Abstract
Insect social parasites, like other parasites, may benefit from inhibiting their host from reproducing (complete or partial parasitic castration) because they can then exploit more of the host’s resources for their own reproduction. In particular, social parasites that kill or expel the host queen need to prevent host workers from reproducing; this is a common worker response to the absence of their queen. Indeed, host workers would benefit from detecting the presence of the parasite and investing in direct and indirect fitness. Studying whether and how social parasites control host worker reproduction can provide information about the degree of integration of the parasite in the host colony and help identify factors regulating workers’ reproductive decisions in social insects. We investigated whether the paper wasp social parasite, Polistes sulcifer, suppresses Polistes dominula (host) worker reproduction as efficiently as the dominant host female does in queen-right colonies by comparing worker reproductive efforts in parasitized and non-parasitized (control) colonies. Our results show that 6 weeks after usurpation of their colony by the social parasite, parasitized workers (1) had more developed ovaries than control workers and (2) laid more eggs as soon as the opportunity arose. This reproductive readiness of parasitized workers was not apparent 2 weeks after colony usurpation. This suggests that P. dominula workers have evolved means to react to social parasitism, as occurs in some ants, and that the parasite has only limited control over host reproduction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insect societies represent valuable resources and, despite efficient cooperative defensive behaviours, are exploited by a vast array of predators and parasites, some of which are themselves social. This is the case for social parasites sensu stricto, i.e. social insect species that parasitize the brood care of other social insects to rear their own offspring (Wilson 1971). In the social Hymenoptera, social parasites have repeatedly evolved from the social ancestors (Lowe et al. 2002) in ants (Hölldobler and Wilson 1990), allodapine bees (Michener 1974), honeybees (Neumann et al. 2001), bumblebees (Alford 1975) and wasps (Cervo 2006). They often parasitize only one or a few related species, to which they are often closely related (Emery’s rule, e.g. Savolainen and Vepsäläinen 2003). Some social parasites live together with host queens, but others kill or expel them, i.e. “usurp” the colony (reviewed in Alford 1975; Brandt et al. 2005; Cervo 2006). In removing the host queen, social parasites also remove the inhibitory effect she has on host worker reproduction. Indeed, workers of many species do not lay eggs in the presence of their queen but retain the ability to do so if their queen is absent or unfertile. In highly eusocial species, workers can only lay unfertilized eggs that develop into males, but in primitively eusocial species, they can mate and hence also lay fertilized eggs that develop into females (e.g. Bourke 2011). Social parasites would therefore benefit from preventing host workers from reproducing because they could then exploit more of the host colony’s resources for their own reproduction. Indeed, complete or partial castration of the host is a common feature of parasitism across many taxa (e.g. Baudoin 1975; Hurd 2001).
Many social parasites "fool" host workers by behaviourally and chemically mimicking the fool ousted host queen(s) (Alford 1975; Lorenzi 2006; Martin et al. 2010). However, this is not always perfect (e.g. Bagnères et al. 1996; Turillazzi et al. 2000) and workers, given the high fitness cost of social parasitism, may be selected to detect social parasites and to behave so as to regain some fitness. For instance, Temnothorax ant workers enslaved by Protomognathus americanus kill two thirds of the pupae of the social parasite, and this may increase their fitness if it lowers the parasite pressure (raids) against related neighbouring colonies (Achenbach and Foitzik 2009). Experimental brood exchange shows that this “rebellion” is stronger against parasite pupae from allopatric populations, suggesting that parasite pupae are under strong selection to chemically mimic sympatric host pupae (Achenbach et al. 2010). A more direct route for workers to secure some fitness would be to lay eggs and/or rear the brood of related reproducing workers. The extent of control exercised by the social parasite on worker reproduction varies depending on the host–parasite system examined. Four out of five social parasite bumblebees and two out of three social parasite wasps examined fully suppress worker reproduction (Fisher 1983, 1984; Jeanne 1977; Greene et al. 1978; Cervo and Lorenzi 1996; Vergara et al. 2003; Zimma et al. 2002; Kreuter et al. 2012). Understanding whether social parasites succeed in controlling host worker reproduction is a key step to understand the degree of parasite integration into the host colony. In addition, analysing the reproductive responses of workers to social parasitism may give clues to the factors regulating division of labour in social insects. Division of labour, including the division of reproduction favoured by kin selection, is the key feature of insect societies and is largely responsible for their great ecological success (Fittkau and Klinge 1973; Wilson 1990).
