Abstract
The productivity of social groups depends critically on effective regulation of work effort among group members. In social insect colonies, regulation of work may be decentralised or alternatively may be controlled by one or a few individuals (‘pacemakers’) within the colony. Social parasites, which usurp host colonies and replace the dominant as the principal reproductive, similarly depend on efficient regulation of work by hosts to rear parasite offspring, but few studies have explored the strategies used by parasites to achieve this. We compared the role of the social parasite Polistes semenowi in regulating host activity with that of the dominant individual on unparasitized nests of the host species, P. dominula. Dominant foundresses acted as pacemakers within unparasitized colonies, interacting frequently with colony members to initiate activity bursts and foraging trips, whereas parasites did not initiate more activity than the average colony member. Nonetheless, overall activity levels were similar in parasitized and unparasitized colonies, indicating that parasites may use other, indirect means to control the host activity. Colony activity did not change significantly following the removal of parasites or dominant host foundresses, perhaps because other individuals rapidly assumed the dominant position, or because of persistent indirect effects on colony activity. The role of P. semenowi in regulating the host activity differs strikingly from that reported for a second Polistes social parasite, P. atrimandibularis, suggesting that different Polistes social parasites may have fundamentally different social roles within host colonies, despite being closely phylogenetically related to one another.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Effective regulation of work among group members is crucial for the survival and productivity of the social groups. Within social insect colonies, the number of offspring reared to maturity depends upon the investment by the adult colony members in foraging and other brood care, as well as in the construction, maintenance and defence of the nest. In colonies of advanced eusocial species, which may comprise hundreds or thousands of individuals, workers are frequently specialized on particular tasks, and the decision to work is made by each individual based on simple behavioural rules in response to changes in local conditions (Anderson and McShea 2001; Camazine et al. 2001; Jeanne 2003). By contrast, in the smaller colonies characteristic of many primitively eusocial wasps and bees, there is a greater scope for centralised control of colony activity by one or a few individuals. Within the colony, such individuals may act as ‘pacemakers’, initiating interactions with colony members at regular intervals to regulate work across the colony (Reeve and Gamboa 1983, 1987).
Paper wasps of the genus Polistes have proved an attractive system for exploring the importance of centralised versus decentralised control of work effort in primitively eusocial societies (e.g. Reeve and Gamboa 1983, 1987; Gamboa et al. 1990; Jha et al. 2006; De Souza and Prezoto 2012). In temperate species, nests are founded by mated females (foundresses), either singly or in small groups (typically 2–10 co-foundresses). Within multiple-foundress colonies, the dominant foundress monopolises reproduction, while her subordinates forage for building materials and food for the developing brood. The first female offspring to mature assume worker roles, foraging and maintaining and defending the nest, while those maturing towards the end of the annual nesting cycle leave the nest to mate and overwinter, emerging from hibernation to found nests the following spring (Reeve 1991).
In Polistes, the dominant foundress spends the majority of her time on the nest, and so could plausibly control the activity of other colony members while on the nest, as well as the frequency of foraging trips made by workers. Within Polistes colonies, activity is episodic, occurring largely within clearly defined activity bursts (Reeve and Gamboa 1983; Jha et al. 2006). Research has, therefore, focused on how these activity bursts are regulated, and thus how the timing and rate of work undertaken within the colony is controlled (it is important to note, however, that the precise relationship between the frequency and duration of activity bursts and colony productivity is yet to be determined). Following removal or cooling of the dominant foundress from single-foundress P. fuscatus colonies, both the overall work rate and the degree of synchronisation in activity among workers were observed to decrease, indicating a role for the foundress in regulating the activity of her workers (Reeve and Gamboa 1983, 1987). However, there was no relationship between worker activity and the rate of interactions between dominants and workers, implying that dominant control is achieved indirectly, rather than through physical interactions with workers. In other studies of Polistes, however, dominant directed control of colony activity is not supported (O’Donnell 1998; Jha et al. 2006; De Souza and Prezoto 2012). Following worker emergence in single-foundress colonies of P. dominula and P. instabilis, Jha et al. (2006) found that foundresses did not initiate more activity bursts or foraging trips than the average worker, while removal of the dominant did not cause a decrease in worker activity. Similarly, there is no evidence that dominants in the neotropical P. versicolor function as pacemakers within their colonies (De Souza and Prezoto 2012). Differences in results between these studies may partly reflect methodological differences (see Jha et al. 2006), but may also point to variation among Polistes species in the function of the dominant in regulating the colony activity.
