Abstract
Endocrine therapy represents a mainstay adjuvant treatment of estrogen receptor-positive (ER+) breast cancer in clinical practice with an overall survival (OS) benefit. However, the emergence of resistance is inevitable over time and is present in one-third of the ER+ breast tumors. Several mechanisms of endocrine resistance in ER+/HER2− advanced breast cancers, through ERα itself, receptor tyrosine signaling, or cell cycle pathway, have been identified to be pivotal in endocrine therapy. The epigenetic alterations also contribute to ensuring tumor cells’ escape from endocrine therapies. The strategy of combined hormone therapy with targeted pharmaceutical compounds has shown an improvement of progression-free survival or OS in clinical practice, including three different classes of drugs: CDK4/6 inhibitors, selective inhibitor of PI3Kα and mTOR inhibitors. Many therapeutic targets of cell cycle pathway and cell signaling and their combination strategies have recently entered clinical trials. This review focuses on Cyclin D–CDK4/6–RB axis, PI3K pathway and HDACs. Additionally, genomic evolution is complex in tumors exposed to hormonal therapy. We highlight the genomic alterations present in ESR1 and PIK3CA genes to elucidate adaptive mechanisms of endocrine resistance, and discuss how these mutations may inform novel combinations to improve clinical outcomes in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ER signaling is a well-established addictive oncogenic pathway in breast cancer cells. Approximately, 70% of breast cancers are classed as ER-positive breast cancers [1,2,3]. Endocrine therapies (ETs) targeting estrogen action have dramatically decreased mortality from breast cancer. Typical ETs that are currently used worldwide include selective estrogen receptor down-regulators (e.g., fulvestrant); selective estrogen receptor modulators (e.g., tamoxifen); and aromatase inhibitors (AIs, e.g., letrozole). However, their efficacy is limited by intrinsic and acquired endocrine resistance [4]. For example, one-third of patients exposed to tamoxifen will eventually relapse with endocrine-resistant advanced tumors within 15 years [1]. After 5 years of scheduled endocrine therapy, the breast cancer recurrences emerged at a stable rate throughout the study period from 5 to 20 years [5]. In a meta-analysis of outcomes of randomized trials of AIs compared with tamoxifen in breast tumors, the 5-year recurrence rate of tamoxifen was 12.6% and that of AIs was 9.6% [6]. Recently, in 692 cases of hormone receptor-positive (HR+) post-endocrine therapy breast cancers, clinical genomic analysis results provided perspicacity in the genomic aberrations present in advanced HR+/HER2− breast cancers [7]. Endocrine-resistant breast tumors were divided into four groups according to the genomic alterations, 18% of the tumors bearing ESR1 alterations, 13% of tumors harboring lesions in the mitogen-activated protein kinase (MAPK) pathway, 9% of the tumors with mutations in the transcriptional factors, and 60% of the endocrine-resistant cases with undiscovered mechanisms [7].
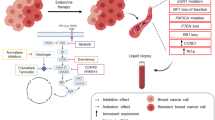
Several well-described mechanisms of ET resistance are focused on the dysregulation of the ER pathway itself. The major form of estrogen receptor in breast cancer is ERα. ERα contains a central DNA-binding domain flanked by two activation domains [8]. The AF-2 domain is located in the ligand-binding domain and its activity is estrogen dependent; moreover, the AF-2 domain interacts with co-activators which promote ERα transcriptional activity [8, 9], whereas AF-1 is activated by phosphorylation of ER [10]. ERα, a transcription factor-dominating gene correlated with cell proliferation, plays key regulatory roles during mammary gland development and in breast carcinogenesis. Blockade of ER signaling function is the mainstay therapeutics for ER+/HER2− breast cancer. But the occurrence of endocrine resistance is inevitable with advanced breast cancer. Given that endocrine therapy can be well tolerated with durations of response extending into years, the clinical benefit rate declines to around 30% for second- or third-line ETs for patients who benefited from the first-line treatment [11]. Therefore, the challenge is to improve our understanding of the mechanisms of ET resistance and to develop therapeutic strategies to extend the duration of effective therapy while minimizing toxicity. In this review, we discuss both the genetic and epigenetic rationale of ET resistance in HR+/HER2− advanced breast cancer, and highlight the striking success of the treatment developed to overcome the specific resistance mechanism.
ESR1 mutations
Dysregulation of various components of the ER signaling associated with endocrine resistance includes dysregulation of ESR1 expression [12, 13], ESR1 mutations, expression of truncated isoforms of ERα [14,15,16], post-translational modification of ERα [17,18,19,20,21,22], abnormality of differential recruitment of ER co-regulators [23, 24] and downstream regulations of ER target genes via receptor tyrosine signaling and other signaling pathways [25,26,27,28]. Much literature exist detailing these mechanisms [29, 30]. The primary mechanism of endocrine resistance in breast cancer is lack of expression of ER, however, two studies showed that less than 10% of the cases appeared to show loss of the ER expression [31, 32], therefore, the ER pathway functions in the majority of cases of ETs resistance. Moreover, several sequencing analysis studies revealed that up to 20% of endocrine-resistant breast cancers harbored ESR1 mutations, while mutations of ESR1 appeared to be rarely happen in primary tumors [33,34,35,36]. The study of genomic landscape of ETs-resistant breast cancer showed that 18% of the tumors harbored mutations of ESR1 [7]. Taken together, the mutation rate in ESR1 in ET resistance breast cancer is 20% approximately. Yates et al. found that the driver coding mutations in ESR1 gene change significantly between primary and metastatic luminal breast cancer [37]. The driver coding mutations in the ESR1 gene confer allele-specific neomorphic properties [33, 38, 39], especially the ER Y537S and D538G mutants have unique transcriptomes and cistromes which drive endocrine resistance and metastases [40]. Spoerke et al. found that multiple different ESR1 mutations coexist in distinct drug-resistant subclonal tumor cells in patients who suffered a failure of endocrine therapy [41]. Moreover, distinct ESR1 mutations differentially affect the efficacy of ER antagonists [42,43,44,45]. Therefore, biopsy and sequencing of advanced ER+ breast cancer may be helpful in some cases, because most ET resistance acquire additional driver mutations not seen in the primary. Razavi et al. identified that ESR1 mutations and lesions in the MAPK pathway, representing different categories of endocrine resistance breast cancer [7]. Although the mutations in the multiple effectors of MAPK signaling or in MYC or other transcription factors were mutually exclusive with hotspot mutations in ESR1 at the level of individual cases in the prospective sequencing cohort, these distinct mutations were observed to coexist in a patient whose multiple tumors were available for analysis [7]. Hence, multiple biologically distinct mechanisms of ET resistance are likely to coexist in subclone cancer cells in individual patients.
Cyclin D–CDK4/6–RB pathway and CDK4/6 inhibitors
Role of cyclin D–CDK4/6–RB axis in G1–S phase transition
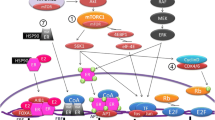
Genetic and biochemical characterization of D-type cyclins and their partner cyclin-dependent kinases (CDK4 and CDK6) have been extensively investigated and revealed how mammalian cells regulate G1–S phase transition in a retinoblastoma protein (RB)-dependent manner [46, 47]. RB, which undergoes periodic phosphorylation during cell division cycle, is a well-known master-regulator of the G1/S-checkpoint. RB is dephosphorylated in the mitosis phase and progressively re-phosphorylated first by cyclin D/CDK4/6 complex and later by cyclin E/CDK2 complex in the G1 phase [48]. Hypophosphorylated RB inhibits the transcriptional activation of E2F target genes as cells rested in the G0 or early G1 phase [49, 50]. RB becomes hyperphosphorylated (inactivated) in the late G1 phase, resulting in the loss of its proliferation-suppressive function and promoting the G1–S phase transition [51]. The enzymatic activities of CDK4/6 in the G1 phase are governed by cyclin D expressed in response to various extracellular signals [52, 53]. Therefore, the cyclin D–CDK4/6–RB axis is downstream of multiple mitogenic cascades, making it a valuable target for drug development [54].
The cyclin D–CDK4/6–RB axis deranged in ER+ breast cancer
The RB tumor suppressor gene is functionally inactivated in approximately 20–30% of breast cancers [55], and loss of RB expression is more commonly observed in triple-negative breast cancer [56, 57]. Therefore, RB is proficient in the majority of HR+ breast cancer [58]. Protein p16INK4, which acts as a brake on the activation of CDK4/6 in RB-proficient cells [59], is found to be inactivated in half of invasive breast cancers [60].While the activated cyclin D–CDK4/6 complex plays a central role in the G1/S phase transition in response to oncogenic pathways and cyclin D1 acts as mitogen sensor to govern G1 phase progression, activating mutations in cyclin D and CDK4/6 rarely existed. Many studies reported that overexpression of cyclin D occurred in over half of all breast cancers with or without cyclin D1 gene amplification [61,62,63,64,65,66,67]. Moreover, amplification of cyclin D1 is especially high in ER+ breast cancers (58% in luminal B subtype and 29% in luminal A subtype, respectively) [68]. Amplification of CDK4 is identified in 25% of luminal B cancers and 14% of luminal A cancers [68]. Additionally, amplification of both cyclin D1 and CDK4 is high in HER2-enriched subtype (38% and 24%, respectively) [68]. While ER and HER2 signaling seems to be drivers in the biology of about 70% and 20% of breast cancers, respectively [23, 69], the two pathways share the same downstream or end points on the cyclin D–CDK4/6–Rb axis. That is, the receptor tyrosine kinases (RTKs) signaling can potentiate cyclin D–CDK4/6–Rb axis in an ER-independent fashion.