In this paper, we investigated whether the obligate workerless social parasite paper wasp Polistes sulcifer suppresses the reproduction of its Polistes dominula host workers. P. dominula is a functionally monogynous species: colonies are founded by one or several foundresses, but only one female is behaviourally dominant and nearly monopolizes reproduction (Queller et al. 2000). If the dominant foundress dies, another foundress takes over reproduction, whereas if all foundresses die, one or a few workers reproduce (Pardi 1946; Strassmann et al. 2004; Monnin et al. 2009). Indeed, Polistes workers can mate, reproduce sexually and start new colonies (Reeve et al. 1998; Strassmann et al. 2004; Dapporto et al. 2005; Liebig et al. 2005). However, workers do not normally reproduce in the presence of a fertile foundress, unless many cells are empty, which presumably indicates that the foundress has recently disappeared or her fertility is decreasing (Liebig et al. 2005). Workers reproduce in orphaned colonies, even if most cells contain brood (Pardi 1946; Strassmann et al. 2004). Therefore, the presence of both a fertile foundress and abundant brood seems necessary to suppress worker reproduction.
P. sulcifer invades P. dominula colonies through violent fights. The dominant host foundress is usually expelled or killed but sometimes remains as a subordinate, non-reproducing foundress, while the parasite becomes the exclusive or nearly exclusive breeder. The parasite behaves like a dominant foundress and mimics the chemical cues of the ousted foundress (Turillazzi et al. 1990; Dapporto et al. 2004; Cervo 2006). Host workers are apparently fooled by the social parasite: they remain on the nest and rear her offspring (Cervo 2006). However, whether they are completely deceived or detect that something has changed remains to be seen. Host workers do not reproduce when their foundress is replaced by a conspecific and familiar foundress, even if the latter is less or not related to the workers (Monnin et al. 2009). Nonetheless, they may be more likely to detect invasion by a heterospecific and unfamiliar parasite. This opens the possibility that they may react to social parasitism, as Temnothorax ants do (Achenbach and Foitzik 2009), for instance, by reproducing more when their colony is parasitized than when it is not or by readying themselves to reproduce at any opportunity.
Extensive knowledge on the reproductive biology of the host and on the strategies employed by the parasite to enter and be accepted by a host colony allows us to make predictions about the ability of the parasite to control host worker reproduction. Since P. sulcifer chemically and behaviourally mimics the foundress, we might expect that she suppresses host worker reproduction as efficiently as the host foundress did. However, previous preliminary studies provide contradictory evidence: Turillazzi et al. (1991) demonstrated that worker ovarian development did not increase in parasitized colonies, suggesting efficient control by the parasite, whereas Dapporto et al. (2004) showed that parasitized colonies produced a few host females at the end of the season, therefore indicating incomplete castration of the host foundresses and/or host workers by the parasite. Here, we study whether the social parasite suppresses host worker reproduction as efficiently as the dominant foundress does by comparing worker ovarian development and egg laying between parasitized and non-parasitized (control) colonies.
Material and methods
Collection of colonies and laboratory rearing
Monogynous P. dominula colonies (N = 60) in the pre-worker phase were collected in Central Italy, in the surroundings of Florence, during the springs of 2010 (n = 40), 2011 (n = 8) and 2012 (n = 12). We maintained them in the laboratory for the entire experimental period. Each colony was reared in glass cages (15 × 15 × 15 cm) with ad libitum water, sugar and fly maggots as food, under natural light–dark cycle, and warmed with additional artificial light (from 8 a.m. to 5 p.m.). P. sulcifer females (n = 30) were collected in Central Italy (Sibillini Mountains) at the beginning of May (2010, n = 20; 2011, n = 4 and 2012, n = 6), during the last part of their overwintering phase. This ensured that the parasites had not yet made usurpation attempts. Parasites were kept in the laboratory under overwintering-like conditions (4 °C) until late May, when usurpations usually take place in the wild. Parasites were then activated at warm temperature for 5–16 days, following Ortolani et al. (2008), and offered a host nest to usurp.
We used allopatric populations of parasites and host for two reasons. First, P. sulcifer is a rare species and we could not find enough parasite females for each host population. Second, it is difficult to define sympatric populations of the parasite and its host. This is because the parasite reproduces in low lands but hibernates on mountain tops (Cervo 2006) and because it is not known whether parasites return to the host population from which they emerged or go down the mountain to any surrounding host population. Therefore, when collecting hibernating parasites, one does not know which population of hosts it comes from and which population it would naturally parasitize. To avoid mixing parasites that may be sympatric of our host colonies and parasites that may be allopatric, we used allopatric host and parasite populations only (more than 200 km apart).
We obtained 30 parasitized colonies by allowing parasites to usurp randomly selected host colonies. The 30 remaining host colonies were used as controls. Colonies were reared for either 2 weeks (early season experiment; 10 controls and 10 parasitized colonies; years, 2011 and 2012) or 6 weeks (late season experiment; 20 controls and 20 parasitized colonies; year, 2010) after usurpation. The behaviour of the parasite was monitored to confirm the success of the usurpation in both experiments. All usurpations were successful: the parasite became dominant and she produced offspring in the late season experiment. At the end of the experiments, all nests were full of brood which shows that they were healthy. Controls and parasitized colonies were balanced across years.
