Abstract
Social insects face strong selection from parasites because the conditions of group living often favor the transmission of infection among nestmates. However, there is little detailed information on the effects of parasite infection in the host species. Workers of Polybia species, neotropical swarm-founding wasps, are commonly infected by gregarines, protozoans that are exclusively parasitic on invertebrates. Previous studies showed that high rates of gregarine infection in workers of Polybia occidentalis (Olivier) have negative effects on their colony performance. However, the effect of seasonality on infection rates throughout the year or between wet and dry seasons has not been examined. Host-parasite interactions cannot be understood without consideration of the overall population dynamic. We compared rates of gregarine infection in workers of Polybia paulista (Ihering) between wet and dry seasons and among months. The 35% rate was by far the highest of the four wet seasons sampled, but the rates declined in the mid-wet season and were very low during the dry season. Strong seasonal differences in infection rates were also observed between the dry and wet seasons. Several potential factors affecting the seasonal differences are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parasites induce physiological, behavioral, and morphological effects on their hosts. Additionally, the co-evolution of parasites and their hosts often leads to drastic changes in the life history of the host species (e.g., Avilés et al2006). Social insects face strong selection from parasites, as shown by the following common observations among colony mates of high-colony genetic relatedness, frequent interactions, and grooming and hygienic behavior (reviewed by Schmid-Hempel 1998, Pie et al2004). Thus, it is important to elucidate the relationships between social insects and their parasites in order to understand the evolution of social behavior.
In social wasps, well-documented interactions with gregarines have been investigated in colonies of Polybia occidentalis (Olivier), a common swarm-founding wasp of the American tropics (Howard & Jeanne 2004, Bouwma et al2005, Howard & Jeanne 2005, Kudô et al2011). Gregarines (Phylum: Apicomplexa, Order: Eugregarinida) are common and diverse protozoans that are exclusively parasitic on invertebrates, especially arthropods. Jeanne and others investigated the relationships between foraging activities and rates of gregarine infection in workers of P. occidentalis (Howard & Jeanne 2004, 2005, Bouwma et al2005). Howard & Jeanne (2004) showed that infection by gregarines increased brood development time, independently of colony size, possibly due to reduced foraging success of infected workers. Bouwma et al (2005) also showed the negative effect of gregarine infections on per-capita brood production and hypothesized that foraging rates per brood may be a more direct result than those per larva. Howard & Jeanne (2005) partially supported this interpretation and showed in pre-emergence colonies that foraging rates per larva did not correlate with infection rates by gregarine. In P. occidentalis, the effect of gregarine infection on worker body size was examined. Kudô et al (2011) found that workers infected by gregarines were larger than uninfected workers. The authors hypothesized that infection by gregarines makes larvae hungrier and induces them to solicit more food from adults.
The same effect on worker body size by gregarine infection has been shown in a swarm-founding polygynous wasp P. paulista (Ihering), a consubgeneric species of P. occidentalis (Kudô et al2004). These authors collected four colonies of this species during the wet season in Ribeirão Preto, southeastern Brazil, and showed that infection rates varied among colonies (0–14%). Infection rates by gregarines were shown to change throughout the wet season in a Finca Las Pumas population (Costa Rica) of P. occidentalis during 1999 and 2000 (Bouwma et al2005). The authors showed that intra-colony infection rates declined from the early wet season to the late wet season in 1999, beginning low in the wet season, increasing in the mid-wet season, and then declining in the late wet season in 2000. These previous works suggest that infection rates by gregarines vary among colonies as well as seasons. However, the effect of seasonality on infection rates by gregarines throughout the year or between wet and dry seasons has not yet been examined. Because gregarines may be an important source of selection in the behavioral ecology and life history of Polybia species (Bouwma et al2005), information about the long-term changes of the infection rates is needed.
Here, we examine seasonal changes in the rates of gregarine infection in workers of P. paulista. First, we collected workers from several colonies at regular intervals throughout a year and compared the rates of gregarine infection between wet and dry seasons and among months. Next, we collected colonies of this species in wet and dry seasons and compared rates of gregarine infection between seasons.
Materials and Methods
Wasp sampling at regular intervals
Every two months between November 2000 and November 2001, we collected wasps with insect nets from a total of 10 colonies at the campus of Universidade de São Paulo (USP) in Ribeirão Preto city (21°11′S, 47°48′W), after these nests were partially destroyed. In each colony, collections were carried out until the colony was destroyed by unknown predators (absconding swarming) or exhibited swarm emigration (reproductive swarming or emigration swarming). The numbers of wasps in each colony collected by sampling month are summarized in Table 1. In our study locality, P. paulista exhibits colony activity throughout the year, but the activity is commonly higher between October and April (hereafter referred to as “wet season”) than between May and September (hereafter referred to as “dry season”) (Kudô et al2003, 2011). Our sampling was carried out four times during the wet season and three times during the dry season (Table 1). On average, 233.7 ± 33.9 wasps (± SE; range, 20–168 wasps) from 4.1 ± 0.7 colonies were collected in each sampling month and immersed in 99% ethanol for later dissection.