CDK4/6 inhibitors’ clinical development in patients with breast cancer
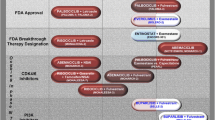
Over the past 4 years, three orally available approved CDK4/6 inhibitors (palbociclib, ribociclib and abemaciclib) have been demonstrated to result in significant clinical benefit when combined with ETs in HR+/HER2− advanced breast cancers in the clinical settings [70,71,72,73,74,75]. Palbociclib was the first CDK4/6 inhibitor approved. The clinical trial NCT01684215 (Phase I/II PALOMA-1 study) evaluated the safety and tolerability of the combination of letrozole plus palbociclib in the first-line treatment of HR+/HER2− advanced breast cancer in postmenopausal women (Table 1). The clinical trial NCT00721409 (Phase II PALOMA-1/TRIO 18 study) revealed an impressive improvement in progression-free survival (PFS) in the palbociclib plus letrozole arm (20.2 versus 10.2 months, p = 0.0004) [72]. The OS for NCT00721409 has not been reported. Consistent with findings from the clinical trial NCT00721409, in the clinical trial NCT01942135 (Phase III PALOMA-3 study), the median PFS showed significant improvement in patients treated for 9.5 months in the palbociclib plus fulvestrant group compared with 4.6 months in the placebo plus fulvestrant group. Moreover, the median OS was 34.9 months (28.8–40.0) in the palbociclib plus fulvestrant arm and 28.0 months (23.6–34.6) in the placebo plus fulvestrant arm (HR = 0.81; p = 0.09) [76]. However, all the three drugs inhibit the proliferation of RB-positive tumor cells to induce cell cycle exit and are inactive in RB-negative tumor cells. Currently, CDK4/6 inhibitors are being increasingly employed in clinical trials combined with signaling pathway inhibitors against epidermal growth factor, phosphoinositide 3 kinase (PI3K), or others that upregulated the expression of cyclin D1 or CDK4/6 (Table 2) [77,78,79,80,81]. These combination therapy strategies designed to increase therapeutic efficiency have been extensively and comprehensively reviewed [74, 82]; moreover, the outcomes of some recent investigations showed that the CDK4/6 inhibitors strengthened the cytostatic effect induced by several signaling pathway inhibitors [83]. Further, CDK4/6 inhibition can also affect the tumor microenvironment. For example, CDK4/6 inhibition triggered antitumor immunity in patient-derived breast cancer cell xenografts model and an MMTV-HER2 mouse [84]. Cdk4/6 inhibitor plus an AI or fulvestrant was listed as the preferred treatment option in HR+/HER2− metastatic breast cancer. Therefore, an applied understanding of the outcomes of CDK4/6 inhibitors and practice patterns may generate a hypothesis for subsequent treatments to deal with the coming challenges. Given palbociclib in combination with hormone therapy led to mPFS of 20.7, 12.8, and 4.0 months when administered in the first-line, second-line and third-line in the real-world palbociclib practice pattern [85, 86], one challenge in the treatment of HR+/HER2− advanced breast cancer is deciding the optimal time to introduce a CDK4/6 inhibitor. Moreover, the cytostatic effects of CDK4/6 inhibitors are limited by primary and acquired resistance. Several studies in preclinical settings have demonstrated primary and acquired resistance to CDK4/6 inhibitors mediated by amplification of CDK6 or CCNE1 or FGFR1 gene, and loss of RB1 or FAT1 gene [87,88,89,90]. In the PALOMA-3 clinical trial, evolution of driver gene mutations (such as RB1 mutations, p = 0.041; PIK3CA mutations, p = 0.00069; and ESR1 Y537S mutation, p = 0.003) was common in patients progressing later on palbociclib combined with fulvestrant treatment [91, 92]. The other challenge is to discover how complex the outcomes would be in patients treated with additional CDK4/6 inhibitor therapy after progression. Recently, the treatment with CDK4/6 inhibitors after disease progression is under active investigation in prospective clinical trials, such as the ongoing PACE trial (NCT03147287), a randomized phase II study comparing the median PFS for fulvestrant alone versus fulvestrant + palbociclib versus fulvestrant + palbociclib + avelumab, and the TRINITI-1 trial (NCT01857193), a single-arm phase II trial assessing the antitumor activity of ribociclib + exemestane + everolimus.
PI3K pathway inhibitors’ clinical development
PI3K pathway is frequently hyperactivated in HR+/HER2− advanced breast cancer and has been implicated in resistance to ETs [93,94,95]. Furthermore, genomic alterations in PIK3CA are common in ER+/HER2− metastatic breast cancer [7, 91, 96,97,98,99]. Thus, the PI3K pathway has emerged as an important therapeutic window for intervention in endocrine-resistant breast cancer. Several PI3K inhibitors combined with various endocrine therapies have been tested in the clinical trial in HR+/HER2− metastatic breast cancer. Pan-Class I PI3K inhibitors (such as buparlisib and pictilisib) have shown modest efficacy in clinical trials [100]. Several clinical trials evaluated the safety and efficacy of buparlisib plus fulvestrant in patients with HR+/HER2− metastatic breast cancer who were pretreated with everolimus plus exemestane (NCT01610284, NCT01633060) [101, 102]. Although the median PFS was significantly improved in the buparlisib versus placebo group (3.9 months vs 1.8 months; HR = 0.67, p = 0.0003), the serious adverse events generated from the off-target effects of the pan-PI3K inhibitors limited the clinical practice of these drug compounds [101, 102]. PI3Kα, which has the most frequent genomic alterations among the class I PI3K isoforms in breast tumors [7, 91, 96,97,98,99], has a prominent role in the PI3K pathway. Selective inhibitors targeting the PI3Kα isoform have been implicated to provide a therapeutic window and to reduce adverse events greatly compared to the Pan-Class I PI3K inhibitors [103]. Alpelisib, an oral selective inhibitor of PI3Kα, was proved to block tumor growth in xenograft models harboring PIK3CA mutations in the preclinical studies [104]. Moreover, alpelisib showed a tolerable clinical safety profile in phase I studies in cohorts of both Western and Japanese patients with PI3KCA-mutated advanced solid cancer (NCT01219699, NCT01387321) [105, 106]. On May 24, 2019, Alpelisib received FDA approval for the treatment of postmenopausal women, and men, with HR+/HER2− , PIK3CA-mutated metastatic breast cancer following progression on or after an endocrine-based regimen. The median OS was 11 months (7.5–14.5) in the alpelisib plus fulvestrant arm and 5.7 months (3.7–7.4) in the placebo plus fulvestrant arm (HR = 0.0.65; p < 0.001) in the cohort of patients with PI3KCA-mutated cancer, and no significant clinical benefit was observed with alpelisib on median PFS in the cohort of patients without a PI3KCA mutation [107, 108].
Histone deacetylases as a therapeutic target in HR+ breast cancer
Preclinical activity of the HDAC inhibitors
In addition to genetic alterations, epigenetic alteration including histone hypoacetylation is a putative mechanism by which tumor cells can develop drug resistance [109,110,111,112]. Aberrant histone deacetylase (HDAC) activity has been demonstrated in breast cancer. In breast cancer core biopsy specimens from 200 patients, HDAC1 expression was associated with estrogen receptor and progesterone receptor expression, and HDAC1 expression predicted significantly better disease-free survival [113]. Muller et al. presented the results of HDACs expression in a large cohort of primary breast cancer cases (n = 238) [114]. HDAC1 was increased in HR+ tumors, while HDAC2 and HDAC3 were strongly expressed in hormone receptor-negative subgroups of tumors with features of a high grade and more aggressiveness [114]. Four ERα corepressors (nuclear corepressor (NCoR), silencing mediator for retinoid or thyroid hormone receptors (SMRT), COUP-TF II and SPEN) have been shown to potentiate endocrine sensitivity in breast cancers [115]. NCoR and SMRT both repress the ERα transcriptional activation depending on HDAC3 activity [116]. COUP-TF II and SPEN attenuate hormonal responses by recruiting HDAC1 to the ERα complex at the genomic sites recognized by ERα [117, 118]. The loss of any of the four corepressors leads to abnormal recruitment of HDACs to ERα-target genes and results in endocrine resistance in breast cancer [115]. These studies have prompted the clinical testing of HDAC inhibitors as anticancer therapeutics in breast cancer [119]. A vast array of both natural and synthetic chemical compounds functioning as HDAC inhibitors were initially discovered based on drug screens for differentiation inducers in leukemias [120, 121]. The HDAC inhibitors have been investigated as therapeutic agents in cancers; for example, romidepsin, vorinostat and belinostat have been approved by the US FDA for treatment of cutaneous or peripheral T cell lymphoma. Panobinostat combined with bortezomib has been approved for the treatment of drug-resistant multiple myeloma. Tucidinostat has been approved in China for relapsed or refractory peripheral T cell lymphoma.
Laboratory research to date supports the investigation of HDAC inhibitors for the treatment of HR+ breast cancer. Several HDAC inhibitors could induce G1 and G2/M cell cycle arrest and subsequent apoptosis or differentiation of both ER-positive and ER-negative breast cancer cell lines [122,123,124,125]. HDAC inhibitors are thought to be able to relieve transcriptional repression in preclinical breast cancer models. Reactivation of silenced ER was observed with vorinostat treatment in preclinical models in hormone receptor-negative tumors [126]. The significance of re-expression of silenced ERα and restoration of sensitivity to endocrine therapy such as AIs were demonstrated in triple-negative breast cancer xenografts following treatment with both HDAC and DNMT inhibitors [127,128,129]. Moreover, entinostat sensitized triple-negative breast cancer xenografts to letrozole [130]. In addition, a significant growth inhibition was also observed in HER2-positive xenograft mouse models following treatment with entinostat plus lapatinib. Mechanistic studies revealed that these effects resulted from downregulation of HER2 and phosphorylated AKT [131, 132]. These experiments provided a strong rationale for combining HADC inhibitors with hormone therapy in advanced HR+ breast cancer clinical trials.