Measurement of worker egg laying
Two or 6 weeks after the onset of the experiment, the dominant host foundress of control colonies and the parasite and supplanted dominant host foundress (when present) of parasitized colonies were removed and killed by freezing. In addition, in each nest, 10 cells located in the central part of the comb were emptied of the brood. Brood removal triggers worker egg laying (Liebig et al. 2005) and removal of the breeder stops policing by oophagy, so that the worker’s readiness to lay eggs could be measured by counting the number of eggs laid into emptied cells 1 day after brood removal (in addition, workers were killed by freezing and then dissected, see below). While this is not a measure of egg laying in the presence of the breeder, it gives an indication of the physiological and behavioural promptness of workers to lay eggs whenever an opportunity arises. All nests were filled with brood before experimental brood removal; hence, the results cannot be attributed to pre-existing differences in the number of empty cells.
Measurement of ovarian development
All individuals (parasites, foundresses and workers) from the 60 colonies (n = 681 wasps) were dissected. For each individual, we calculated as a reliable index of ovarian development the mean length of the six longest oocytes (Cini et al. 2013). We then compared the mean ovarian development of workers of each colony. Ovaries of 35 out of the 681 wasps were rotten due to poor storage conditions and could not be dissected (all workers from one parasitized and one control colony, 14 workers and 12 parasites/foundresses scattered in other colonies).
Data analysis
Since both the number of cells and the number of workers are indices of colony size (and positively correlated: early season: Pearson r = 0.730, p < 0.001, N = 20; late season: Pearson r = 0.528, p < 0.001, N = 40), we used principal component analysis (separately for each experiment) to produce a single “colony size” predictor explaining most variance of the two original colonial features (87 and 82 % of variance explained for early and late season experiments, respectively; worker number and cell number had loadings greater than 0.86 and 0.90, respectively).
Data were analysed using general linear models (GLMs). The dependent variables were mean ovarian development of workers for each colony and the number of eggs laid by workers in each colony. We performed preliminary model selections based on the Akaike Information Criterion (AIC) by starting with a parameter-rich model which included the following explanatory variables: “colony size” (as a covariate), presence/absence of the former alpha foundress in parasitized colonies (as a factor), the parasitized/control category (as a factor) and the meaningful interaction between category and colony size. We then used the best model identified by AIC to detect effects for selected predictors.
We applied a GLM on the normally distributed log-transformed data for ovarian development (homogeneity of variance was respected: early season: Levene’s test F = 0.23; df1,2 = 1, 18, p = 0.880; late season: Levene’s test F = 2.00, df1,2 = 1, 36, p = 0.166). Egg laying data were not normally distributed, even after transformation, and therefore analysed with a generalized linear model (GLZ) with quasipoisson distribution and log-link function. Quasipoisson models also allow for possible over-dispersion of data. GLM, GLZ and AIC were carried out using the “glm” and “step” function of the “stats” R package.
In addition to comparing the mean ovarian development of workers between parasitized and control colonies (above), we also compared the pattern of worker ovarian development within each colony by analysing the bias in individual degree of ovarian development by computing the B index (Nonacs 2000) using Skew Calculator 2003 (http://www.eeb.ucla.edu/Faculty/Nonacs). B equals 0 when ovarian developments vary randomly between individuals within a colony, is positive when ovarian development varies more than expected from a binomial distribution and is negative when ovarian development is more similar than as expected from a binomial distribution. The 99 % confidence intervals for B indices were compared with expected B values for random, even and monopolized distribution for each colony (as suggested by Nonacs 2000). Since B can be non-linear, we used non-parametric statistics to compare B among groups. SPSS 20.0 was used for statistical analysis. The joint GLM and B index analyses allowed us to evaluate whether parasitized and control colonies differed in their average level and pattern of worker ovarian development.
Results
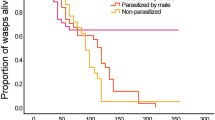
In the early season experiment, neither worker ovarian development nor worker egg laying was significantly affected by parasitism (Table 1, Fig. 1a, c). Worker ovarian development was significantly affected by colony size (workers from larger colonies had less developed ovaries on average, Fig. 1a) while worker egg laying was not (Fig. 1c).
In contrast, in the late season experiment, both worker ovarian development and egg laying were higher (approximately 1.5 and twice as high, respectively) in parasitized colonies than in control colonies (Table 1, Fig. 1d, f). Neither ovarian development nor egg laying was affected by colony size (Table 1, Fig. 1d, f).
Colony size of parasitized and control colonies did not differ 2 weeks after usurpation (T test, t = 0.244, df = 18, p = 0.810), but it was significantly bigger in control colonies 6 weeks after usurpation (T test, t = 2.173, df = 38, p = 0.036).