Colonies of social insects, including Polistes, are vulnerable to attack by inquiline brood parasites (‘social’ parasites) which live and reproduce within the host colony, exploiting the parental care of hosts to rear parasite offspring (Cervo 2006). Securing access to a host colony is vital for the success of parasites, and much attention has been given to strategies used to achieve this (for reviews see e.g. Lorenzi 2006; Guillem et al. 2014). Once established within the host colony, however, parasites must ensure that hosts continue to forage and to provision the parasite offspring that have replaced their own brood within the nest. Many social parasites replace the original dominant in the host colony, often killing or evicting her (Cervo 2006). In small-colony host species where the dominant normally acts as a pacemaker, parasites may then be forced to assume this role themselves, regulating the activity of the host workforce through direct physical interactions or possibly through more covert means, for example through pheromonal manipulation. By contrast, where colony activity is decentralised, parasites may instead focus exclusively on reproduction and rely on hosts to organise work among them. In such cases, however, parasites might still be expected to take an active role in controlling activity as a means of boosting productivity or counteracting any strategic decrease in effort by hosts that recognise that they are parasitized.
Though effective control of host activity is critical to the fitness of social parasites, few studies have explored how such control is achieved. Here, we investigate the role of a Polistes social parasite in regulating activity among its host workforce. Individual females of Polistes social parasites (P. atrimandibularis, P. semenowi and P. sulcifer) usurp host colonies just prior to worker emergence, and replace the dominant foundress as the principal reproductive in the colony (Lorenzi et al. 1992; Green et al. 2014). Though the three species comprise a monophyletic group (Choudhary et al. 1994), there is a striking diversity in the strategies that each employs, including differences in preferred host species and behavioural interactions with hosts (Cervo 2006), which are likely to have important consequences for how activity within parasitized colonies is controlled. Observing activity within P. biglumis colonies, Fucini et al. (2014) found that single foundresses parasitized by P. atrimandibularis were more active and foraged at a higher rate than unparasitized foundresses (see also Fucini and Lorenzi 2004). Subsequent removal of the parasite resulted in a decrease in host activity, indicating that the parasite itself is responsible for controlling the host work effort. However, it is unclear whether this result holds for two the other Polistes inquilines, P. semenowi and P. sulcifer, which exploit a different host (P. dominula) and preferentially attack large, multiple-foundress colonies (Cervo and Turillazzi 1996; Shreeves et al. 2003). Exploitation of larger colonies may effectively preclude parasite control through repeated behavioural interactions, and instead, favour either more indirect means of colony control, or decentralised control. On the other hand, the high levels of aggression shown by P. semenowi and P. sulcifer to hosts during initial nest invasion (Zacchi et al. 1996; Cini et al. 2011), as well as their near continuous presence on the host nest, may permit effective centralised control of host work effort, via aggression and/or other means. Understanding how the different social parasite species control the activity of their hosts will provide insights into the diversification of parasitic strategies within Polistes, particularly in the period following usurpation. Furthermore, comparison of the strategies used by the parasite with those used by the dominant host in unparasitized colonies is important for understanding the evolutionary trajectory from a non-parasitic ancestor to obligate brood parasitism in Polistes. For instance, P. semenowi parasites resemble the host dominant in terms of monopolisation of colony reproduction (Green et al. 2014), but it is unclear whether the parasite also expresses the same repertoire of behaviour that characterises social interactions between dominant host foundresses and their subordinates.
In this study, we explore how activity is regulated within P. dominula colonies parasitized by P. semenowi. The study of P. atrimandibularis by Fucini et al. (2014) suggested that increased host activity could be the result of frequent interactions with the parasite on the nest; however, the specific outcomes of these interactions were not reported, making this hypothesis difficult to test. To determine the precise manner in which P. semenowi regulates the host activity, we explored the parasite’s role in initiating the activity bursts and foraging trips. In addition, we compared the parasite’s role in regulating activity to that of the dominant foundress in unparasitized colonies to determine whether parasites use similar strategies of control to those of their hosts. Finally, to test how the presence of the parasite on the nest affects the overall work effort of hosts, we compared activity patterns on host nests before and after the experimental removal of the parasite.
Methods
Field methods
Parasitized and unparasitized P. dominula multiple-foundress colonies were studied in 2004 and 2005 at two nearby field sites within 5 km of Conil de la Frontera, in Southern Spain (Cant and Field 2001; Shreeves et al. 2003; Leadbeater et al. 2011). Parasitism of P. dominula by P. semenowi at these sites has been recorded since 1994. During the nest-founding phase in late February–early March, we located individually labelled all nests at both sites. Nest censuses were then performed every 3–4 days at night when wasps were cool and inactive. All foundresses present on nests were briefly removed and individually marked using enamel paints. We continued to census nests throughout March and April, recording and marking any P. semenowi females found on nests. After this point, we checked nests sporadically throughout the season, marking any worker offspring that we found on nests with a dot of white paint.
