H-benzo[f]chromen-2-yl)-1-(2,4-dimethoxyphenyl) propyl-2-en-1-one (2s)
Yield: 33.5%, m.p. 139.8–141.9 °C. 1H-NMR (300 MHz, CDCl3): δ 3.86 (d, 3H, –OCH3), 3.89 (d, 3H, –OCH3),5.11 (s, 2H, –CH2), 6.55–6.58 (m, 2H, Ar–H), 6.96 (d, J = 15.78 Hz, 1H, = CH), 7.11 (s, 1H, = CH), 7.36–7.45 (m, 2H, Ar–H), 7.53 (d, J = 15.78 Hz, 1H, = CH), 7.57–7.84 (m, 4H, Ar–H), 7.99 (m, J = 8.33 Hz, 1H, Ar–H). 13C-NMR (75 MHz, CDCl3):55.6, 55.7, 65.3, 98.7, 105.3, 115.3, 117.3, 121.2, 124.2, 125.8, 126.2, 127.1, 127.3, 128.0, 128.7, 129.5, 130.2, 131.2, 132.9, 139.4, 153.4, 160.4, 164.3, 189.9. IR (KBr) cm−1: 1654 (C=O), 1546 (C–O). HRMS m/z: calcd for C24H20O4 372.14, found 372.17 (M + H+).
Animals
The processing programs of all the animals were carried out according to the supervision standards established by the Government of China. The Animal Research Laboratory, School of Pharmacy, Zhejiang Academy of Medical Sciences (Zhejiang, China) provided the ICR mice (20 ± 2 g). All animals were allowed to adapt to the environment for a week before the experiment. Mice were placed in a temperature controlled (23 ± 2 °C) environment and maintained on standard tap water and food pellets (unless stated otherwise) and a light-dark cycle of 12 h before and during the experiment.
Forced swimming test (FST)
Male ICR mice (20 ± 2 g) were allowed to drink and eat normally before the experiment, and eight mice were raised in each group. The 19 3H-benzo[f]chromen chalcone derivatives and the positive drug, fluoxetine hydrochloride, were dissolved in DMSO. The negative control was the carrier solvent and the positive control was fluoxetine. On the day of the experiment, 30 min after intraperitoneal administration of the drugs, mice were placed into a Plexiglass™ cylinder containing 10 cm of water (height, 25 cm; diameter, 10 cm; temperature, 22 ± 3 °C) one at a time for ~6 min. The mice struggled slightly in the first 2 min and then entered the observation range of immobile time. The immobility duration was recorded in the last 4 min of the test. The time for rest was considered as the time spent by mice floating in the water without struggling and stroking its limbs only to maintain its head floating above the water [24, 43].
Tail suspension test (TST)
Mice were hung by their tails (clipped 2 cm from the end) 5 cm above the base of a box (25 × 25 × 30 cm). The test was conducted in a dark room with little-to-no background noise. All mice were suspended for 6 min to observe the immobility duration and immobility was measured in the last 4 min of the test. Video cameras were fixed directly above the box to record all the test phenomena. Two professional observers watched the videos and scored them. Mice were considered to be immobile only when they hung passively and were completely motionless [47].
Maximum electroconvulsive seizure (MES) test
The power supply parameters of the maximum electroconvulsive instrument were set to 50 mA, 60 Hz alternating current, single electrode stimulation voltage of 110 V, time 0.3 s. The experiment was started 30 min after intraperitoneal administration in each group. Normal saline was evenly applied to the edges of the mouse’s ears, then the edges of the mouse’s ears were clamped with the alligator clip provided with the instrument. Mice were given a single 0.3 s electrical stimulation. After the mouse was electrically stimulated, if the hind limbs were rigid, the mouse was used in the next step. If the hind limbs are not rigid, it indicates that the compound has antimaximal electroconvulsive activity [48].
Neurotoy screening
The compound’s neurovirulence was evaluated by the rotating rod test. Before the experiment, the mice were trained to stay on a speed rotary bar (diameter, 3.2 cm) which is rotated at 10 revolutions per min. Whether the mice in each experiment maintained balance on the rod for at least 1 min was used as the evaluation criterion for neurotoxicity [49]. Trained mice were administered 3H-benzo[f]chromen chalcone derivative 2a–2s intraperitoneally 30 min before the test, and were then placed on the accelerating rotating rod. If the mice maintained their balance for 1 min without falling in three consecutive experiments, the compound was considered not to show neurotoxicity at that dose.