B indices of all colonies suggested a generally significant but limited skew in worker ovarian development: 52 out of 58 colonies had a B index significantly higher than zero (99 % confidence intervals of B did not include zero). Reproductive skew did not differ significantly between early and late seasons (T test, t = −0.671, df = 56, p = 0.505) and was not different between parasitized and control colonies in both early and late seasons (Table 2, Fig. 1b, e). There was no correlation between B index and colony size in early (Pearson r = 0.040, p = 0.840, n = 19) or late season (Pearson r = −0.288, p = 0.079, n = 38).
Ovarian development of workers did not correlate with that of the parasite and/or alpha foundress, whether in the early season (Pearson r = −0.176, p = 0.486, N = 20) or late season experiment (Pearson r = −0.11, p = 0.593, N = 28). Worker egg laying was not correlated with the ovarian development of the parasite and/or alpha foundress in the early season experiment (Pearson r = 0.081, p = 0.749, N = 20), while it was correlated (but not highly significant) in the late season experiment (Pearson r = 0.382, p = 0.049, N = 29). This suggests that colonies with more fertile dominant breeders may experience more worker egg laying because of their larger number of workers (itself a consequence of breeder fertility). Indeed, when considering parasitized and control nests separately, the positive correlation between breeder ovarian development and worker egg laying holds in control nests where worker number depends on the foundress fertility (Pearson r = 0.749, p = 0.007, n = 11), but not in parasitized nests (Pearson r = 0.239, p = 0.392, n = 15), as expected.
At the end of the experiments, parasite females did not differ in ovarian development from the host alpha foundress of the control colonies (early season (mean ± std. dev.): 1.07 ± 0.42 mm (N = 10) vs. 1.27 ± 0.35 mm (N = 9), T test, t = −0.292, p = 0.773; late season (mean ± std. dev.): 1.84 ± 0.84 mm (N = 16) vs. 1.69 ± 0.21 mm (N = 12), T test, t = 0.288, p = 0.776).
Discussion
Our results show that workers of P. dominula colonies usurped by the social parasite P. sulcifer have a heightened readiness to reproduce. Specifically, they have more developed ovaries than workers from control colonies, and they lay eggs more rapidly when given the opportunity. This effect was apparent 6 weeks after parasitism (late season, Table 2), but not 2 weeks after parasitism (early season, Table 1) even though 6 days is enough to allow workers to develop their ovaries (Monnin et al. 2009). Our results therefore suggest that the social parasite initially suppresses host worker reproduction but does not, in the long term, maintain this suppression as efficiently as the host dominant foundress does.
Our results also indicate that ovarian development was higher in larger colonies, which can result from a more efficient division of labour so that larger colonies can sustain reproductive workers, whereas smaller colonies cannot. The presence of the former dominant foundress in the colony did not affect ovarian development or egg laying. Indeed, when she was not expelled, she stopped behaving as a dominant individual. On the contrary, she often remained motionless on the back of the nest and was sometimes submissive to workers. The presence of the parasite did not affect the bias in ovarian development among workers (Table 2), that is, parasitism did not result in more or fewer workers developing their ovaries. This is consistent with the notion that reproductive workers can be costly because they contribute less to colony efficiency (e.g. Monnin et al. 2003; Heinze 2008), so that most enslaved workers may gain more inclusive fitness by supporting related workers than by attempting to reproduce themselves.
When colonies are parasitized, some workers are therefore ready to reproduce, which could be advantageous if the parasite dies or becomes old. In our experimental setting, alternative strategies such as absconding the nest to found new colonies were impossible. Workers would benefit from expelling the parasite but did not do so. This could be because the parasite has high fighting abilities and can defeat up to 11 workers (Cervo and Turillazzi 1996) or because workers may benefit more by adopting a covert than an overt response to the presence of the parasite.
From the parasite perspective, workers’ investment in reproduction should be minimized in order to maximize their investment in rearing the parasite’s brood. This could be achieved by directly punishing reproductive workers through aggressions or, more likely, indirectly punishing through oophagy (Wenseleers and Ratnieks 2006). Direct punishment of reproductive workers has been shown in some eusocial species (e.g. Monnin and Ratnieks 2001; Monnin et al. 2002; Wenseleers et al. 2004; Ratnieks et al. 2006; Teseo et al. 2013), but not in primitively eusocial Polistes wasps (Liebig et al. 2005). This is probably due to the lack of reliable cues for fertility in this species (Dapporto et al. 2007). Moreover, when the colony is parasitized, the odour differences among colony members tend to decrease (Dapporto et al. 2004) and the parasite is thus likely unable to recognize fertile workers. However, she nevertheless monopolizes most of the colony reproductive potential through dominance behaviour and efficient egg policing (Turillazzi and Cervo 1996). Indeed, the report that parasitized colonies produce only a few host reproductives (Dapporto et al. 2004) suggests that only a few host eggs were laid and escaped parasite control.