HDAC inhibitors’ clinical development in patients with breast cancer
Several HDAC inhibitors have been evaluated or being evaluated in a number of Phase I/II/III trials in patients with breast cancer. Vorinostat, which targets classes 1 and 2 HDACs, was the first HDAC inhibitor available for investigator-initiated trails. In a Phase II trial of single-agent vorinostat in patients with advanced breast cancer [133], 14 patients received vorinostat at a dose of 200 mg oral twice daily for 14 days of each 21-day cycle. The clinical trial revealed no complete or partial responses, and the study was terminated after the first stage. Although the study did not meet its primary end point, stable disease was observed in almost 30% (4 of 14) of the patients. The therapy was well tolerated with the most common adverse events. Given vorinostat was found to enhance the anti-proliferative actions of tamoxifen on breast cancer cells [134], a Phase II clinical trial (NCT00365599) of vorinostat plus tamoxifen treatment was designed in the hormone therapy-resistant breast cancer setting. 43 women with hormone-resistant breast cancer received oral vorinostat 400 mg daily (21 days of a 28 day cycle) and tamoxifen 20 mg daily [135]. The results showed that the objective response rate was 19% and the clinical benefit rate was 40%. In addition, the combination of these two agents was well tolerated [135]. Entinostat is a synthetic benzamide derivative HDAC inhibitor, which potently inhibits class 1 and class 4 HDAC enzymes. Several clinical trials revealed that oral entinostat was well tolerated in patients with both solid tumors and hematologic malignancies [136,137,138]. ENCORE 301 (NCT00676663) was a Phase II randomized, double-blind, placebo-controlled study of the addition of entinostat to exemestane in patients with HR+ advanced breast cancer with disease progression after prior non-steroidal aromatase inhibitor [139]. The study demonstrated a significant improvement in PFS in the entinostat arm versus placebo (median 4.3 versus 2.3 months, p = 0.055), and an impressive improvement in OS was also observed in the entinostat arm versus placebo (28.1 versus 19.8 months, p = 0.036) [139, 140]. The follow-up randomized Phase III confirmatory study (E2112, NCT02115282) is ongoing [140]. On October 25, 2018, a press release by the Syndax Pharmaceuticals stated that the Phase III breast cancer trial E2112 failed to achieve its statistical hurdle for the co-primary end point of improvement in PFS [141]. However, the final data of the findings from the PFS analysis will not be available until report of the final OS results. Tucidinostat, an oral benzamide class of HDAC inhibitor, selectively inhibits HDAC1, HDAC2, HDAC3 and HDAC10 enzymes. The ACE study (NCT02482753) was a randomized, double-blind, placebo-controlled Phase III clinical trial of tucidinostat plus exemestane [142]. The clinical study revealed a significant improvement in PFS in the tucidinostat arm versus placebo (median 7.4 versus 3.8 months, p = 0.033). The following up for investigation of overall survival is ongoing. Serious adverse events were observed more common in the tucidinostat plus exemestane group (21%, 51 of 244 patients) than in the placebo plus exemestane group (6%, 7 of 121 patients).
Why are the PFS results from E2112 study seemingly divergent to the PFS results from both the ENCORE 301 study and the ACE study? In view of differences among the three clinical trials, the practice pattern, especially the exposure to previous systemic regimens, could be the major factor affecting the clinical outcomes. Patients enrolled in the E2112 study are more likely to have received previous CDK4/6 inhibitors. In China, neither CDK4/6 inhibitors nor everolimus was approved during the enrollment period (July 20, 2015 to June 26, 2017), thus only seven patients in the ACE study had previously received palbociclib and none of the 130 patients in the ENCORE 301 study had taken CDK4/6 inhibitors. The molecular mechanisms governing resistance to CDK4/6 inhibitor combination with endocrine therapy could be distinct from those facilitating resistance to anti-estrogen monotherapy [143]. Therefore, previous exposure to CDK4/6 inhibitors could modulate the therapeutic benefit with subsequent HDAC inhibitor treatment. Similarly, a Phase I/II clinical trial (NCT00258349) was developed to evaluate the response rate after treatment with vorinostat and trastuzumab in patients with HER2-overexpressing metastatic breast cancer with trastuzumab-resistant progressive disease. The results revealed that none of the patients in the primary analysis set responded to combination vorinostat and trastuzumab treatment [144]. Moreover, Kim et al. reported that pretreatment of various tumor cell lines with trichostatin A or vorinostat increased the cytotoxicity of chemotherapy, while administering the HDAC inhibitors after chemotherapy did not achieve the same results [145]. Hence, additional research is needed to determine the optimal treatment sequencing of HDAC inhibitors and the schedule of administration should ideally be modeled preclinically prior to the initiation of clinical trials. Overall, although both entinostat and tucidinostat have not been approved for clinical use by any regulatory agency for the management of HR+ advanced breast cancer, the results of the clinical trials (NCT00676663, NCT02115282 and NCT02482753) represented an important step forward in the development of epigenetic therapy for endocrine-resistant breast cancer.
Conclusions and future directions
Intrinsic and acquired resistance to hormonal therapy results in cancer recurrence and limits clinical benefit on HR+/HER2− advanced breast cancer. Recently, genomic mutations in the ESR1 gene were found in approximately 18% of endocrine-resistant HR+ breast cancers [7, 33,34,35,36]. Importantly, ESR1 mutations differentially affect the efficacy of ER antagonists [42,43,44,45]. Therefore, the ER signaling pathway for tumor progression remains to be elucidated further. While the ESR1 alterations offer beneficial advantageous insights into the genomic evolution of HR+ breast cancers under the selective pressure of drugs, pan-wild-type tumors with unknown mechanisms of ETs accounted for around 60% of patients. Therefore, more research data are required to provide evidence informing optimal sequencing of available therapies for the guidance to develop therapeutic approaches to overcome resistance. Although ESR1 mutations, MAPK alterations and PI3KCA aberrations were mutually exclusive at the level of individual cases in the prospective sequencing cohort according to the taxonomy, they could coexist in the metastatic tumors from one patient. Thus, multiple biologically distinct mechanism of ET resistance probably coexist in distinct tumor subclones in individual patients. Thereby, the effective overcome of ET resistance will be achieved by combination therapies that affect the cell cycle regulation, ER signaling and other compensatory mechanisms and alternative pathways. However, the phenocopy of coexistent mutations in individual cases makes it challenging to develop combination therapies which could uproot all ET resistance clones; hence, cross talk between these signaling pathways is required to be further investigated. Now, the complex mutational genomic landscape and the extensive genomic heterogeneity changes in ETs-resistant breast cancer have been revealed by large-scale genomics analyses [7, 91, 97, 146,147,148]. Novel essential factors contributing to endocrine resistance are being discovered at the preclinical level: for example, the nuclear envelope anchored protein LEM4, transcriptional factor FOXA, and non-coding RNA genes RMRP and NEAT1 [61, 149]. In parallel, genome sequencing efforts of thousands of uncultured tumors have revealed that more than 50% of human cancers harbor mutations in enzymes (HDACs and HATs, TETs and DNMTs, KDMs and KMTs) that are involved in chromatin organization [150,151,152]. The frequent existence of fascinating interplay between the genetic alterations and epigenetic abnormalities promote tumorigenesis and metastasis; for example, PI3K pathway regulates ER-dependent transcription in breast cancer through the epigenetic regulator KMT2D [28]. All these findings would influence clinical practice to personalize therapeutic regimens for individual patients or inform potential approaches to outcome resistance. Moreover, advances in genomic sequencing and other technologies that allow deeper understanding of the genetic alterations and epigenetic abnormalities of individual tumors and further investigation into the cross-talk between these signaling pathways have yielded a superabundance data both in the preclinical and clinical setting. And it is clear that these data require continued systematic mining to reveal many exciting discoveries to personalize therapeutic strategies for each patient with ER positive breast cancer.