Statistical analyses
Prism version 2.0 (GraphPad, San Diego, CA, USA) was used for statistical analysis of categorical data. Data were represented as the average ± SEM. Differences between the two groups were compared with Student’s t-test, and p < 0.05 was considered statistically significant.
References
Zhang J, Huen JMY, Lew B, Chistopolskaya K, Talib MA, Siau CS. et al. Depression, anxiety, and stress as a function of psychological strains: towards an etiological theory of mood disorders and psychopathologies. J Affect Disord. 2020;271:279–85. https://doi.org/10.1016/j.jad.2020.03.076.
Hijne K, Penninx BW, van Hemert AM, Spinhoven P. The association of changes in repetitive negative thinking with changes in depression and anxiety. J Affect Disord. 2020;275:157–64. https://doi.org/10.1016/j.jad.2020.07.002.
Liu Q, He H, Yang J, Feng X, Zhao F, Lyu J. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. J Psychiatr Res. 2019;126:134–40. https://doi.org/10.1016/j.jpsychires.2019.08.002.
Coppen A, Shaw DM, Malleson A. Changes in 5-hydroxytryptophan metabolism in depression. Br J Psychiatry. 1965;111:105–107. https://doi.org/10.1192/bjp.111.470.105.
Janowsky DS, El-Yousef MK, Davis JM, Sekerke HJ. A cholinergic-ad-renergic hypothesis of mania and depression. Lancet. 1972;300:632–5. https://doi.org/10.1016/s0140-6736(72)93021-8.
Ménard C, Hodes GE, Russo SJ. Pathogenesis of depression: Insights from human and rodent studies. Neuroscience. 2016;321:138–62. https://doi.org/10.1016/j.neuroscience.2015.05.053.
Lee STH. Inflammation, depression, and anxiety disorder: A population-based study examining the association between Interleukin-6 and the experiencing of depressive and anxiety symptoms. Psychiatry Res. 2020;285:112809. https://doi.org/10.1016/j.psychres.2020.112809.
Liu T, Zhou N, Xu R, Cao Y, Zhang Y, Liu Z. et al. A metabolomic study on the anti-depressive effects of two active components from Chrysanthemum morifolium. Artif Cells Nanomed Biotechnol. 2020;48:718–27. https://doi.org/10.1080/21691401.2020.1774597.
Tan S, Wang Y, Chen K, Long Z, Zou J. Ketamine alleviates depressive-like behaviors via down-regulating inflammatory cytokines induced by chronic restraint stress in mice. Biol Pharm Bull. 2017;40:1260–1267. https://doi.org/10.1248/bpb.b17-00131.
Gao W, Wang W, Peng Y, Deng Z. Antidepressive effects of kaempferol mediated by reduction of oxidative stress, proinflammatory cytokines and up-regulation of AKT/β-catenin cascade. Metab Brain Dis. 2019;34:485–94. https://doi.org/10.1007/s11011-019-0389-5.
Zhang YQ, Wang XB, Xue RR, Gao XX, Li W. Ginsenoside Rg1 attenuates chronic unpredictable mild stress-induced depressive-like effect via regulating NF-κB/NLRP3 pathway in rats. Neuroreport. 2019;30:893–900. https://doi.org/10.1097/WNR.0000000000001302.
Ferraz CAA, de Oliveira Júnior RG, Picot L, da Silva Almeida JRG, Nunes XP, Nunes XP. Pre-clinical investigations of β-carboline alkaloids as antidepressant agents: a systematic review. Fitoterapia. 2019;137:104196. https://doi.org/10.1016/j.fitote.2019.104196.
Forthoffer N, Kleitz C, Bilger M, Brissart H. Depression could modulate neuropsychological status in epilepsy. Rev Neurol. 2020;176:456–67. https://doi.org/10.1016/j.neurol.2020.03.015.
Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7:31–40. https://doi.org/10.1038/nrneurol.2010.178.
Yamatogi Y. Principles of antiepileptic drug treatment of epilepsy. Psychiatry Clin Neurosci. 2004;58:S3–6. https://doi.org/10.1111/j.1440-1819.2004.01244_1.x.
Stefan H, Feuerstein TJ. Novel anticonvulsant drugs. Pharm Ther. 2007;113:165–183. https://doi.org/10.1016/j.pharmthera.2006.07.005.
Zhuang C, Zhang W, Sheng C, Zhang W, Xing C, Miao Z. Chalcone: a privileged structure in medicinal chemistry. Chem Rev. 2017;117:7762–810. https://doi.org/10.1021/acs.chemrev.7b00020.