We filmed colony activity on 20 parasitized nests and 21 unparasitized nests on warm, sunny days in May. Each colony was filmed for a single continuous session of 3–4 h from 1100 to 1600. Filming of parasitized colonies occurred 14–55 days following initial invasion by parasites, thereby ensuring that parasites had become established as reproductives within their host colonies (Cervo 2006; Fucini et al. 2014). Prior to filming, the dominant foundress in unparasitized colonies was identified by observing the behavioural interactions between foundresses and by recording individual foraging effort—dominants spend less time away from the nest foraging than their subordinates (for methods see Cant and Field 2001). At the time of filming, parasitized and unparasitized colonies were similar in terms of number of adults (foundresses + parasites; mean ± 1 s.e. = 4.0 ± 0.41 vs. 3.4 ± 0.33; Mann–Whitney test, W = 250, p = 0.29), worker offspring (2.1 ± 0.53 vs. 2.0 ± 0.56; W = 216, p = 0.88), pupae (10.9 ± 1.7 vs. 10.4 vs. 2.1; W = 227, p = 0.67) and nest cells (85.3 ± 7.4 vs. 73.2 ± 5.3; W = 258, p = 0.22).
Behavioural analysis
For each colony, we analysed the first five activity bursts occurring within 30 min of the start of the recording where the parasite/dominant foundress and at least one other wasp were present on the nest (for two of the colonies that we analysed, number of bursts was only 3 or 4). Bursts were defined as occurring when at least 50 % of wasps on the nest became active (walking across the nest surface, building or repairing nest cells or provisioning brood), followed by a period of inactivity lasting at least 30 s. Note that this definition differs slightly from that of Jha et al. (2006), who define activity bursts as occurring when all colony members are active. Our preliminary observations of parasite activity on host nests in the field revealed that parasites are inactive for a much greater amount of time than their hosts (J. P. Green, pers. obs.). Recording only those activity bursts where all individuals were active would, therefore, bias activity initiation in favour of the parasite compared with the dominant foundress on unparasitized nests. In the case of six colonies (five unparasitized colonies and one parasitized colony) where only two individuals (including the parasite/dominant foundress) were present on the nest, however, we recorded an activity burst only when both individuals were active. For each burst, we recorded whether activity was initiated by the parasite/dominant foundress or a subordinate (foundress or worker). Five distinct behaviours initiated activity bursts in both parasitized and unparasitized colonies: walking, darting, antennating, arriving and gaster-wagging [for a description of these behaviours see Jha et al. (2006)]. Mounting, where one individual stands on top of a second individual, also initiated activity, but only on parasitized nests. In addition to recording activity bursts, we also analysed the first five departures made by subordinates from each colony within the same time 30 min period, recording the identity of the last individual to interact with the departing wasp. We excluded the departures where there was only a single wasp present on the nest besides the departing individual. Consequently, for a number of colonies, the total number of recorded departures was <5. Colonies where <2 departures were recorded were excluded from analyses, resulting in a total of 16 parasitized and 15 unparasitized colonies available for analysis.
Statistical analysis
We explored the roles of parasites and dominant foundresses in regulating the colony activity in a number of ways. First, we asked whether the parasites and dominant foundresses initiate more activity bursts than would be expected if activity initiation was distributed equally among all wasps in the colony. To do this, we used 1-sample Mann–Whitney tests to determine whether the ratio of the observed proportion of parasite- or dominant foundress-initiated bursts to the proportion expected if all wasps were equally likely to initiate bursts (equivalent to the proportion of parasites or dominant foundresses in the colony) departed significantly from unity. To calculate the proportion of parasites/dominant foundresses within colonies, we estimated colony size as the mean number of wasps present on the nest at the start of each activity burst (excluding newly emerged workers sat motionless within cells).
Second, we asked whether parasites and dominant foundresses differed in the number of activity bursts they initiated. To do this, we ran a generalised linear model (GLM) with quasibinomial errors with the proportion of bursts initiated by the parasite/dominant foundress in each colony as the response and colony status (parasitized or unparasitized) as a predictor. Colony size (estimated as above) and year (2004 or 2005) were fitted as additional predictors in the model, together with the interaction between colony size and colony status.