Abbreviations
- AIs:
-
Aromatase inhibitors
- CDK4:
-
Cyclin-dependent kinase 4
- CDK6:
-
Cyclin-dependent kinase 6
- DNMT:
-
DNA methyltransferase
- ER:
-
Estrogen receptor
- ETs:
-
Endocrine therapies
- HAT:
-
Histone acetyltransferase
- HDAC:
-
Histone deacetylase
- HR:
-
Hormone receptor-positive
- KDM:
-
Histone demethylase
- MAPK:
-
Mitogen-activated protein kinase
- NCoR:
-
Nuclear corepressor
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- PI3K:
-
Phosphoinositide 3 kinase
- RB:
-
Retinoblastoma protein
- RTKs:
-
Receptor tyrosine kinases
- SMRT:
-
Silencing mediator for retinoid or thyroid hormone receptors
- TET:
-
Ten–eleven translocation
References
Early Breast Cancer Trialists’ Collaborative G (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472):1687–1717. https://doi.org/10.1016/s0140-6736(05)66544-0
Chen WY, Colditz GA (2007) Risk factors and hormone-receptor status: epidemiology, risk-prediction models and treatment implications for breast cancer. Nat Clin Pract Oncol 4(7):415–423. https://doi.org/10.1038/ncponc0851
Bernstein L, Lacey JV Jr (2011) Receptors, associations, and risk factor differences by breast cancer subtypes: positive or negative? J Natl Cancer Inst 103(6):451–453. https://doi.org/10.1093/jnci/djr046
Early Breast Cancer Trialists’ Collaborative G (2015) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 386(10001):1341–1352. https://doi.org/10.1016/s0140-6736(15)61074-1
Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, Peto R, Pritchard KI, Bergh J, Dowsett M, Hayes DF, Ebctcg (2017) 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med 377(19):1836–1846. https://doi.org/10.1056/nejmoa1701830
Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J, Buyse M, Baum M, Buzdar A, Colleoni M, Coombes C, Snowdon C, Gnant M, Jakesz R, Kaufmann M, Boccardo F, Godwin J, Davies C, Peto R (2010) Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol 28(3):509–518. https://doi.org/10.1200/jco.2009.23.1274
Razavi P, Chang MT, Xu G, Bandlamudi C, Ross DS, Vasan N, Cai Y, Bielski CM, Donoghue MTA, Jonsson P, Penson A, Shen R, Pareja F, Kundra R, Middha S, Cheng ML, Zehir A, Kandoth C, Patel R, Huberman K, Smyth LM, Jhaveri K, Modi S, Traina TA, Dang C, Zhang W, Weigelt B, Li BT, Ladanyi M, Hyman DM, Schultz N, Robson ME, Hudis C, Brogi E, Viale A, Norton L, Dickler MN, Berger MF, Iacobuzio-Donahue CA, Chandarlapaty S, Scaltriti M, Reis-Filho JS, Solit DB, Taylor BS, Baselga J (2018) The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell 34(3):427–438. https://doi.org/10.1016/j.ccell.2018.08.008
Jordan VC, O’Malley BW (2007) Selective estrogen-receptor modulators and antihormonal resistance in breast cancer. J Clin Oncol 25(36):5815–5824. https://doi.org/10.1200/jco.2007.11.3886
Perissi V, Rosenfeld MG (2005) Controlling nuclear receptors: the circular logic of cofactor cycles. Nat Rev Mol Cell Biol 6(7):542–554. https://doi.org/10.1038/nrm1682
Lannigan DA (2003) Estrogen receptor phosphorylation. Steroids 68(1):1–9
Ellis MJ, Llombart-Cussac A, Feltl D, Dewar JA, Jasiowka M, Hewson N, Rukazenkov Y, Robertson JF (2015) Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: overall survival analysis from the phase II FIRST Study. J Clin Oncol 33(32):3781–3787. https://doi.org/10.1200/jco.2015.61.5831
Zhang J, Zhou C, Jiang H, Liang L, Shi W, Zhang Q, Sun P, Xiang R, Wang Y, Yang S (2017) ZEB1 induces ER-alpha promoter hypermethylation and confers antiestrogen resistance in breast cancer. Cell Death Dis 8(4):e2732. https://doi.org/10.1038/cddis.2017.154
Clarke R, Liu MC, Bouker KB, Gu Z, Lee RY, Zhu Y, Skaar TC, Gomez B, O’Brien K, Wang Y, Hilakivi-Clarke LA (2003) Antiestrogen resistance in breast cancer and the role of estrogen receptor signaling. Oncogene 22(47):7316–7339. https://doi.org/10.1038/sj.onc.1206937
Hartmaier RJ, Trabucco SE, Priedigkeit N, Chung JH, Parachoniak CA, Vanden Borre P, Morley S, Rosenzweig M, Gay LM, Goldberg ME, Suh J, Ali SM, Ross J, Leyland-Jones B, Young B, Williams C, Park B, Tsai M, Haley B, Peguero J, Callahan RD, Sachelarie I, Cho J, Atkinson JM, Bahreini A, Nagle AM, Puhalla SL, Watters RJ, Erdogan-Yildirim Z, Cao L, Oesterreich S, Mathew A, Lucas PC, Davidson NE, Brufsky AM, Frampton GM, Stephens PJ, Chmielecki J, Lee AV (2018) Recurrent hyperactive ESR1 fusion proteins in endocrine therapy-resistant breast cancer. Ann Oncol 29(4):872–880. https://doi.org/10.1093/annonc/mdy025
Wang Q, Jiang J, Ying G, Xie XQ, Zhang X, Xu W, Zhang X, Song E, Bu H, Ping YF, Yao XH, Wang B, Xu S, Yan ZX, Tai Y, Hu B, Qi X, Wang YX, He ZC, Wang Y, Wang JM, Cui YH, Chen F, Meng K, Wang Z, Bian XW (2018) Tamoxifen enhances stemness and promotes metastasis of ERalpha36(+) breast cancer by upregulating ALDH1A1 in cancer cells. Cell Res 28(3):336–358. https://doi.org/10.1038/cr.2018.15
Shi L, Dong B, Li Z, Lu Y, Ouyang T, Li J, Wang T, Fan Z, Fan T, Lin B, Wang Z, Xie Y (2009) Expression of ER-{alpha}36, a novel variant of estrogen receptor {alpha}, and resistance to tamoxifen treatment in breast cancer. J Clin Oncol 27(21):3423–3429. https://doi.org/10.1200/jco.2008.17.2254
Zheng XQ, Guo JP, Yang H, Kanai M, He LL, Li YY, Koomen JM, Minton S, Gao M, Ren XB, Coppola D, Cheng JQ (2014) Aurora-A is a determinant of tamoxifen sensitivity through phosphorylation of ERalpha in breast cancer. Oncogene 33(42):4985–4996. https://doi.org/10.1038/onc.2013.444
Holz MK (2012) The role of S6K1 in ER-positive breast cancer. Cell Cycle 11(17):3159–3165. https://doi.org/10.4161/cc.21194
Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H (2001) Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem 276(13):9817–9824. https://doi.org/10.1074/jbc.m010840200
Cui Y, Zhang M, Pestell R, Curran EM, Welshons WV, Fuqua SA (2004) Phosphorylation of estrogen receptor alpha blocks its acetylation and regulates estrogen sensitivity. Can Res 64(24):9199–9208. https://doi.org/10.1158/0008-5472.can-04-2126
Yu L, Liang Y, Cao X, Wang X, Gao H, Lin SY, Schiff R, Wang XS, Li K (2017) Identification of MYST3 as a novel epigenetic activator of ERalpha frequently amplified in breast cancer. Oncogene 36(20):2910–2918. https://doi.org/10.1038/onc.2016.433
Jia X, Li C, Li L, Liu X, Zhou L, Zhang W, Ni S, Lu Y, Chen L, Jeong LS, Yu J, Zhang Y, Zhang J, He S, Hu X, Sun H, Yu K, Liu G, Zhao H, Zhang Y, Jia L, Shao ZM (2019) Neddylation inactivation facilitates FOXO3a nuclear export to suppress estrogen receptor transcription and improve fulvestrant sensitivity. Clin Cancer Res. https://doi.org/10.1158/1078-0432.ccr-18-2434
Jin K, Park S, Teo WW, Korangath P, Cho SS, Yoshida T, Gyorffy B, Goswami CP, Nakshatri H, Cruz LA, Zhou W, Ji H, Su Y, Ekram M, Wu Z, Zhu T, Polyak K, Sukumar S (2015) HOXB7 is an ERalpha cofactor in the activation of HER2 and multiple ER target genes leading to endocrine resistance. Cancer Discov 5(9):944–959. https://doi.org/10.1158/2159-8290.cd-15-0090
Patten DK, Corleone G, Gyorffy B, Perone Y, Slaven N, Barozzi I, Erdos E, Saiakhova A, Goddard K, Vingiani A, Shousha S, Pongor LS, Hadjiminas DJ, Schiavon G, Barry P, Palmieri C, Coombes RC, Scacheri P, Pruneri G, Magnani L (2018) Enhancer mapping uncovers phenotypic heterogeneity and evolution in patients with luminal breast cancer. Nat Med 24(9):1469–1480. https://doi.org/10.1038/s41591-018-0091-x
Sherr CJ (1996) Cancer cell cycles. Science 274(5293):1672–1677
Hay N (2005) The Akt-mTOR tango and its relevance to cancer. Cancer Cell 8(3):179–183. https://doi.org/10.1016/j.ccr.2005.08.008
Steelman LS, Martelli AM, Cocco L, Libra M, Nicoletti F, Abrams SL, McCubrey JA (2016) The therapeutic potential of mTOR inhibitors in breast cancer. Br J Clin Pharmacol 82(5):1189–1212. https://doi.org/10.1111/bcp.12958
Toska E, Osmanbeyoglu HU, Castel P, Chan C, Hendrickson RC, Elkabets M, Dickler MN, Scaltriti M, Leslie CS, Armstrong SA, Baselga J (2017) PI3K pathway regulates ER-dependent transcription in breast cancer through the epigenetic regulator KMT2D. Science 355(6331):1324–1330. https://doi.org/10.1126/science.aah6893
Musgrove EA, Sutherland RL (2009) Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer 9(9):631–643. https://doi.org/10.1038/nrc2713
Liu CY, Wu CY, Petrossian K, Huang TT, Tseng LM, Chen S (2017) Treatment for the endocrine resistant breast cancer: current options and future perspectives. J Steroid Biochem Mol Biol 172:166–175. https://doi.org/10.1016/j.jsbmb.2017.07.001