Szliszka E, Czuba ZP, Mazur B, Paradysz A, Krol W. Chalcones and dihydrochalcones augment TRAIL-mediated apoptosis in prostate cancer cells. Molecules. 2010;15:5336–53. https://doi.org/10.3390/molecules15085336.
Aoki N, Muko M, Ohta E, Ohta S. C-geranylated chalcones from the stems of Angelica keiskei with superoxide-scavenging activity. J Nat Prod. 2008;71:1308–10. https://doi.org/10.1021/np800187f.
Sivakumar PM, Ganesan S, Veluchamy P, Doble M. Novel chalcones and 1,3,5-triphenyl-2-pyrazoline derivatives as antibacterial agents. Chem Biol Drug Des. 2010;76:407–11. https://doi.org/10.1111/j.1747-0285.2010.01020.x.
de Mello MVP, Abrahim-Vieira BA, Domingos TFS, de Jesus JB, de Sousa ACC, Rodrigues CR. et al. A comprehensive review of chalcone derivatives as antileishmanial agents. Eur J Med Chem. 2018;150:920–9. https://doi.org/10.1016/j.ejmech.2018.03.047.
Tadigoppula N, Korthikunta V, Gupta S, Kancharla P, Khaliq T, Soni A. et al. Synthesis and insight into the structure-activity relationships of chalcones as antimalarial agents. J Med Chem. 2013;56:31–45. https://doi.org/10.1021/jm300588j.
Mojzis J, Varinska L, Mojzisova G, Kostova I, Mirossay L. Antiangiogenic effects of flavonoids and chalcones. Pharm Res. 2008;57:259–65. https://doi.org/10.1016/j.phrs.2008.02.005.
Gomes MN, Braga RC, Grzelak EM, Neves BJ, Muratov E, Ma R. et al. QSAR-driven design, synthesis and discovery of potent chalcone derivatives with antitubercular activity. Eur J Med Chem. 2017;137:126–38. https://doi.org/10.1016/j.ejmech.2017.05.026.
Guan LP, Zhao DH, Chang Y, Sun Y, Ding XL, Jiang JF. Design, synthesis and antidepressant activity evaluation 2’-hydroxy-4’,6’-diisoprenyloxychalcone derivatives. Med Chem Res. 2013;22:5218–26. https://doi.org/10.1007/s00044-013-0517-4.
Yamamoto T, Yoshimura M, Yamaguchi F, Kouchi T, Tsuji R, Saito M. et al. Anti-allergic Activity of Naringenin Chalcone from a Tomato Skin Extract. Biosci Biotechnol Biochem. 2004;68:1706–11. https://doi.org/10.1271/bbb.68.1706.
Birari RB, Gupta S, Mohan CG, Bhutani KK. Antiobesity and lipid lowering effects of Glycyrrhiza chalcones: experimental and computational studies. Phytomedicine. 2011;18:795–801. https://doi.org/10.1016/j.phymed.2011.01.002.
Sato Y, He JX, Nagai H, Tani T, Akao T. Isoliquiritigenin, one of the antispasmodic principles of Glycyrrhiza ularensis roots, acts in the lower part of intestine. Biol Pharm Bull. 2007;30:145–9. https://doi.org/10.1248/bpb.30.145.
Nowakowska Z. A review of anti-infective and anti-inflammatory chalcones. Eur J Med Chem. 2007;42:125–37. https://doi.org/10.1016/j.ejmech.2006.09.019.
Wang W, Hu X, Zhao Z, Liu P, Hu Y, Zhou J. et al. Antidepressant-like effects of liquiritin and isoliquiritin from Glycyrrhiza uralensis in the forced swimming test and tail suspension test in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1179–1184. https://doi.org/10.1016/j.pnpbp.2007.12.021.
Sahu NK, Balbhadra SS, Choudhary J, Kohli DV. Exploring pharmacological significance of chalcone scaffold: a review. Curr Med Chem. 2012;19:209–25. https://doi.org/10.2174/092986712803414132.
Singh P, Anand A, Kumar V. Recent developments in biological activities of chalcones: a mini review. Eur J Med Chem. 2014;85:758–77. https://doi.org/10.1016/j.ejmech.2014.08.033.
Sinha S, Medhi B, Sehgal R. Challenges of drug-resistant malaria. Parasite. 2014;21:61. https://doi.org/10.1051/parasite/2014059.