Incomplete castration of workers by the social parasite may occur because of incomplete control and/or because of “reproductive concessions” of the parasite to workers. If workers somehow detect that their colony is being parasitized, they may collectively start reproducing so as to regain some fitness. The parasite may be unable to destroy all worker-laid eggs, but she may also concede some fitness to workers in order to prevent them from leaving the nest to attempt starting new colonies (see review of reproductive skew theory by Nonacs and Hager 2011). Incomplete control and reproductive concessions may not necessarily be exclusive, and they may not necessarily occur at the same time in the colony annual cycle. Indeed, given that the parasite brood develops faster than the host brood (Cervo et al. 2004) and that its reproductive cycle is shorter than that of its host (Cervo et al. 2004), the parasite needs worker help mostly in the early season. In contrast, workers gain fitness in the late season, when producing males and future foundresses (Reeve 1991). However, sexual production obviously depends on the workforce available, which becomes greatly reduced by the parasite through a combination of usurpation and oophagy of foundress-laid eggs.
The known triggers of worker reproduction in P. dominula colonies are the lack of dominant foundress (Pardi 1946; Monnin et al. 2009) and/or the presence of empty cells (Liebig et al. 2005). Worker reproduction was therefore not expected given that the parasite did not differ in ovarian development from the dominant foundress and that parasitized colonies did not have more empty cells than control colonies. It is thus evident that the parasite does not completely fool workers, who somehow realize that their colony is being parasitized even though they may not be able to identify the parasite as such. Several factors may allow workers to detect parasitism. First, the chemical mimicry of the ousted dominant foundress by the parasite (Dapporto et al. 2004; Cervo 2006) is not perfect (Turillazzi et al. 2000). Second, the facial visual pattern of the parasite differs conspicuously from the host (Ortolani et al. 2010), in which facial markings are involved in communication (Tibbetts et al. 2010; Ortolani et al. 2010, but see Cervo et al. 2008b; Green and Field 2011; Cini et al. 2011). Third, the brood of the parasite may differ from the brood of the host, as evidences suggest in other social parasite–social host systems (Chernenko et al. 2013). However, eggs of P. sulcifer do not differ chemically from P. dominula eggs (Dani et al. 2004), P. sulcifer larvae are chemically insignificant (Cervo et al. 2008a) and there is no evidence that parasitized workers kill parasite pupae unlike what occurs in Temnothorax/Protomagnathus interactions (Achenbach and Foitzik 2009). Nevertheless, newly emerged P. sulcifer wasps have their own specific chemical profile (Dani et al., unpublished results), and they are typically present in the colony from around the fifth to sixth week after usurpation, which is when parasitized workers showed increased ovarian development and readiness to lay eggs. The possibility that the odour of young adult parasites is the cue to which host workers react is an intriguing hypothesis to be tested.
It is thus possible that workers perceive the presence of a heterospecific parasite several weeks after usurpation, when the parasite has realized most of her fitness and reproductive competition with her sharply decreases, and that they react by developing their ovaries. If the cue of usurpation is the emergence of young parasite wasps, then it would explain why workers do not react similarly when their colony is usurped by an unrelated yet conspecific foundress (Monnin et al. 2009), because cuticular cues may not allow reliable recognition of kinship (Dani et al. 2004).
Several studies have shown co-evolution of sympatric social hosts and social parasites, with local adaptation either favouring the exploitation of the host by the parasite or the defence of the host against its parasite (Foitzik et al. 2001, 2003; Fischer and Foitzik 2004; Ruano et al. 2011; Lorenzi and Thompson 2011; Pamminger et al. 2013). The complex biology of P. sulcifer, with its annual migration between breeding in P. dominula colonies in low lands and hibernating on mountain tops, prevented the use of sympatric populations. We were constrained in using allopatric hosts and parasites, which is artificial. Our results are nevertheless likely to remain valid for sympatric populations, because previous studies showed that local adaptation had a quantitative effect on host–parasite interactions rather than a qualitative effect. Determining whether post-hibernation parasites return to the host population where they emerged or parasitize any neighbouring host population would be useful to better understand this host–parasite system.