Third, we explored whether subordinates on parasitized and unparasitized nests left the nest following contact with the parasite or dominant foundress, and what behaviours initiated departures by subordinates. As for the analysis of activity bursts, we tested whether the parasites and dominant foundresses initiated more departures than expected if all wasps in the colony were equally likely to initiate departures using 1-sample Mann–Whitney tests (see above). We also tested whether parasites and dominant foundresses differed in the number of departures they initiated using a GLM with quasibinomial errors with the proportion of departures initiated by the parasite/dominant foundress as the response and colony status (parasitized or unparasitized) as a predictor. Colony size and year were fitted as additional predictors in the model, together with the interaction between colony size and colony status. In these analyses, colony size was estimated as the mean number of wasps present on the nest at the time of each departure, again excluding newly emerged workers sitting within cells.
Fourth, for a subset of 12 parasitized and unparasitized colonies we compared activity before and after the removal of the parasite or dominant foundress. For this subset, parasitized and unparasitized colonies were again similar in terms of the number of adults (foundresses + parasites; 3.7 ± 0.39 vs. 3.9 ± 0.54; W = 69, p = 0.88), worker offspring (1.9 ± 0.60 vs. 1.8 ± 0.57; W = 74, p = 0.95), pupae (8.5 ± 2.08 vs. 12.6 ± 3.39; W = 54, p = 0.31) and nest cells (83.3 ± 10.7 vs. 78.3 ± 8.5; W = 75, p = 0.89). Parasites/dominant foundresses were removed from nests after the first day of filming and colonies were then filmed for a second time 24 h later, following Jha et al. (2006). In a few cases where bad weather prevented filming, colony activity was recorded 48 h after the removal of the parasite or dominant foundress. For each colony, we recorded the number of activity bursts (defined as above) occurring within the first 30 min of filming, as well as the duration of the first five bursts. Burst duration (log-transformed) was analysed in a generalised linear mixed model (GLMM) with Normal errors (‘lme’ function in nlme package), while the number of bursts was analysed in a GLMM with Poisson errors (‘lmer’ function). Colony status (parasitized or unparasitized), removal status (before or after removal), colony size and year were fitted as fixed effects in both models, together with the interaction between colony status and removal status. In both models, nest ID was fitted as a random effect to account for any similarity in activity levels within individual colonies.
Data were analysed in R v. 3.1.1 (R Core Team 2014). For GLM and GLMM analyses, model simplification proceeded by backwards deletion of nonsignificant terms until further removals led to significant (p < 0.05) increases in deviance, assessed by log-likelihood ratios for Normal errors, χ 2 values for Poisson errors and F ratios for quasibinomial errors. Year of study was not a significant predictor in any of the analyses, and is omitted from the results.
Results
Regulation of colony activity
Dominant foundresses initiated 2.7 ± 0.34 (mean ± s.e.) times as many activity bursts as expected by chance (W = 226, p = 0.0001; Fig. 1a). In contrast, parasites did not initiate more bursts than expected by chance (W = 147, p = 0.12). Dominant foundresses initiated on average 1.9 times more bursts than did parasites (F 1,38 = 11.76, p = 0.001; Fig. 1b). The proportion of dominant foundress- or parasite-initiated activity bursts decreased with increasing group size (F 1,38 = 5.31, p = 0.03; parasitism × group size interaction: F 1,37 = 0.67, p = 0.42). Excluding the six colonies with a group size of two did not change the results. Overall, the frequency distribution of activity-initiating behaviours by parasites and dominant foundresses was similar (Fig. 2). However, parasites and dominant foundresses differed in the frequency of specific activity-initiating behaviours. Mounting of subordinates by parasites initiated activity bursts in 7 % cases, but mounts were never observed to initiate activity in unparasitized colonies. In contrast, gaster-wagging by dominant foundresses initiated activity bursts in a small proportion (14 %) of cases, but gaster-wagging by parasites was not observed to initiate activity in parasitized colonies.
Initiation of colony activity by parasites and dominant foundresses. a, c Observed proportion of activity bursts and departures initiated by parasites and dominant foundresses divided by the expected proportion if initiation was distributed equally among colony members. b, d The proportion of activity bursts and departures initiated by parasites and dominant foundresses. Central lines represent median values, the top and bottom lines of the box represent the first and third quartiles and vertical lines represent approximately two standard deviations around the interquartile range (circles denote outliers)
Dominant foundresses initiated 1.96 ± 0.40 times as many departures as expected by chance (W = 100.5, p = 0.02). In contrast, parasites did not initiate more departures than expected by chance (W = 85, p = 0.12; Fig. 1c). However, the proportion of departures initiated by dominant foundresses was not significantly higher than that initiated by parasites (F 1,28 = 1.61, p = 0.21; Fig. 1d). The proportion of dominant foundress- or parasite-initiated departures decreased with increasing colony size (F 1,29 = 7.22, p = 0.02; parasitism x group size interaction: F 1,27 = 0.96, p = 0.40). The majority (79 %) of departures in both parasitized and unparasitized colonies were triggered by antennations.