Matos MJ, Vazquez-Rodriguez S, Uriarte E, Santana L. Potential pharmacological uses of chalcones: a patent review (from June 2011 - 2014). Expert Opin Ther Pat. 2015;25:351–66. https://doi.org/10.1517/13543776.2014.995627.
Mohan M, Attarde D, Momin R, Kasture S. Antidepressant, anxiolytic and adaptogenic activity of torvanol A: an isoflavonoid from seeds of Solanum torvum. Nat Prod Res. 2013;27:2140–3. https://doi.org/10.1080/14786419.2013.778853.
Yan L, Hu Q, Mak MS, Lou J, Xu SL, Bi CW. et al. A Chinese herbal decoction, reformulated from Kai-Xin-San, relieves the depression-like symptoms in stressed rats and induces neurogenesis in cultured neurons. Sci Rep.2016;6:30014. https://doi.org/10.1038/srep30014.
Lu P, Mamiya T, Lu L, Mouri A, Niwa M, Kim HC. et al. Silibinin attenuates cognitive deficits and decreases of dopamine and serotonin induced by repeated methamphetamine treatment. Behav Brain Res. 2010;207:387–393. https://doi.org/10.1016/j.bbr.2009.10.024.
Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. https://doi.org/10.1038/nri.2015.5.
Costa M, Dias TA, Brito A, Proença F. Biological importance of structurally diversified chromenes. Eur J Med Chem. 2016;123:487–507. https://doi.org/10.1016/j.ejmech.2016.07.057.
Chen Y, Kong LD, Xia X, Kung HF, Zhang L. Behavioral and biochemical studies of total furocoumarins from seeds of Psoralea corylifolia in the forced swimming test in mice. J Ethnopharmacol. 2005;96:451–9. https://doi.org/10.1016/j.jep.2004.09.033.
Ariza SY, Rueda DC, Javier RV, Linares EL, Guerrero MF. Pharmacological effects on the central nervous system induced by coumarin, isolated from hygrophila tyttha leonard. Vitae. 2007;14:51–8. https://doi.org/10.1590/S0121-40042007000200007.
Vergel NE, López JL, Orallo F, Viña D, Buitrago DM, del Olmo E. et al. Antidepressant-like profile and MAO-A inhibitory activity of 4-propyl-2H-benzo[h]-chromen-2-one. Life Sci. 2010;86:819–24. https://doi.org/10.1016/j.lfs.2010.04.001.
Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–36.
Jin HG, Zhou M, Jin QH, Liu BY, Guan LP. Antidepressant-like effects of saringosterol, a sterol from Sargassum fusiforme by performing in vivo behavioral tests. Med Chem Res. 2017;26:909–15. https://doi.org/10.1007/s00044-017-1804-2.
Zhang XW, Zhao DH, Quan YC, Sun L, Yin XM, Guan LP. Synthesis and evaluation of antiinflammatory activity of substituted chalcone derivatives. Med Chem Res. 2009;19:403–12. https://doi.org/10.1007/s00044-009-9202-z.
Zhao LM, Jin HS, Sun LP, Piao HR, Quan ZS. Synthesis and evaluation of antiplatelet activity of trihydroxychalcone derivatives. Bioorg Med Chem Lett. 2005;15:5027–9. https://doi.org/10.1016/j.bmcl.2005.08.039.
Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–70. https://doi.org/10.1007/bf00428203.
Klein BD, Jacobson CA, Metcalf CS, Smith MD, Wilcox KS, Hampson AJ. et al. Evaluation of Cannabidiol in Animal Seizure Models by the Epilepsy Therapy Screening Program (ETSP). Neurochem Res. 2017;42:1939–48. https://doi.org/10.1007/s11064-017-2287-8.
Zhang L, Guan LP, Sun XY, Wei CX, Chai KY, Quan ZS. Synthesis and anticonvulsant activity of 6-alkoxy-[1,2,4]triazolo[3,4-a]phthalazines. Chem Biol Drug Des. 2009;73:313–9. https://doi.org/10.1111/j.1747-0285.2009.00776.x.
Acknowledgements
This work was supported by Zhejiang Province Public Technology Application Project of China (No. 2017C33131). We thank Arshad Makhdum, PhD, from LiwenBianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tan, QW., He, LY., He, ZW. et al. Design, synthesis, and antidepressant/anticonvulsant activities of 3H-benzo[f]chromen chalcone derivatives. Med Chem Res 30, 1427–1437 (2021). https://doi.org/10.1007/s00044-021-02742-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-021-02742-5