Our findings challenge the idea that P. sulcifer is capable of perfectly mimicking the host foundress she overthrows and monopolizing all the resources of the host colony to her own needs. They suggest that the P. dominula–P. sulcifer social host–social parasite system is dynamic, with host workers fine-tuning their reproductive effort, and adds complexity to our understanding of workers’ reproductive decisions. Unlike P. dominula workers, Temnothorax workers do not react to social parasitism by readying themselves to reproduce at any opportunity but rebel by killing parasite pupae (Achenbach and Foitzik 2009). This may be because their parasite cannot defend its pupae given that they are spread out across nest chambers, while in Polistes, they are grouped in a small comb. It may also be that an overt reaction is the only option for enslaved Temnothorax. P. dominula workers may obtain fitness after their parasite has completed its annual cycle, but this is not an option for Temnothorax whose parasite is long lived. It would be interesting however to determine whether enslaved Temnothorax also pursues a more direct route to secure some fitness, i.e. by laying eggs or readying themselves to lay as P. dominula does. This work also underlines the importance of social parasites as key models to unravel the proximate and ultimate factors that regulate worker reproduction in insect societies.
References
Achenbach A, Foitzik S (2009) First evidence for slave rebellion: enslaved ant workers systematically kill the brood of their social parasite. Evolution 63:1068–1075
Achenbach A, Witte V, Foitzik S (2010) Brood exchange experiments and chemical analyses shed light on slave rebellion in ants. Behav Ecol 21:948–956
Alford DV (1975) Bumble bees. Davis-Poynter, London
Bagnères AG, Lorenzi MC, Dusticier G, Turillazzi S, Clément JL (1996) Chemical usurpation of a nest by paper wasp parasites. Science 272:889–892
Baudoin M (1975) Host castration as a parasitic strategy. Evolution 29:335–352
Bourke AFG (2011) Principles of social evolution. Oxford series in ecology and evolution. Oxford University Press, Oxford
Brandt M, Foitzik S, Fischer-Blass B, Heinze J (2005) The coevolutionary dynamics of obligate ant social parasite systems: between prudence and antagonism. Biol Rev 80:1–17
Cervo R (2006) Polistes wasps and their social parasites: an overview. Ann Zool Fenn 43:531–549
Cervo R, Lorenzi C (1996) Inhibition of host queen reproductive capacity by the obligate social parasite Polistes atrimandibularis (Hymenoptera, Vespidae). Ethology 102:1042–1047
Cervo R, Turillazzi S (1996) Host nest preference and nest choice in the cuckoo paper wasp Polistes sulcifer (Hymenoptera, Vespidae). J Insect Behav 9:297–306
Cervo R, Macinai V, Dechigi TS (2004) Fast growth of immature brood in a social parasite wasp: a convergent evolution between avian and insect cuckoos. Am Nat 164:814–820. doi:10.1086/425987
Cervo R, Dani FR, Cotoneschi C, Scala C, Lotti I, Strassmann JE, Queller DC, Turillazzi S (2008a) Why are larvae of the social parasite wasp Polistes sulcifer not removed from the host nest? Behav Ecol Sociobiol 62:319–1331
Cervo R, Dapporto L, Beani L, Strassmann JE, Turillazzi S (2008b) On status badges and quality signals in the paper wasp Polistes dominulus: body size, facial colour patterns and hierarchical rank. Proc R Soc B 275:1189–1196
Chernenko A, Vidal-Garcia M, Helanterä H, Sundström L (2013) Colony take-over and brood survival in temporary social parasites of the ant genus Formica. Behav Ecol Sociobiol 67:727–735
Cini A, Bruschini C, Poggi L, Cervo R (2011) Fight or fool? Physical strength, instead of sensory deception, matters in host nest invasion by a wasp social parasite. Anim Behav 81(6):1139–1145
Cini A, Meconcelli S, Cervo R (2013) Ovarian indexes as indicators of reproductive investment and egg-laying activity in social insects: a comparison among methods. Insect Soc 60:393–402
Dani FR, Giovannotti M, Cervo R, Turillazzi S (2004) Esiste integrazione chimica fra la prole del parassita sociale Polistes sulcifer e quella del suo ospite P. dominulus (Hymenoptera: Vespidae)? In: XIX Congresso Nazionale Italiano di Entomologia, Catania, giugno, pp. 377–380
Dapporto L, Cervo R, Sledge MF, Turillazzi S (2004) Rank integration in dominance hierarchies of host colonies by the paper wasp social parasite Polistes sulcifer (Hymenoptera, Vespidae). J Insect Physiol 50:217–223