Effect of parasite/dominant removal on colony activity
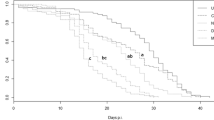
Removal of the parasite or dominant foundress did not affect colony activity, either in terms of the number of bursts (removal: \( \chi_{1}^{2} \) = 0.004, p = 0.95; removal × parasitism interaction: \( \chi_{3}^{2} \) = 0.26, p = 0.97) or the duration of bursts (removal: L = 0.32, p = 0.57; removal × parasitism interaction: L = 1.48, p = 0.69; Fig. 3). Combining data from before and after parasite/dominant foundress removal, there was no significant difference between parasitized and unparasitized colonies in either the number (\( \chi_{1}^{2} \) = 0.25, p = 0.62) or duration of bursts (L = 0.80, p = 0.37; Fig. 3). Bursts were longer in larger colonies (L = 10.57, p = 0.001), but not more frequent (\( \chi_{1}^{2} \) = 0.60, p = 0.44).
Duration (untransformed) and number of activity bursts in 12 parasitized and unparasitized colonies before (dark grey) and after (light grey) removal of the parasite or dominant foundress. Central lines represent median values, the top and bottom lines of the box represent the first and third quartiles and vertical lines represent approximately two standard deviations around the interquartile range (circles denote outliers)
Discussion
There was no evidence that the social parasite P. semenowi behaved as a pacemaker within the host colony. Parasites were not more likely than the average colony member to initiate either activity bursts or foraging trips through behavioural interactions. This is in marked contrast to the dominant P. dominula foundress in unparasitized nests, which initiated almost twice as many foraging trips and three times as many activity bursts as the average colony member. Dominant foundresses initiated significantly more activity bursts than parasites, but the difference in the number of foraging trips initiated was not significant. Our results, which suggest that dominant foundresses function as pacemakers within unparasitized multiple-foundress colonies, contrasts with a previous study by Jha et al. (2006), which found that worker activity in single-foundress P. dominula colonies was not directed by the foundress. Although the definitions of activity bursts differed slightly between the two studies, there is no reason to believe that this accounts for the difference in results. Rather, one possible explanation for the difference in results is that the colonies studied by Jha et al. were larger than those used in the present study (8–43 vs. 2–12 adults). A distinction has often been drawn between the large eusocial insect colonies, where behavioural regulation of colony activity by one or a small number of dominant individuals is unlikely to be feasible, and the smaller colonies of primitively eusocial species, where such dominant control may be possible. While we have found support for this in multiple-foundress colonies containing only a small number of workers, it is possible that as group size increases with the emergence of further workers throughout the season, dominant foundresses are no longer able to function as pacemakers, with colony activity instead becoming increasingly self-directed. A second possible explanation for the difference in results between our study and that of Jha et al. may relate to the genetic structure of the colonies under study. Within the single-foundress colonies studied by Jha et al., workers are the offspring of the foundress, whereas in the pre-worker, multiple-foundress colonies of P. dominula we studied kin relationships are more variable, with a significant proportion of colonies containing subordinates that are unrelated to the dominant (Zanette and Field 2008; Leadbeater et al. 2010). Recent research indicates that relatedness to the dominant does influence subordinate work rate in this species: relatives of the dominant that are smaller in size (and hence lower down the inheritance queue) forage more, but no such pattern is observed for unrelated subordinates (Leadbeater et al. 2014). From the perspective of the dominant, the differences in work rate according to kinship and the prospect of inheritance among subordinate co-foundresses may favour more direct control of activity within multiple-foundress groups. If so, this may explain why subordinate activity in pre-worker, multiple-foundress colonies appears to be subject to greater dominant control (this study) than worker activity in single-foundress colonies (Jha et al. 2006). We lack data on genetic relationships within the co-foundress groups used in this study, but exploring whether control of subordinate work effort by dominants varies with dominant-subordinate relatedness would be an interesting focus for future studies.
The fact that P. semenowi did not interact with its hosts to initiate activity at a greater rate than expected by chance could suggest that the parasite has no control over its host workforce, a scenario that is also supported by the absence of any change in host activity following parasite removal. However, activity levels in parasitized colonies were similar to those in unparasitized colonies where dominant foundresses were observed to function as behavioural pacemakers, implying that parasites may indeed control host behaviour, through means other than direct physical interactions. One possibility is that parasites manipulate host activity through the use of pheromones. Pheromonal control of worker behaviour is well described in insect species exhibiting advanced eusociality (for a recent review, see Richard and Hunt 2013), but to our knowledge there is no current evidence for pheromonal control of colony activity by dominants in primitively eusocial species, or their social parasites. However, recent findings that P. sulcifer venom volatiles trigger intracolonial aggression in P. dominula (Bruschini and Cervo 2011) appear to indicate that chemical manipulation of host behaviour by Polistes social parasites can occur, though whether parasite-derived pheromones play a role in controlling the host activity beyond the initial usurpation stage remains to be explored.