Ellis MJ, Tao Y, Luo J, A’Hern R, Evans DB, Bhatnagar AS, Chaudri Ross HA, von Kameke A, Miller WR, Smith I, Eiermann W, Dowsett M (2008) Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst 100(19):1380–1388. https://doi.org/10.1093/jnci/djn309
Sighoko D, Liu J, Hou N, Gustafson P, Huo D (2014) Discordance in hormone receptor status among primary, metastatic, and second primary breast cancers: biological difference or misclassification? Oncologist 19(6):592–601. https://doi.org/10.1634/theoncologist.2013-0427
Toy W, Shen Y, Won H, Green B, Sakr RA, Will M, Li Z, Gala K, Fanning S, King TA, Hudis C, Chen D, Taran T, Hortobagyi G, Greene G, Berger M, Baselga J, Chandarlapaty S (2013) ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet 45(12):1439–1445. https://doi.org/10.1038/ng.2822
Robinson DR, Wu YM, Vats P, Su F, Lonigro RJ, Cao X, Kalyana-Sundaram S, Wang R, Ning Y, Hodges L, Gursky A, Siddiqui J, Tomlins SA, Roychowdhury S, Pienta KJ, Kim SY, Roberts JS, Rae JM, Van Poznak CH, Hayes DF, Chugh R, Kunju LP, Talpaz M, Schott AF, Chinnaiyan AM (2013) Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet 45(12):1446–1451. https://doi.org/10.1038/ng.2823
Merenbakh-Lamin K, Ben-Baruch N, Yeheskel A, Dvir A, Soussan-Gutman L, Jeselsohn R, Yelensky R, Brown M, Miller VA, Sarid D, Rizel S, Klein B, Rubinek T, Wolf I (2013) D538G mutation in estrogen receptor-alpha: a novel mechanism for acquired endocrine resistance in breast cancer. Can Res 73(23):6856–6864. https://doi.org/10.1158/0008-5472.can-13-1197
Jeselsohn R, Yelensky R, Buchwalter G, Frampton G, Meric-Bernstam F, Gonzalez-Angulo AM, Ferrer-Lozano J, Perez-Fidalgo JA, Cristofanilli M, Gomez H, Arteaga CL, Giltnane J, Balko JM, Cronin MT, Jarosz M, Sun J, Hawryluk M, Lipson D, Otto G, Ross JS, Dvir A, Soussan-Gutman L, Wolf I, Rubinek T, Gilmore L, Schnitt S, Come SE, Pusztai L, Stephens P, Brown M, Miller VA (2014) Emergence of constitutively active estrogen receptor-alpha mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin Cancer Res 20(7):1757–1767. https://doi.org/10.1158/1078-0432.ccr-13-2332
Yates LR, Knappskog S, Wedge D, Farmery JHR, Gonzalez S, Martincorena I, Alexandrov LB, Van Loo P, Haugland HK, Lilleng PK, Gundem G, Gerstung M, Pappaemmanuil E, Gazinska P, Bhosle SG, Jones D, Raine K, Mudie L, Latimer C, Sawyer E, Desmedt C, Sotiriou C, Stratton MR, Sieuwerts AM, Lynch AG, Martens JW, Richardson AL, Tutt A, Lonning PE, Campbell PJ (2017) Genomic evolution of breast cancer metastasis and relapse. Cancer Cell 32(2):169–184. https://doi.org/10.1016/j.ccell.2017.07.005
McDonnell DP, Norris JD, Chang CY (2018) Neomorphic ERalpha mutations drive progression in breast cancer and present a challenge for new drug discovery. Cancer Cell 33(2):153–155. https://doi.org/10.1016/j.ccell.2018.01.014
Harrod A, Fulton J, Nguyen VTM, Periyasamy M, Ramos-Garcia L, Lai CF, Metodieva G, de Giorgio A, Williams RL, Santos DB, Gomez PJ, Lin ML, Metodiev MV, Stebbing J, Castellano L, Magnani L, Coombes RC, Buluwela L, Ali S (2017) Genomic modelling of the ESR1 Y537S mutation for evaluating function and new therapeutic approaches for metastatic breast cancer. Oncogene 36(16):2286–2296. https://doi.org/10.1038/onc.2016.382
Jeselsohn R, Bergholz JS, Pun M, Cornwell M, Liu W, Nardone A, Xiao T, Li W, Qiu X, Buchwalter G, Feiglin A, Abell-Hart K, Fei T, Rao P, Long H, Kwiatkowski N, Zhang T, Gray N, Melchers D, Houtman R, Liu XS, Cohen O, Wagle N, Winer EP, Zhao J, Brown M (2018) Allele-specific chromatin recruitment and therapeutic vulnerabilities of ESR1 activating mutations. Cancer Cell 33(2):173–186. https://doi.org/10.1016/j.ccell.2018.01.004
Spoerke JM, Gendreau S, Walter K, Qiu J, Wilson TR, Savage H, Aimi J, Derynck MK, Chen M, Chan IT, Amler LC, Hampton GM, Johnston S, Krop I, Schmid P, Lackner MR (2016) Heterogeneity and clinical significance of ESR1 mutations in ER-positive metastatic breast cancer patients receiving fulvestrant. Nat Commun 7:11579. https://doi.org/10.1038/ncomms11579
Jeselsohn R, Buchwalter G, De Angelis C, Brown M, Schiff R (2015) ESR1 mutations-a mechanism for acquired endocrine resistance in breast cancer. Nat Rev Clin Oncol 12(10):573–583. https://doi.org/10.1038/nrclinonc.2015.117
Fribbens C, O’Leary B, Kilburn L, Hrebien S, Garcia-Murillas I, Beaney M, Cristofanilli M, Andre F, Loi S, Loibl S, Jiang J, Bartlett CH, Koehler M, Dowsett M, Bliss JM, Johnston SR, Turner NC (2016) Plasma ESR1 mutations and the treatment of estrogen receptor-positive advanced breast cancer. J Clin Oncol 34(25):2961–2968. https://doi.org/10.1200/jco.2016.67.3061
Toy W, Weir H, Razavi P, Lawson M, Goeppert AU, Mazzola AM, Smith A, Wilson J, Morrow C, Wong WL, De Stanchina E, Carlson KE, Martin TS, Uddin S, Li Z, Fanning S, Katzenellenbogen JA, Greene G, Baselga J, Chandarlapaty S (2017) Activating ESR1 mutations differentially affect the efficacy of ER antagonists. Cancer Discov 7(3):277–287. https://doi.org/10.1158/2159-8290.cd-15-1523
Martin LA, Ribas R, Simigdala N, Schuster E, Pancholi S, Tenev T, Gellert P, Buluwela L, Harrod A, Thornhill A, Nikitorowicz-Buniak J, Bhamra A, Turgeon MO, Poulogiannis G, Gao Q, Martins V, Hills M, Garcia-Murillas I, Fribbens C, Patani N, Li Z, Sikora MJ, Turner N, Zwart W, Oesterreich S, Carroll J, Ali S, Dowsett M (2017) Discovery of naturally occurring ESR1 mutations in breast cancer cell lines modelling endocrine resistance. Nat Commun 8(1):1865. https://doi.org/10.1038/s41467-017-01864-y
Malumbres M, Barbacid M (2009) Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 9(3):153–166. https://doi.org/10.1038/nrc2602
Burkhart DL, Sage J (2008) Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat Rev Cancer 8(9):671–682. https://doi.org/10.1038/nrc2399
Sherr CJ (1995) D-type cyclins. Trends Biochem Sci 20(5):187–190
Trimarchi JM, Lees JA (2002) Sibling rivalry in the E2F family. Nat Rev Mol Cell Biol 3(1):11–20. https://doi.org/10.1038/nrm714
Dyson N (1998) The regulation of E2F by pRB-family proteins. Genes Dev 12(15):2245–2262. https://doi.org/10.1101/gad.12.15.2245
Weinberg RA (1995) The retinoblastoma protein and cell cycle control. Cell 81(3):323–330
Zwijsen RM, Wientjens E, Klompmaker R, van der Sman J, Bernards R, Michalides RJ (1997) CDK-independent activation of estrogen receptor by cyclin D1. Cell 88(3):405–415
Sherr CJ, Roberts JM (1999) CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 13(12):1501–1512. https://doi.org/10.1101/gad.13.12.1501
Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, Albassam M, Zheng X, Leopold WR, Pryer NK, Toogood PL (2004) Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther 3(11):1427–1438
Bosco EE, Knudsen ES (2007) RB in breast cancer: at the crossroads of tumorigenesis and treatment. Cell Cycle 6(6):667–671. https://doi.org/10.4161/cc.6.6.3988
Arima Y, Inoue Y, Shibata T, Hayashi H, Nagano O, Saya H, Taya Y (2008) Rb depletion results in deregulation of E-cadherin and induction of cellular phenotypic changes that are characteristic of the epithelial-to-mesenchymal transition. Can Res 68(13):5104–5112. https://doi.org/10.1158/0008-5472.can-07-5680
Trere D, Brighenti E, Donati G, Ceccarelli C, Santini D, Taffurelli M, Montanaro L, Derenzini M (2009) High prevalence of retinoblastoma protein loss in triple-negative breast cancers and its association with a good prognosis in patients treated with adjuvant chemotherapy. Ann Oncol 20(11):1818–1823. https://doi.org/10.1093/annonc/mdp209
Musgrove EA, Sutherland RL (2010) RB in breast cancer: differential effects in estrogen receptor-positive and estrogen receptor-negative disease. Cell Cycle 9(23):4607. https://doi.org/10.4161/cc.9.23.13889
Medema RH, Herrera RE, Lam F, Weinberg RA (1995) Growth suppression by p16ink4 requires functional retinoblastoma protein. Proc Natl Acad Sci USA 92(14):6289–6293. https://doi.org/10.1073/pnas.92.14.6289
Lee JJ, Ko E, Cho J, Park HY, Lee JE, Nam SJ, Kim DH, Cho EY (2012) Methylation and immunoexpression of p16(INK4a) tumor suppressor gene in primary breast cancer tissue and Their quantitative p16(INK4a) hypermethylation in plasma by real-time PCR. Korean J Pathol 46(6):554–561. https://doi.org/10.4132/koreanjpathol.2012.46.6.554
Gao A, Sun T, Ma G, Cao J, Hu Q, Chen L, Wang Y, Wang Q, Sun J, Wu R, Wu Q, Zhou J, Liu L, Hu J, Dong JT, Zhu Z (2018) LEM4 confers tamoxifen resistance to breast cancer cells by activating cyclin D–CDK4/6–Rb and ERalpha pathway. Nat Commun 9(1):4180. https://doi.org/10.1038/s41467-018-06309-8
Kenny FS, Hui R, Musgrove EA, Gee JM, Blamey RW, Nicholson RI, Sutherland RL, Robertson JF (1999) Overexpression of cyclin D1 messenger RNA predicts for poor prognosis in estrogen receptor-positive breast cancer. Clin Cancer Res 5(8):2069–2076