Dapporto L, Sledge MF, Turillazzi S (2005) Dynamics of cuticular chemical profiles of Polistes dominulus workers in orphaned nests. J Insect Physiol 51:969–973
Dapporto L, Dani FR, Turillazzi S (2007) Social dominance molds cuticular and egg chemical blends in a paper wasp. Curr Biol 17:504–505. doi:10.1016/j.cub.2007.05.002
Fischer B, Foitzik S (2004) Local coadaptation leading to a geographical mosaic of coevolution in a social parasite system. J Evol Biol 17:1026–1034
Fisher RM (1983) Inability of the social parasite Psithyrus ashtoni to suppress ovarian development in workers of Bombus affinis (Hymenoptera; Apidae). J Kans Entomol Soc 56:69–73
Fisher RM (1984) Dominance by a bumble bee social parasite (Psithyrus citrinus) over workers of its host (Bombus impatiens). Anim Behav 32:304–305
Fittkau EJ, Klinge H (1973) On biomass and trophic structure of the central Amazonian rain forest ecosystem. Biotropica 5:2–14
Foitzik S, DeHeer CJ, Hunjan DN, Herbers JM (2001) Coevolution in host–parasite systems: behavioural strategies of slave-making ants and their hosts. Proc R Soc B 268:1139–1146
Foitzik S, Fischer B, Heinze J (2003) Arms races between social parasites and their hosts: geographic patterns of manipulation and resistance. Behav Ecol 14:80–88
Green JP, Field J (2011) Assessment between species: information gathering in usurpation contests between a paper wasp and its social parasite. Anim Behav 81(6):1263–1269
Greene A, Akre RD, Landolt PJ (1978) Behavior of the yellowjacket social parasite, Dolichovespula arctica (Rohwer) (Hymenoptera: Vespidae). Melanderia 29:1–28
Heinze J (2008) Hierarchy length in orphaned colonies of the ant Temnothorax nylanderi. Naturwissenschaften 95:757–760
Hölldobler B, Wilson EO (1990) The ants. Springer, Berlin
Hurd H (2001) Host fecundity reduction: a strategy for damage limitation? Trends Parasitol 17:363–368
Jeanne RL (1977) Behavior of the obligate social parasite Vespula arctica (Hymenoptera: Vespidae). J Kans Entomol Soc 50:541–557
Kreuter K, Bunk E, Lückemeyer A, Twele R, Francke W, Ayasse M (2012) How the social parasitic bumblebee Bombus bohemicus sneaks into power of reproduction. Behav Ecol Sociobiol 66:475–486
Liebig J, Monnin T, Turillazzi S (2005) Direct assessment of queen quality and lack of worker suppression in a paper wasp. Proc R Soc Lond B 272:1339–1344. doi:10.1098/rspb.2005.3073
Lorenzi MC (2006) The result of an arms race: the chemical strategies of Polistes social parasites. Ann Zool Fenn 43:550–563
Lorenzi MC, Thompson JN (2011) The geographic structure of selection on a coevolving interaction between social parasitic wasps and their hosts hampers social evolution. Evolution 65:3527–3542. doi:10.1111/j.1558-5646.2011.01403.x
Lowe RM, Ward SA, Crozier RH (2002) The evolution of parasites from their hosts: intra- and interspecific parasitism and Emery’s rule. Proc R Soc Lond B 269:1301–1305
Martin SJ, Carruthers JM, Williams PH, Drijfhout FP (2010) Host specific social parasites (Psithyrus) indicate chemical recognition system in bumblebees. J Chem Ecol 36:855–863
Michener CD (1974) The social behavior of the bees: a comparative study. The Belknap Press of Harvard University Press, Cambridge, Massachussetts
Monnin T, Ratnieks FLW (2001) Policing in queenless ponerine ants. Behav Ecol Sociobiol 50:97–108
Monnin T, Ratnieks FLW, Jones GR, Beard R (2002) Pretender punishment induced by chemical signalling in a queenless ant. Nature 419:61–65
Monnin T, Ratnieks FLW, Brandão CRF (2003) Reproductive conflict in animal societies: hierarchy length increases with colony size in queenless ponerine ants. Behav Ecol Sociobiol 54:71–79
Monnin T, Cini A, Lecat V, Fédérici P, Doums C (2009) No actual conflict over colony inheritance despite high potential conflict in the social wasp Polistes dominulus. Proc R Soc B 276:1593–1601. doi:10.1098/rspb.2008.1739
Neumann P, Radloff SE, Moritz RFA, Hepburn HR, Reece SL (2001) Social parasitism by honeybee workers (Apis mellifera capensis Escholtz): host finding and resistance of hybrid host colonies. Behav Ecol 12:419–428
Nonacs P (2000) Measuring and using skew in the study of social behaviour and evolution. Am Nat 156:577–589. doi:10.1086/316995
Nonacs P, Hager R (2011) The past, present and future of reproductive skew theory and experiments. Biol Rev 86:271–298
Ortolani I, Turillazzi S, Cervo R (2008) Spring usurpation restlessness: a wasp social parasite adapts its seasonal activity to the host cycle. Ethology 114:782–788