Whatever the mechanism, it is clear that colony control by P. semenowi does not result in hosts working at a significantly higher rate than subordinates in unparasitized colonies. This is in marked contrast to the behaviour of P. biglumis foundresses parasitized by P. atrimandibularis, which spend more time off the nest foraging than unparasitized foundresses, both prior to, and after, worker emergence (Fucini et al. 2014). One explanation for this difference could be that, while P. atrimandibularis parasitises single-foundress colonies, P. semenowi preferentially usurps larger, multi-foundress colonies. Though the activity of multiple foundresses ensures that such colonies are more productive (i.e. a greater number of parasite offspring reared), increased colony size may at the same time limit the scope for parasites to manipulate the host activity levels, either directly or indirectly. Examining strategies of host activity regulation by the third Polistes inquiline, P. sulcifer, which also targets multiple-foundress colonies, would help to determine the role of host colony size in shaping strategies of host control among social parasites.
Despite identifying a key role for the dominant P. dominula foundress as a pacemaker, we found no effect of her removal on colony activity. There was also no effect of removal of the parasite on activity in parasitized colonies. Performing similar removal experiments, Fucini et al. reported pronounced shifts in colony activity that occurred just a few hours following the removal of P. atrimandibularis parasites (Fucini and Lorenzi 2004; Fucini et al. 2014). Therefore, it seems unlikely that our failure to detect changes in colony activity 24 h after the removal of the dominant foundress or parasite was due to hosts not having sufficient time to respond to the manipulation. Instead, it is possible that by this time the vacant dominant position in parasitized and unparasitized colonies had already been assumed by the Rank 2 foundress, who then maintained colony activity at the previously observed levels. Alternatively, in the case of parasitized colonies, it is possible that the apparent indirect effects of parasitism on colony activity suggested by our results may persist for some time even in the absence of the parasite herself.
In many host-brood parasite systems, it is challenging to determine from host behavioural responses to parasites whether hosts are deceived by parasites or whether parasites are recognised, but nonetheless accepted because the costs of rejection exceed those of acceptance (Kilner and Langmore 2011). Parasitism by P. semenowi provides a good case in point: the violent usurpation of host nests by the parasite (Zacchi et al. 1996; Green and Field 2011) would seem to provide a clear cue to hosts, supported by the fact that many hosts subsequently desert the nest for a short period (Zacchi et al. 1996; J. P. Green, pers. obs.). However, following the return of the majority of foundress groups to their nests, it is unclear whether hosts are still able to recognise parasites, which following usurpation adopt the cuticular hydrocarbon profile of the host colony, and specifically that of the former dominant (Lorenzi et al. 2004). Preferential feeding of own versus parasite brood provides some evidence that P. semenowi hosts can detect parasitism (Almond 2007), but the opposite feeding bias in hosts parasitized by P. sulcifer points instead to host manipulation by parasites (Cervo et al. 2004). Increased rates of ovarian development in host workers following the removal of P. sulcifer versus dominant foundresses from unparasitized colonies could also indicate the recognition of parasites, but could alternatively be a consequence of weaker suppression of host reproduction by the parasite (Cini et al. 2014). Exploration of host activity patterns in parasitized colonies provides an additional context in which to assess host recognition of parasitism. Our finding that parasites and dominant foundresses differed in the frequency of their behavioural interactions with subordinates suggests that the pattern of social interaction in parasitized colonies is altered, which might allow hosts to detect parasitism. However, the overall similarity in the behaviours used to initiate the activity by parasites and dominant foundresses indicates that recognition based on specific interactions with parasites is unlikely. In addition, hosts that are able to recognise parasites might be expected to avoid rearing parasite offspring, instead preserving energy for possible future reproductive opportunities (e.g. inheritance of the dominant position from the parasite; Lorenzi et al. 1992). The similarity between parasitized and unparasitized colonies in activity levels does not by itself provide convincing evidence against recognition, since any strategic reductions in helping effort may be countered by parasite manipulation of host activity (see above). Thus, while the differing roles of parasites and dominant foundresses in regulating activity suggest that parasitized and unparasitized hosts may experience different social conditions within the colony, it remains unclear whether these or other differences associated with parasitism allow hosts to recognise that parasitism has occurred.