Gillett C, Fantl V, Smith R, Fisher C, Bartek J, Dickson C, Barnes D, Peters G (1994) Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Can Res 54(7):1812–1817
Buckley MF, Sweeney KJ, Hamilton JA, Sini RL, Manning DL, Nicholson RI, deFazio A, Watts CK, Musgrove EA, Sutherland RL (1993) Expression and amplification of cyclin genes in human breast cancer. Oncogene 8(8):2127–2133
Bartkova J, Lukas J, Muller H, Lutzhoft D, Strauss M, Bartek J (1994) Cyclin D1 protein expression and function in human breast cancer. Int J Cancer 57(3):353–361
Dickson C, Fantl V, Gillett C, Brookes S, Bartek J, Smith R, Fisher C, Barnes D, Peters G (1995) Amplification of chromosome band 11q13 and a role for cyclin D1 in human breast cancer. Cancer Lett 90(1):43–50
An HX, Beckmann MW, Reifenberger G, Bender HG, Niederacher D (1999) Gene amplification and overexpression of CDK4 in sporadic breast carcinomas is associated with high tumor cell proliferation. Am J Pathol 154(1):113–118. https://doi.org/10.1016/s0002-9440(10)65257-1
Cancer Genome Atlas N (2012) Comprehensive molecular portraits of human breast tumours. Nature 490(7418):61–70. https://doi.org/10.1038/nature11412
Tinoco G, Warsch S, Gluck S, Avancha K, Montero AJ (2013) Treating breast cancer in the 21st century: emerging biological therapies. J Cancer 4(2):117–132. https://doi.org/10.7150/jca.4925
Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, Ettl J, Patel R, Pinter T, Schmidt M, Shparyk Y, Thummala AR, Voytko NL, Fowst C, Huang X, Kim ST, Randolph S, Slamon DJ (2015) The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 16(1):25–35. https://doi.org/10.1016/s1470-2045(14)71159-3
Gelbert LM, Cai S, Lin X, Sanchez-Martinez C, Del Prado M, Lallena MJ, Torres R, Ajamie RT, Wishart GN, Flack RS, Neubauer BL, Young J, Chan EM, Iversen P, Cronier D, Kreklau E, de Dios A (2014) Preclinical characterization of the CDK4/6 inhibitor LY2835219: in vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs 32(5):825–837. https://doi.org/10.1007/s10637-014-0120-7
Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, Gauthier E, Lu DR, Randolph S, Dieras V, Slamon DJ (2016) Palbociclib and letrozole in advanced breast cancer. N Engl J Med 375(20):1925–1936. https://doi.org/10.1056/nejmoa1607303
Tripathy D, Bardia A, Sellers WR (2017) Ribociclib (LEE011): mechanism of action and clinical impact of this selective cyclin-dependent kinase 4/6 inhibitor in various solid tumors. Clin Cancer Res 23(13):3251–3262. https://doi.org/10.1158/1078-0432.ccr-16-3157
Layman RM (2019) CDK4/6 inhibitors for advanced hormone receptor-positive breast cancer, 2019 and beyond. J Natl Compr Canc Netw 17(2):190–192. https://doi.org/10.6004/jnccn.2018.7271
Eggersmann TK, Degenhardt T, Gluz O, Wuerstlein R, Harbeck N (2019) CDK4/6 inhibitors expand the therapeutic options in breast cancer: palbociclib, Ribociclib and abemaciclib. BioDrugs 33(2):125–135. https://doi.org/10.1007/s40259-019-00337-6
Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, Iwata H, Harbeck N, Loibl S, Andre F, Puyana Theall K, Huang X, Giorgetti C, Huang Bartlett C, Cristofanilli M (2018) Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med 379(20):1926–1936. https://doi.org/10.1056/nejmoa1810527
Vijayaraghavan S, Karakas C, Doostan I, Chen X, Bui T, Yi M, Raghavendra AS, Zhao Y, Bashour SI, Ibrahim NK, Karuturi M, Wang J, Winkler JD, Amaravadi RK, Hunt KK, Tripathy D, Keyomarsi K (2017) CDK4/6 and autophagy inhibitors synergistically induce senescence in Rb positive cytoplasmic cyclin E negative cancers. Nat Commun 8:15916. https://doi.org/10.1038/ncomms15916
Yam C, Hung MC, Hortobagyi GN (2018) CDK4/6 inhibitors in hormone receptor-positive, human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer: are we at the finish line? Oncotarget 9(76):34193–34195. https://doi.org/10.18632/oncotarget.26134
Malumbres M (2019) CDK4/6 inhibitors: what is the best cocktail? Clin Cancer Res 25(1):6–8. https://doi.org/10.1158/1078-0432.ccr-18-2177
Formisano L, Lu Y, Servetto A, Hanker AB, Jansen VM, Bauer JA, Sudhan DR, Guerrero-Zotano AL, Croessmann S, Guo Y, Ericsson PG, Lee KM, Nixon MJ, Schwarz LJ, Sanders ME, Dugger TC, Cruz MR, Behdad A, Cristofanilli M, Bardia A, O’Shaughnessy J, Nagy RJ, Lanman RB, Solovieff N, He W, Miller M, Su F, Shyr Y, Mayer IA, Balko JM, Arteaga CL (2019) Aberrant FGFR signaling mediates resistance to CDK4/6 inhibitors in ER+ breast cancer. Nat Commun 10(1):1373. https://doi.org/10.1038/s41467-019-09068-2
Daniell KM, Bardia A, Sun F, Roberts SA, Brunelle CL, Gillespie TC, Sayegh HE, Naoum GE, Juric D, Isakoff SJ, Fitzgerald DM, Taghian AG (2019) Incidence of peripheral edema in patients receiving PI3K/mTOR/CDK4/6 inhibitors for metastatic breast cancer. Breast Cancer Res Treat 175(3):649–658. https://doi.org/10.1007/s10549-019-05206-y
Sherr CJ, Beach D, Shapiro GI (2016) Targeting CDK4 and CDK6: from discovery to therapy. Cancer Discov 6(4):353–367. https://doi.org/10.1158/2159-8290.cd-15-0894
Klein ME, Kovatcheva M, Davis LE, Tap WD, Koff A (2018) CDK4/6 inhibitors: the mechanism of action may not be as simple as once Thought. Cancer Cell 34(1):9–20. https://doi.org/10.1016/j.ccell.2018.03.023
Goel S, DeCristo MJ, Watt AC, BrinJones H, Sceneay J, Li BB, Khan N, Ubellacker JM, Xie S, Metzger-Filho O, Hoog J, Ellis MJ, Ma CX, Ramm S, Krop IE, Winer EP, Roberts TM, Kim HJ, McAllister SS, Zhao JJ (2017) CDK4/6 inhibition triggers anti-tumour immunity. Nature 548(7668):471–475. https://doi.org/10.1038/nature23465
Kish JK, Ward MA, Garofalo D, Ahmed HV, McRoy L, Laney J, Zanotti G, Braverman J, Yu H, Feinberg BA (2018) Real-world evidence analysis of palbociclib prescribing patterns for patients with advanced/metastatic breast cancer treated in community oncology practice in the USA one year post approval. Breast Cancer Res 20(1):37. https://doi.org/10.1186/s13058-018-0958-2
Xi J, Oza A, Thomas S, Ademuyiwa F, Weilbaecher K, Suresh R, Bose R, Cherian M, Hernandez-Aya L, Frith A, Peterson L, Luo J, Krishnamurthy J, Ma CX (2019) Retrospective analysis of treatment patterns and effectiveness of palbociclib and subsequent regimens in metastatic breast cancer. J Natl Compr Canc Netw 17(2):141–147. https://doi.org/10.6004/jnccn.2018.7094
Herrera-Abreu M, Palafox M, Asghar U (2016) Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Can Res 76:2301–2313. https://doi.org/10.1158/0008-5472.can-15-0728
Yang C, Li Z, Bhatt T (2017) Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene 36:2255–2264. https://doi.org/10.1038/onc.2016.379
Formisano L, Lu Y, Jansen V (2017) Abstract 1008: gain-of-function kinase library screen identifies FGFR1 amplification as a mechanism of resistance to antiestrogens and CDK4/6 inhibitors in ER+ breast cancer [abstract]. Can Res. https://doi.org/10.1158/1538-7445.am2017-1008
Li Z, Razavi P, Li Q, Toy W, Liu B, Ping C, Hsieh W, Sanchez-Vega F, Brown DN, Da Cruz Paula AF, Morris L, Selenica P, Eichenberger E, Shen R, Schultz N, Rosen N, Scaltriti M, Brogi E, Baselga J, Reis-Filho JS, Chandarlapaty S (2018) Loss of the FAT1 tumor suppressor promotes resistance to CDK4/6 inhibitors via the Hippo pathway. Cancer Cell 34(6):893–905. https://doi.org/10.1016/j.ccell.2018.11.006
O’Leary B, Cutts RJ, Liu Y, Hrebien S, Huang X, Fenwick K, Andre F, Loibl S, Loi S, Garcia-Murillas I, Cristofanilli M, Huang Bartlett C, Turner NC (2018) The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov 8(11):1390–1403. https://doi.org/10.1158/2159-8290.cd-18-0264
Condorelli R, Spring L, O’Shaughnessy J (2018) Polyclonal RB1 mutations and acquired resistance to CDK 4/6 inhibitors in patients with metastatic breast cancer. Ann Oncol 29:640–645. https://doi.org/10.1093/annonc/mdx784
Miller TW, Hennessy BT, Gonzalez-Angulo AM, Fox EM, Mills GB, Chen H, Higham C, Garcia-Echeverria C, Shyr Y, Arteaga CL (2010) Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest 120(7):2406–2413. https://doi.org/10.1172/jci41680
Bosch A, Li Z, Bergamaschi A, Ellis H, Toska E, Prat A, Tao JJ, Spratt DE, Viola-Villegas NT, Castel P, Minuesa G, Morse N, Rodon J, Ibrahim Y, Cortes J, Perez-Garcia J, Galvan P, Grueso J, Guzman M, Katzenellenbogen JA, Kharas M, Lewis JS, Dickler M, Serra V, Rosen N, Chandarlapaty S, Scaltriti M, Baselga J (2015) PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Sci Transl Med 7(283):283ra251. https://doi.org/10.1126/scitranslmed.aaa4442
Miller TW, Balko JM, Arteaga CL (2011) Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. J Clin Oncol 29(33):4452–4461. https://doi.org/10.1200/jco.2010.34.4879
Kang S, Bader AG, Vogt PK (2005) Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc Natl Acad Sci USA 102(3):802–807. https://doi.org/10.1073/pnas.0408864102