Ortolani I, Zecchini L, Turillazzi S, Cervo R (2010) Recognition of a paper wasp social parasite by its host: evidence for a visual signal reducing host aggressiveness. Anim Behav 80:683–688. doi:10.1016/j.anbehav.2010.07.003
Pamminger T, Leingärtner A, Achenbach A, Kleeberg I, Pennings PS, Foitzik S (2013) Geographic distribution of the anti-parasite trait “slave rebellion”. Evol Ecol 27:39–49
Pardi L (1946) Ricerche sui Polistini VII. La “dominazione” ed il ciclo ovarico annuale di Polistes gallicus (L.). Boll Ist Entomol Univ Bologna 15:25–84
Queller DC, Zacchi F, Cervo R, Turillazzi S, Henshaw MT, Santorelli LA, Strassman JE (2000) Unrelated helpers in a social insect. Nature 405:784–787. doi:10.1038/35015552
Ratnieks FLW, Foster KR, Wenseleers T (2006) Conflict resolution in insect societies. Ann Rev Entomol 51:581–608
Reeve HK (1991) Polistes. In: Ross K, Matthews R (eds) The social biology of wasps. Cornell University Press, Ithaca, pp 99–148
Reeve HK, Peters JM, Nonacs P, Starks PT (1998) Dispersal of first ‘workers’ in social wasps: causes and implications of an alternative reproductive strategy. Proc Natl Acad Sci U S A 95:13737–13742. doi:10.1073/pnas.95.23.13737
Ruano F, Devers S, Sanllorente O, Errard C, Tinaut A, Lenoir A (2011) A geographical mosaic of coevolution in a slave‐making host–parasite system. J Evolution Biol 24:1071–1079
Savolainen R, Vepsäläinen K (2003) Sympatric speciation through intraspecific social parasitism. Proc Natl Acad Sci U S A 100:7169–7174
Strassmann JE, Fortunato A, Cervo R, Turillazzi S, Damon JM, Queller DC (2004) The cost of queen loss in the social wasp Polistes dominulus (Hymenoptera: Vespidae). J Kansas Entomol Soc 77:343–355
Teseo S, Kronauer DJ, Jaisson P, Châline N (2013) Enforcement of reproductive synchrony via policing in a clonal ant. Current Biol 23:328–332
Tibbetts EA, Mettler A, Levy S (2010) Mutual assessment via visual status signals in Polistes dominulus wasps. Biol Lett 6:10–13. doi:10.1098/rsbl.2009.0420
Turillazzi S, Cervo R (1996) Oofagy and infanticide in colonies of social wasps. In: Parmigiani S, Vom Saal FS (eds) Infanticide and parental care. Harwood Academic, Newark, pp 213–236
Turillazzi S, Cervo R, Cavallari I (1990) Invasion of the nest of Polistes dominulus by the social parasite Sulcopolistes sulcifer (Hymenoptera, Vespidae). Ethology 84:47–59
Turillazzi S, Cervo R, Zanobetti L (1991) Control of host reproduction by social parasite Sulcopolistes sulcifer (Hymenoptera, Vespidae). Act Coll Insect S 7:97–102
Turillazzi S, Sledge MF, Dani FR, Cervo R, Massolo A, Fondelli L (2000) Social hackers: integration in the host chemical recognition system by a paper wasp social parasite. Naturwissenschaften 87:172–176
Vergara CH, Schroder S, Almanza MT, Wittmann D (2003) Suppression of ovarian development of Bombus terrestris workers by B. terrestris queens, Psithyrus vestalis and Psithyrus bohemicus females. Apidologie 34:563–568
Wenseleers T, Ratnieks FLW (2006) Enforced altruism in insect societies. Nature 444:50. doi:10.1038/444050a
Wenseleers T, Helanterä H, Hart A, Ratnieks FLW (2004) Worker reproduction and policing in insect societies: an ESS analysis. J Evol Biol 17:1035–1047
Wilson EO (1971) The insect societies. Harvard University Press, Cambridge, p 548
Wilson EO (1990) Success and dominance in ecosystems: the case of the social insects. Ecology Institute, Oldendorf/Luhe
Zimma BO, Ayasse M, Tengö J, Ibarra F, Franke W (2002) The role of semiochemicals in the reproductive biology of the social parasitic bumblebee Psithyrus norvegicus. In: Proc XIV international congress of IUSSI, Sapporo, Japan, p 136
Acknowledgments
We thank the referees for their helpful comments and Laura Aquiloni, Claudie Doums, Stefano Turillazzi and all members of “Gruppo Vespe” for the logistical support and useful comments on an earlier version of the paper. We are grateful to Carlotta Cini for the language revision and to Adam Cronin for both the useful comments and language revision. Funds were provided by the University of Florence (to RC) and the Fondation Fyssen (to AC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Cremer
Rights and permissions
About this article
Cite this article
Cini, A., Nieri, R., Dapporto, L. et al. Almost royal: incomplete suppression of host worker ovarian development by a social parasite wasp. Behav Ecol Sociobiol 68, 467–475 (2014). https://doi.org/10.1007/s00265-013-1661-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-013-1661-z