Nest collection
We collected 11 colonies in August 2012 (dry season) and 12 colonies in January to March 2013 (wet season) on the campus of USP and its adjacent area. All wasps were immersed in 99% ethanol for later dissection.
Dissections
The abdomen was dissected from all wasps collected in 2000 and 2001 (N = 1636) under a binocular microscope (Olympus, SZ-61), whereas the abdomen was dissected from 100 randomly selected wasps from each of 23 colonies collected in 2012 and 2013 (N = 2300). As Noll & Zucchi (2000) noted in P. paulista, the following three development types were recognized in the ovary: type A with filamentous ovarioles bearing no visible or slightly developed oocytes, type B with at least some mature or nearly mature oocytes, and type C with well-developed and longer ovarioles each bearing from two to several mature oocytes. Since only type C females contained sperm in the spermatheca, females with ovaries A, B, and C were characterized as workers, intermediates, and queens, respectively. By dissection of the abdomen, we found milky white spheres in the host wasps (length and width: ca. 500 μm), which were the gametocysts of the gregarine (Kudô et al2004). We treated these wasps as “parasitized by gregarines,” but did not measure the infection intensities (numbers of the gametocysts) or developmental stages of the parasites.
Results
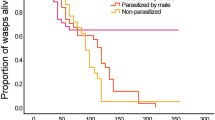
The numbers of queens, intermediates, and workers collected in 2000 and 2001 were 7, 19, and 1610, respectively. No queens or intermediates were parasitized by the gregarines. The proportion of parasitized workers per colony changed drastically (Fig 1); more than 30% of workers were parasitized in November 2000 (early wet season), and the proportion decreased gradually through the mid-wet to the late wet season. There were very few parasitized workers between March and July 2001, and so the proportion per colony was particularly low in those months. After September 2001, however, the proportion of parasitized workers per colony increased again. Proportion of infected workers in individual colonies changed consistently; no infected workers were observed over four months spanning wet to dry season in two colonies (PP0011 and PP0034) (Fig 2), while proportion of infected workers spanning dry to early wet season in one of other two colonies (PP0151 and PP0152) increased gradually (Fig 3).
In 11 colonies collected in the dry season (2012), mean proportion of infected workers per colony was only 0.09% (± 0.09%, ± SE). In addition, four of 17 queens were parasitized by the gregarines, but no intermediates (N = 85) were parasitized. In 12 colonies collected in the wet season (2013), mean proportion of infected workers per colony was 1.95% (± 0.98%, ± SE), but no queens (N = 34) or intermediates (N = 22) were parasitized. The mean proportion of parasitized workers per colony during the wet season (2013) was significantly larger than the dry season (2012) (Mann-Whitney U test, z = 2.388, P = 0.017).
Discussion
We studied seasonal differences in the rates of gregarine infection in workers of the swarm-founding wasp P. paulista. Two different sampling methods consistently showed that infection rates were (1) higher during wet seasons than during dry seasons and (2) not constant throughout the wet season.
Bouwma et al (2005) investigated intra-colony infection rates by gregarines during wet seasons in the Costa Rican P. occidentalis, but the infection rates over a year to include the dry season had not previously been examined. This is the first study to examine changes in infection rates by gregarines throughout the year and whether the rates differ between wet and dry seasons in social wasps. Because the mode of transmission for gregarines is not known, there are no data bearing directly on the mechanism for the observed change in infection rates between seasons. One possibility is that colonies with high gregarine infection rates in workers during the wet season are unable to survive for a long duration, at least over seasons. Because infected workers commonly forage at lower rates than uninfected workers (Bouwma et al2005), it appears that the wasp population in such colonies is not maintained. In fact, demographic data of P. paulista workers indicated that even colonies with several thousand workers would quickly be reduced to very small numbers unless new workers were produced to replace old workers (Kudô et al2011). Another possibility for the observed change in infection rates between seasons could be associated with environmental temperature and temperature variation. Several recent studies indicated immediate and longer-lasting effects of environmental temperature and temperature variation in the outcome of future host-parasite interactions (Eggert et al2015, Pamminger et al2016). If this is the case, the temperature difference between seasons in our study locality would affect the susceptibility of workers to infection.
We found very few workers infected by gregarines from late in the wet season to the middle of the dry season. These very low infection rates could be associated with a longer lifespan of workers infected late in the wet season. In Ribeirão Preto, 60–70% of P. paulista colonies do not rear broods during the dry season (K. Kudô unpublished data), suggesting that the life span of such infected workers is prolonged. Additionally, because infected workers commonly forage at lower rates than uninfected workers (Bouwma et al2005), it appears that very few P. paulista workers infected by gregarines at the end of the wet season would have survived longer than a few months.
Infection rates by the gregarines were not constant throughout the wet seasons in the Ribeirão Preto population of P. paulista during 2000–2001. Bouwma et al (2005) analyzed the relationship between infection rates by gregarines and collection date (from early to late wet season) in P. occidentalis and found different patterns in the relationships between two years; the infection rates declined from the early to late wet season in 1999, whereas in 2000 the rates began in the early wet season, increased in the mid-wet season, and then declined in the late wet season. Again, because the mode of transmission is not known, it is unclear why the pattern of the gregarine infection rates throughout the wet seasons differed between years.