Banerji S, Cibulskis K, Rangel-Escareno C, Brown KK, Carter SL, Frederick AM, Lawrence MS, Sivachenko AY, Sougnez C, Zou L, Cortes ML, Fernandez-Lopez JC, Peng S, Ardlie KG, Auclair D, Bautista-Pina V, Duke F, Francis J, Jung J, Maffuz-Aziz A, Onofrio RC, Parkin M, Pho NH, Quintanar-Jurado V, Ramos AH, Rebollar-Vega R, Rodriguez-Cuevas S, Romero-Cordoba SL, Schumacher SE, Stransky N, Thompson KM, Uribe-Figueroa L, Baselga J, Beroukhim R, Polyak K, Sgroi DC, Richardson AL, Jimenez-Sanchez G, Lander ES, Gabriel SB, Garraway LA, Golub TR, Melendez-Zajgla J, Toker A, Getz G, Hidalgo-Miranda A, Meyerson M (2012) Sequence analysis of mutations and translocations across breast cancer subtypes. Nature 486(7403):405–409. https://doi.org/10.1038/nature11154
Sabine VS, Crozier C, Brookes CL, Drake C, Piper T, van de Velde CJ, Hasenburg A, Kieback DG, Markopoulos C, Dirix L, Seynaeve C, Rea DW, Bartlett JM (2014) Mutational analysis of PI3K/AKT signaling pathway in tamoxifen exemestane adjuvant multinational pathology study. J Clin Oncol 32(27):2951–2958. https://doi.org/10.1200/jco.2013.53.8272
Mayer IA, Abramson VG, Formisano L, Balko JM, Estrada MV, Sanders ME, Juric D, Solit D, Berger MF, Won HH, Li Y, Cantley LC, Winer E, Arteaga CL (2017) A phase Ib study of alpelisib (BYL719), a PI3Kalpha-specific inhibitor, with letrozole in ER+/HER2− Metastatic breast cancer. Clin Cancer Res 23(1):26–34. https://doi.org/10.1158/1078-0432.ccr-16-0134
Pirali T, Ciraolo E, Aprile S, Massarotti A, Berndt A, Griglio A, Serafini M, Mercalli V, Landoni C, Campa CC, Margaria JP, Silva RL, Grosa G, Sorba G, Williams R, Hirsch E, Tron GC (2017) Identification of a potent phosphoinositide 3-kinase pan inhibitor displaying a strategic carboxylic acid group and development of its prodrugs. ChemMedChem 12(18):1542–1554. https://doi.org/10.1002/cmdc.201700340
Baselga J, Im SA, Iwata H, Cortes J, De Laurentiis M, Jiang Z, Arteaga CL, Jonat W, Clemons M, Ito Y, Awada A, Chia S, Jagiello-Gruszfeld A, Pistilli B, Tseng LM, Hurvitz S, Masuda N, Takahashi M, Vuylsteke P, Hachemi S, Dharan B, Di Tomaso E, Urban P, Massacesi C, Campone M (2017) Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 18(7):904–916. https://doi.org/10.1016/s1470-2045(17)30376-5
Di Leo A, Johnston S, Lee KS, Ciruelos E, Lonning PE, Janni W, O’Regan R, Mouret-Reynier MA, Kalev D, Egle D, Csoszi T, Bordonaro R, Decker T, Tjan-Heijnen VCG, Blau S, Schirone A, Weber D, El-Hashimy M, Dharan B, Sellami D, Bachelot T (2018) Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 19(1):87–100. https://doi.org/10.1016/s1470-2045(17)30688-5
Fruman DA, Rommel C (2014) PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov 13(2):140–156. https://doi.org/10.1038/nrd4204
Fritsch C, Huang A, Chatenay-Rivauday C, Schnell C, Reddy A, Liu M, Kauffmann A, Guthy D, Erdmann D, De Pover A, Furet P, Gao H, Ferretti S, Wang Y, Trappe J, Brachmann SM, Maira SM, Wilson C, Boehm M, Garcia-Echeverria C, Chene P, Wiesmann M, Cozens R, Lehar J, Schlegel R, Caravatti G, Hofmann F, Sellers WR (2014) Characterization of the novel and specific PI3Kalpha inhibitor NVP-BYL719 and development of the patient stratification strategy for clinical trials. Mol Cancer Ther 13(5):1117–1129. https://doi.org/10.1158/1535-7163.mct-13-0865
Juric D, Rodon J, Tabernero J, Janku F, Burris HA, Schellens JHM, Middleton MR, Berlin J, Schuler M, Gil-Martin M, Rugo HS, Seggewiss-Bernhardt R, Huang A, Bootle D, Demanse D, Blumenstein L, Coughlin C, Quadt C, Baselga J (2018) Phosphatidylinositol 3-kinase alpha-selective inhibition with alpelisib (BYL719) in PIK3CA-altered solid tumors: results from the first-in-human study. J Clin Oncol 36(13):1291–1299. https://doi.org/10.1200/jco.2017.72.7107
Ando Y, Iwasa S, Takahashi S, Saka H, Kakizume T, Natsume K, Suenaga N, Quadt C, Yamada Y (2019) Phase I study of alpelisib (BYL719), an alpha-specific PI3K inhibitor, in Japanese patients with advanced solid tumors. Cancer Sci 110(3):1021–1031. https://doi.org/10.1111/cas.13923
Juric D, Janku F, Rodon J, Burris HA, Mayer IA, Schuler M, Seggewiss-Bernhardt R, Gil-Martin M, Middleton MR, Baselga J, Bootle D, Demanse D, Blumenstein L, Schumacher K, Huang A, Quadt C, Rugo HS (2018) Alpelisib plus fulvestrant in PIK3CA-altered and PIK3CA-wild-type estrogen receptor-positive advanced breast cancer: a phase 1b clinical trial. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2018.4475
Andre F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, Iwata H, Conte P, Mayer IA, Kaufman B, Yamashita T, Lu YS, Inoue K, Takahashi M, Papai Z, Longin AS, Mills D, Wilke C, Hirawat S, Juric D, Group S-S, the S-SG (2019) Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med 380(20):1929–1940. https://doi.org/10.1056/nejmoa1813904
Guerriero JL, Sotayo A, Ponichtera HE, Castrillon JA, Pourzia AL, Schad S, Johnson SF, Carrasco RD, Lazo S, Bronson RT, Davis SP, Lobera M, Nolan MA, Letai A (2017) Class IIa HDAC inhibition reduces breast tumours and metastases through anti-tumour macrophages. Nature 543(7645):428–432. https://doi.org/10.1038/nature21409
Jones PA, Issa JP, Baylin S (2016) Targeting the cancer epigenome for therapy. Nat Rev Genet 17(10):630–641. https://doi.org/10.1038/nrg.2016.93
Haven B, Heilig E, Donham C, Settles M, Vasilevsky N, Owen K, Reproducibility Project: Cancer B, Reproducibility Project Cancer B (2016) Registered report: a chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Elife 5:5. https://doi.org/10.7554/elife.09462
Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, McDermott U, Azizian N, Zou L, Fischbach MA, Wong KK, Brandstetter K, Wittner B, Ramaswamy S, Classon M, Settleman J (2010) A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell 141(1):69–80. https://doi.org/10.1016/j.cell.2010.02.027
Krusche CA, Wulfing P, Kersting C, Vloet A, Bocker W, Kiesel L, Beier HM, Alfer J (2005) Histone deacetylase-1 and -3 protein expression in human breast cancer: a tissue microarray analysis. Breast Cancer Res Treat 90(1):15–23. https://doi.org/10.1007/s10549-004-1668-2
Muller BM, Jana L, Kasajima A, Lehmann A, Prinzler J, Budczies J, Winzer KJ, Dietel M, Weichert W, Denkert C (2013) Differential expression of histone deacetylases HDAC1, 2 and 3 in human breast cancer–overexpression of HDAC2 and HDAC3 is associated with clinicopathological indicators of disease progression. BMC Cancer 13:215. https://doi.org/10.1186/1471-2407-13-215
Legare S, Basik M (2016) Minireview: the link between eralpha corepressors and histone deacetylases in tamoxifen resistance in breast cancer. Mol Endocrinol 30(9):965–976. https://doi.org/10.1210/me.2016-1072
Li J, Wang J, Wang J, Nawaz Z, Liu JM, Qin J, Wong J (2000) Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3. EMBO J 19(16):4342–4350. https://doi.org/10.1093/emboj/19.16.4342
Smirnov DA, Hou S, Ricciardi RP (2000) Association of histone deacetylase with COUP-TF in tumorigenic Ad12-transformed cells and its potential role in shut-off of MHC class I transcription. Virology 268(2):319–328. https://doi.org/10.1006/viro.1999.0181
Shi Y, Downes M, Xie W, Kao HY, Ordentlich P, Tsai CC, Hon M, Evans RM (2001) Sharp, an inducible cofactor that integrates nuclear receptor repression and activation. Genes Dev 15(9):1140–1151. https://doi.org/10.1101/gad.871201
Connolly R, Stearns V (2012) Epigenetics as a therapeutic target in breast cancer. J Mammary Gland Biol Neoplasia 17(3–4):191–204. https://doi.org/10.1007/s10911-012-9263-3
Richon VM, Emiliani S, Verdin E, Webb Y, Breslow R, Rifkind RA, Marks PA (1998) A class of hybrid polar inducers of transformed cell differentiation inhibits histone deacetylases. Proc Natl Acad Sci USA 95(6):3003–3007. https://doi.org/10.1073/pnas.95.6.3003
Falkenberg KJ, Johnstone RW (2014) Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat Rev Drug Discov 13(9):673–691. https://doi.org/10.1038/nrd4360
Said TK, Moraes RC, Sinha R, Medina D (2001) Mechanisms of suberoylanilide hydroxamic acid inhibition of mammary cell growth. Breast Cancer Res 3(2):122–133. https://doi.org/10.1186/bcr284
Munster PN, Troso-Sandoval T, Rosen N, Rifkind R, Marks PA, Richon VM (2001) The histone deacetylase inhibitor suberoylanilide hydroxamic acid induces differentiation of human breast cancer cells. Cancer Res 61(23):8492–8497
Torres-Adorno AM, Lee J, Kogawa T, Ordentlich P, Tripathy D, Lim B, Ueno NT (2017) Histone deacetylase inhibitor enhances the efficacy of MEK inhibitor through NOXA-mediated MCL1 degradation in triple-negative and inflammatory breast cancer. Clin Cancer Res 23(16):4780–4792. https://doi.org/10.1158/1078-0432.ccr-16-2622