Polistes social parasites comprise a monophyletic clade, with the three inquilines more closely related to one another than to their hosts (Choudhary et al. 1994; Carpenter 1997). Given that parasites did not evolve in sympatry with their hosts, an interesting question is thus how similar parasites are to each other versus their host species. Here, we have found that P. semenowi differs markedly from its host dominant in its role as pacemaker within the host colony. At the same time, however, the behaviours used by the parasite and its host to initiate activity were found to be very similar, and were similar to those used to initiate the interactions by a second parasite species, P. atrimandibularis (Fucini et al. 2014). One interpretation of this is that the repertoire of social behaviours expressed by parasites in interactions with hosts is conserved and shared with the more distantly related host species, but that, at least in the case of P. semenowi, parasites have not necessarily evolved to imitate the precise social functions of the host dominant they replace. Despite the similarity between the two inquilines in the behavioural repertoire used in interactions with hosts, comparison of our results with those of Fucini et al. (2014) also reveals pronounced differences between the two species in terms of control of colony activity with a higher rate of interactions between P. atrimandibularis and its P. biglumis hosts leading to elevated host activity compared with unparasitized colonies. Such differences provide further evidence for divergence between the P. semenowi and P. sulcifer, which are specialists upon P. dominula and the generalist P. atrimandibularis, which shows a number of unique behavioural traits, including a non-aggressive usurpation tactic and active depredation of brood from satellite host nests (reviewed in Cervo 2006). Generally, our results demonstrate that control of colony activity by social parasites can be highly variable, even among closely related inquiline species. Outside of polistine wasps, social parasitism has evolved in several other primitively eusocial taxa, including allodapine and halictid bees (Danforth et al. 2013), but host-parasite interactions beyond the initial usurpation stage have received relatively little attention. The study of such interactions, in particular, how parasites control and coordinate host work effort, will provide a more comprehensive understanding of the strategies used by the social parasites to successfully exploit their hosts.
References
Almond EJ (2007) Interaction between the paper wasp Polistes dominula and its social parasite, Polistes semenowi. Dissertation, University College London
Anderson C, McShea DW (2001) Individual versus social complexity, with particular reference to ant colonies. Biol Rev 76:211–237
Bruschini C, Cervo R (2011) Venom volatiles of the paper wasp social parasite Polistes sulcifer elicit intra-colonial aggression on the nest of the host species Polistes dominulus. Insect Soc 58:383–390
Camazine S, Deneubourg JL, Franks NR, Sneyd J, Theraulaz G, Bonabeau E (2001) Self-organization in biological systems. Princeton University Press, Princeton
Cant MA, Field J (2001) Helping effort and future fitness in cooperative animal societies. Proc R Soc Lond B 268:1959–1964
Carpenter JM (1997) Phylogenetic relationships among European Polistes and the evolution of social parasitism (Hymenoptera: Vespidae: Polistinae). Mémoires du Muséum national d’histoire naturelle 173:135–161
Cervo R (2006) Polistes wasps and their social parasites: an overview. Ann Zool Fenn 43:531–549
Cervo R, Turillazzi S (1996) Host nest preference and nest choice in the cuckoo paper wasp Polistes sulcifer (Hymenoptera: Vespidae). J Insect Behav 9:297–306
Cervo R, Macinai V, Dechigi F, Turillazzi S (2004) Fast growth of immature brood in a social parasite wasp: a convergent evolution between avian and insect cuckoos. Am Nat 164:814–820
Choudhary M, Strassmann JE, Queller DC, Turillazzi S, Cervo R (1994) Social parasites in Polistine wasps are monophyletic: implications for speciation. Proc R Soc Lond B 257:31–35
Cini A, Bruschini C, Poggi L, Cervo R (2011) Fight or fool? Physical strength, instead of sensory deception, matters in host nest invasion by a wasp social parasite. Anim Behav 81:1139–1145
Cini A, Nieri R, Dapporto L, Monnin T, Cervo R (2014) Almost royal: incomplete suppression of host worker ovarian development by a social parasite wasp. Behav Ecol Sociobiol 68:467–475
Danforth BN, Cardinal S, Praz C, Almeida EAB, Michez D (2013) The impact of molecular data on our understanding of bee phylogeny and evolution. Ann Rev Entomol 58:57–78