Heavy infection by gregarines in workers during wet seasons must have a negative effect on fitness via smaller mature colony size as a consequence of reduced productivity and retarded growth, and there is evidence to support this view. Howard & Jeanne (2004) measured brood development rates in colonies of founding stages in P. occidentalis and showed that infection by gregarines increased brood development time, independently of colony size. Howard & Jeanne (2005) also observed that infected workers of this species forage at lower rates than uninfected workers. In P. paulista, the extranidal activities of workers during the wet season were much higher than those during the dry season (Kudô et al2003). Moreover, reproductive swarms during the wet season occur frequently compared with the dry season in P. paulista (Kudô unpublished results). If infected workers of P. paulista tend to forage at lower rates, as observed in P. occidentalis, the reduced foraging rates during the wet season cause smaller and/or fewer reproductive swarms.
This study showed that in the swarm-founding wasp P. paulista, infection rates by gregarines in workers were higher during the wet season than during the dry season and were not constant throughout the wet season. As previous studies in P. occidentalis suggested, our results suggest that gregarines must be an important selection factor in the behavioral ecology and life history of P. paulista. Further studies on annual and seasonal changes in foraging, individual or colony mortality, and colony productivity are needed to further elucidate on the potential effects of gregarines.
References
Avilés JM, Stokke BG, Parejo D (2006) Relationship between nest predation suffered by host and brown-headed cowbird parasitism: a comparative study. Evol Ecol 20:97–111
Bouwma AM, Howard K, Jeanne RL (2005) Parasitism in a social wasp: effect of gregarines on foraging behavior, colony productivity, and adult mortality. Behav Ecol Sociobiol 59:222–233
Eggert H, Diddens-de Buhr MF, Kurtz J (2015) A temperature shock can lead to trans-generational immune priming in the red flour beetle. Tribolium castaneum. Ecol Entomol 5:1318–1326
Howard KJ, Jeanne RL (2004) Rates of brood development in a social wasp: effects of colony size and parasite infection. Insect Soc 51:179–185
Howard KJ, Jeanne RL (2005) Shifting foraging strategies in colonies of the social wasp Polybia occidentalis (Hymenoptera, Vespidae). Behav Ecol Sociobiol 57:481–489
Kudô K, Zucchi R, Tsuchida K (2003) Initial nest development in the swarm-founding paper wasp, Polybia paulista (Hymenoptera: Vespidae, Epiponini): cases of building multiple initial combs. J New York Entomol Soc 111:151–158
Kudô K, Yamane S, Mateus S, Tsuchida K, Itô Y, Miyano S, Zucchi R (2004) Parasitism affects worker size in the Neotropical swarm-founding social wasp, Polybia paulista (Hymenoptera, Vespidae). Insect Soc 51:221–225
Kudô K, Ohka H, Zucchi R (2011) Modification of morphological characteristics by endoparasites in workers of the swarm-founding wasp Polybia occidentalis. In: Stewart EM (ed) Social insects: structure, function, and behavior. Nova Science Publishers, NY, pp 83–96
Noll FB, Zucchi R (2000) Increasing caste differences related to life progression in some Neotropical swarm-founding polygynic polistine wasps (Hymenoptera Vespidae Epiponini). Ethol Ecol Evol 12:43–65
Pamminger T, Steier T, Tragust S (2016) High temperature and temperature variation undermine future disease susceptibility in a population of the invasive garden ant Lasius neglectus. Sci Nat 103:46
Pie MR, Rosengaus RB, Traniello JFA (2004) Nest architecture, activity pattern, worker density and the dynamics of disease transmission in social insects. Theor Biol 226:45–51
Schmid-Hempel P (1998) Parasites in social insects. Princeton University Press, Princeton, p 392
Acknowledgments
We are grateful to B. Kranz who provided invaluable comments and suggestions for the improvement of the text. A Grant-in-Aid for Scientific Research (C) (No. 24570021) was given to K. Kudô. We thank Eri Kudô, Yuriko Naganuma, and Lucas Oliveira for their kind help during the field collection.
Funding
This study was supported by a JSPS Research fellowship for Young Scientists (No. 02415).
Author information
Authors and Affiliations
Contributions
KK, MH, SM, RZ, and FSN planned and designed field and experimental works; KK, MH, SM, and FSN performed field work; KK and MH performed experimental work; KK and MH analyzed the data; KK wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Edited by Fernando B Noll – UNESP
Rights and permissions
About this article
Cite this article
Kudô, K., Hasegawa, M., Mateus, S. et al. Effect of Seasonality on Rates of Gregarine Infection in Workers of a Social Wasp Polybia Paulista (Hymenoptera, Vespidae). Neotrop Entomol 48, 368–372 (2019). https://doi.org/10.1007/s13744-018-0649-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-018-0649-9