De Souza AR, Prezoto F (2012) Regulation of worker activity in the social wasp Polistes versicolor. Insect Soc 59:193–199
Fucini S, Lorenzi MC (2004) Behavioural counter-adaptations to social parasites in Polistes biglumis, host of P. atrimandibularis (Hymenoptera, Vespidae). Insect Soc Life 5:27–29
Fucini S, Uboni A, Lorenzi MC (2014) Cuckoo wasps manipulate foraging and resting activities in their hosts. Behav Ecol Sociobiol 68:1753–1759
Gamboa GJ, Wacker TL, Scope JA, Cornell TJ, Shellman-Reeve JS (1990) The mechanism of queen regulation of foraging by workers in paper wasps (Polistes fuscatus, Hymenoptera, Vespidae). Ethology 85:335–343
Green JP, Field J (2011) Assessment between species: information gathering in usurpation contests between a paper wasp and its social parasite. Anim Behav 81:1263–1269
Green JP, Cant MA, Field J (2014) Using social parasitism to test reproductive skew models in a primitively eusocial wasp. Proc R Soc Lond B 281:20142016
Guillem RM, Drijfhout F, Martin SJ (2014) Chemical deception among ant social parasites. Curr Zool 60:62–75
Jeanne RL (2003) Social complexity in the Hymenoptera, with special attention to the wasps. In: Kikuchi T, Azuma N, Higashi S (eds) Genes, behaviors and evolution of social insects. Hokkaido University Press, Sapporo, pp 81–131
Jha S, Casey-Ford RG, Pedersen JS, Platt TG, Cervo R, Queller DC, Strassmann JE (2006) The queen is not a pacemaker in the small-colony wasps Polistes instabilis and P. dominulus. Anim Behav 71:1197–1203
Kilner RM, Langmore NE (2011) Cuckoos versus hosts in insects and birds: adaptations, counter-adaptations and outcomes. Biol Rev 86:836–852
Leadbeater E, Carruthers JM, Green JP, van Heusden J, Field J (2010) Unrelated helpers in a primitively eusocial wasp: is helping tailored towards direct fitness? PLoS ONE 5:e11997
Leadbeater E, Carruthers JM, Green JP, Rosser NS, Field J (2011) Nest inheritance is the missing source of direct fitness in a primitively eusocial insect. Science 333:874–876
Leadbeater E, Dapporto L, Turillazzi S, Field J (2014) Available kin recognition cues may explain why wasp behaviour reflects relatedness to nest mates. Behav Ecol 25:344–351
Lorenzi MC (2006) The result of an arms race: the chemical strategies of Polistes social parasites. Ann Zool Fenn 43:550–563
Lorenzi MC, Cervo R, Turillazzi S (1992) Effects of social parasitism of Polistes atrimandibularis on the colony cycle and brood production of Polistes biglumis bimaculatus (Hymenoptera, Vespidae). Ital J Zool 59:267–271
Lorenzi MC, Cervo R, Zacchi F, Turillazzi S, Bagnères AG (2004) Dynamics of chemical mimicry in the social parasite wasp Polistes semenowi (Hymenoptera: Vespidae). Parasitology 129:643–651
O’Donnell S (1998) Effects of experimental forager removals on division of labour in the primitively eusocial wasp Polistes instabilis (Hymenoptera: Vespidae). Behaviour 135:173–193
R Core Team (2014) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/. Accessed Jan 2015
Reeve HK (1991) Polistes. In: Ross KG, Matthews RW (eds) The social biology of wasps. Cornell University Press, Ithaca, pp 99–148
Reeve HK, Gamboa GJ (1983) Colony activity integration in primitively eusocial wasps: the role of the queen (Polistes fuscatus, Hymenoptera: Vespidae). Behav Ecol Sociobiol 13:63–74
Reeve HK, Gamboa GJ (1987) Queen regulation of worker foraging in paper wasps: a social feedback control system (Polistes fuscatus, Hymenoptera: Vespidae). Behaviour 102:147–167
Richard F-J, Hunt JH (2013) Intracolony chemical communication in social insects. Insectes Soc 60:275–291
Shreeves G, Cant MA, Bolton A, Field J (2003) Insurance-based advantages for subordinate co-foundresses in a temperate paper wasp. Proc R Soc Lond B 270:1617–1622
Zacchi F, Cervo R, Turillazzi S, Moli FL, Mori A, Grasso DA (1996) How Polistes semenowi, obligate social parasite, invades the nest of its host, Polistes dominulus (Hymenoptera, Vespidae). Insect Soc Life 1:125–130
Zanette LRS, Field J (2008) Genetic relatedness in early associations of P. dominulus: from related to unrelated helpers. Mol Ecol 17:2590–2597
Acknowledgments
We thank Lorenzo Zanette for assistance in the field. This research was funded by Natural Environment Research Council Grant NE/E017894/1 to JF.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Green, J.P., Almond, E.J., Williamson, J. et al. Regulation of host colony activity by the social parasite Polistes semenowi . Insect. Soc. 63, 385–393 (2016). https://doi.org/10.1007/s00040-016-0478-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-016-0478-y