Ono H, Sowa Y, Horinaka M, Iizumi Y, Watanabe M, Morita M, Nishimoto E, Taguchi T, Sakai T (2018) The histone deacetylase inhibitor OBP-801 and eribulin synergistically inhibit the growth of triple-negative breast cancer cells with the suppression of survivin, Bcl-xL, and the MAPK pathway. Breast Cancer Res Treat 171(1):43–52. https://doi.org/10.1007/s10549-018-4815-x
Zhou Q, Shaw PG, Davidson NE (2009) Inhibition of histone deacetylase suppresses EGF signaling pathways by destabilizing EGFR mRNA in ER-negative human breast cancer cells. Breast Cancer Res Treat 117(2):443–451. https://doi.org/10.1007/s10549-008-0148-5
Sharma D, Saxena NK, Davidson NE, Vertino PM (2006) Restoration of tamoxifen sensitivity in estrogen receptor-negative breast cancer cells: tamoxifen-bound reactivated ER recruits distinctive corepressor complexes. Cancer Res 66(12):6370–6378. https://doi.org/10.1158/0008-5472.can-06-0402
Yang X, Phillips DL, Ferguson AT, Nelson WG, Herman JG, Davidson NE (2001) Synergistic activation of functional estrogen receptor (ER)-alpha by DNA methyltransferase and histone deacetylase inhibition in human ER-alpha-negative breast cancer cells. Cancer Res 61(19):7025–7029
Fan J, Yin WJ, Lu JS, Wang L, Wu J, Wu FY, Di GH, Shen ZZ, Shao ZM (2008) ER alpha negative breast cancer cells restore response to endocrine therapy by combination treatment with both HDAC inhibitor and DNMT inhibitor. J Cancer Res Clin Oncol 134(8):883–890. https://doi.org/10.1007/s00432-008-0354-x
Sabnis GJ, Goloubeva O, Chumsri S, Nguyen N, Sukumar S, Brodie AM (2011) Functional activation of the estrogen receptor-alpha and aromatase by the HDAC inhibitor entinostat sensitizes ER-negative tumors to letrozole. Cancer Res 71(5):1893–1903. https://doi.org/10.1158/0008-5472.can-10-2458
Huang X, Wang S, Lee CK, Yang X, Liu B (2011) HDAC inhibitor SNDX-275 enhances efficacy of trastuzumab in erbB2-overexpressing breast cancer cells and exhibits potential to overcome trastuzumab resistance. Cancer Lett 307(1):72–79. https://doi.org/10.1016/j.canlet.2011.03.019
Lee J, Bartholomeusz C, Mansour O, Humphries J, Hortobagyi GN, Ordentlich P, Ueno NT (2014) A class I histone deacetylase inhibitor, entinostat, enhances lapatinib efficacy in HER2-overexpressing breast cancer cells through FOXO3-mediated Bim1 expression. Breast Cancer Res Treat 146(2):259–272. https://doi.org/10.1007/s10549-014-3014-7
Luu TH, Morgan RJ, Leong L, Lim D, McNamara M, Portnow J, Frankel P, Smith DD, Doroshow JH, Wong C, Aparicio A, Gandara DR, Somlo G (2008) A phase II trial of vorinostat (suberoylanilide hydroxamic acid) in metastatic breast cancer: a California Cancer Consortium study. Clin Cancer Res 14(21):7138–7142. https://doi.org/10.1158/1078-0432.ccr-08-0122
Hodges-Gallagher L, Valentine CD, Bader SE, Kushner PJ (2007) Inhibition of histone deacetylase enhances the anti-proliferative action of antiestrogens on breast cancer cells and blocks tamoxifen-induced proliferation of uterine cells. Breast Cancer Res Treat 105(3):297–309. https://doi.org/10.1007/s10549-006-9459-6
Munster PN, Thurn KT, Thomas S, Raha P, Lacevic M, Miller A, Melisko M, Ismail-Khan R, Rugo H, Moasser M, Minton SE (2011) A phase II study of the histone deacetylase inhibitor vorinostat combined with tamoxifen for the treatment of patients with hormone therapy-resistant breast cancer. Br J Cancer 104(12):1828–1835. https://doi.org/10.1038/bjc.2011.156
Ryan QC, Headlee D, Acharya M, Sparreboom A, Trepel JB, Ye J, Figg WD, Hwang K, Chung EJ, Murgo A, Melillo G, Elsayed Y, Monga M, Kalnitskiy M, Zwiebel J, Sausville EA (2005) Phase I and pharmacokinetic study of MS-275, a histone deacetylase inhibitor, in patients with advanced and refractory solid tumors or lymphoma. J Clin Oncol 23(17):3912–3922. https://doi.org/10.1200/jco.2005.02.188
Kummar S, Gutierrez M, Gardner ER, Donovan E, Hwang K, Chung EJ, Lee MJ, Maynard K, Kalnitskiy M, Chen A, Melillo G, Ryan QC, Conley B, Figg WD, Trepel JB, Zwiebel J, Doroshow JH, Murgo AJ (2007) Phase I trial of MS-275, a histone deacetylase inhibitor, administered weekly in refractory solid tumors and lymphoid malignancies. Clin Cancer Res 13(18 Pt 1):5411–5417. https://doi.org/10.1158/1078-0432.ccr-07-0791
Gore L, Rothenberg ML, O’Bryant CL, Schultz MK, Sandler AB, Coffin D, McCoy C, Schott A, Scholz C, Eckhardt SG (2008) A phase I and pharmacokinetic study of the oral histone deacetylase inhibitor, MS-275, in patients with refractory solid tumors and lymphomas. Clin Cancer Res 14(14):4517–4525. https://doi.org/10.1158/1078-0432.ccr-07-1461
Yardley DA, Ismail-Khan RR, Melichar B, Lichinitser M, Munster PN, Klein PM, Cruickshank S, Miller KD, Lee MJ, Trepel JB (2013) Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J Clin Oncol 31(17):2128–2135. https://doi.org/10.1200/jco.2012.43.7251
Yeruva SLH, Zhao F, Miller KD, Tevaarwerk AJ, Wagner LI, Gray RJ, Sparano JA, Connolly RM (2018) E2112: randomized phase iii trial of endocrine therapy plus entinostat/placebo in patients with hormone receptor-positive advanced breast cancer. NPJ Breast Cancer 4:1. https://doi.org/10.1038/s41523-017-0053-3
Wander SA, Spring LM, Bardia A (2019) Genetics to epigenetics: targeting histone deacetylases in hormone receptor-positive metastatic breast cancer. Lancet Oncol 20(6):746–748. https://doi.org/10.1016/s1470-2045(19)30279-7
Jiang Z, Li W, Hu X, Zhang Q, Sun T, Cui S, Wang S, Ouyang Q, Yin Y, Geng C, Tong Z, Cheng Y, Pan Y, Sun Y, Wang H, Ouyang T, Gu K, Feng J, Wang X, Wang S, Liu T, Gao J, Cristofanilli M, Ning Z, Lu X (2019) Tucidinostat plus exemestane for postmenopausal patients with advanced, hormone receptor-positive breast cancer (ACE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 20(6):806–815. https://doi.org/10.1016/s1470-2045(19)30164-0
Spring LM, Wander SA, Zangardi M, Bardia A (2019) CDK 4/6 inhibitors in breast cancer: current controversies and future directions. Curr Oncol Rep 21(3):25. https://doi.org/10.1007/s11912-019-0769-3
Goldstein LJ, Zhao F, Wang M, Swaby RF, Sparano JA, Meropol NJ, Bhalla KN, Pellegrino CM, Katherine Alpaugh R, Falkson CI, Klein P, Sledge GW (2017) A phase I/II study of suberoylanilide hydroxamic acid (SAHA) in combination with trastuzumab (Herceptin) in patients with advanced metastatic and/or local chest wall recurrent HER2-amplified breast cancer: a trial of the ECOG-ACRIN Cancer Research Group (E1104). Breast Cancer Res Treat 165(2):375–382. https://doi.org/10.1007/s10549-017-4310-9
Kim MS, Blake M, Baek JH, Kohlhagen G, Pommier Y, Carrier F (2003) Inhibition of histone deacetylase increases cytotoxicity to anticancer drugs targeting DNA. Cancer Res 63(21):7291–7300
Ciriello G, Gatza ML, Beck AH, Wilkerson MD, Rhie SK, Pastore A, Zhang H, McLellan M, Yau C, Kandoth C, Bowlby R, Shen H, Hayat S, Fieldhouse R, Lester SC, Tse GM, Factor RE, Collins LC, Allison KH, Chen YY, Jensen K, Johnson NB, Oesterreich S, Mills GB, Cherniack AD, Robertson G, Benz C, Sander C, Laird PW, Hoadley KA, King TA, Network TR, Perou CM (2015) Comprehensive molecular portraits of invasive lobular breast cancer. Cell 163(2):506–519. https://doi.org/10.1016/j.cell.2015.09.033
Gellert P, Segal CV, Gao Q, Lopez-Knowles E, Martin LA, Dodson A, Li T, Miller CA, Lu C, Mardis ER, Gillman A, Morden J, Graf M, Sidhu K, Evans A, Shere M, Holcombe C, McIntosh SA, Bundred N, Skene A, Maxwell W, Robertson J, Bliss JM, Smith I, Dowsett M, Group PTM, Trialists (2016) Impact of mutational profiles on response of primary oestrogen receptor-positive breast cancers to oestrogen deprivation. Nat Commun 7:13294. https://doi.org/10.1038/ncomms13294
Nik-Zainal S, Davies H, Staaf J, Ramakrishna M, Glodzik D, Zou X, Martincorena I, Alexandrov LB, Martin S, Wedge DC, Van Loo P, Ju YS, Smid M, Brinkman AB, Morganella S, Aure MR, Lingjaerde OC, Langerod A, Ringner M, Ahn SM, Boyault S, Brock JE, Broeks A, Butler A, Desmedt C, Dirix L, Dronov S, Fatima A, Foekens JA, Gerstung M, Hooijer GK, Jang SJ, Jones DR, Kim HY, King TA, Krishnamurthy S, Lee HJ, Lee JY, Li Y, McLaren S, Menzies A, Mustonen V, O’Meara S, Pauporte I, Pivot X, Purdie CA, Raine K, Ramakrishnan K, Rodriguez-Gonzalez FG, Romieu G, Sieuwerts AM, Simpson PT, Shepherd R, Stebbings L, Stefansson OA, Teague J, Tommasi S, Treilleux I, Van den Eynden GG, Vermeulen P, Vincent-Salomon A, Yates L, Caldas C, van’t Veer L, Tutt A, Knappskog S, Tan BK, Jonkers J, Borg A, Ueno NT, Sotiriou C, Viari A, Futreal PA, Campbell PJ, Span PN, Van Laere S, Lakhani SR, Eyfjord JE, Thompson AM, Birney E, Stunnenberg HG, van de Vijver MJ, Martens JW, Borresen-Dale AL, Richardson AL, Kong G, Thomas G, Stratton MR (2016) Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature 534(7605):47–54. https://doi.org/10.1038/nature17676
Rheinbay E, Parasuraman P, Grimsby J, Tiao G, Engreitz JM, Kim J, Lawrence MS, Taylor-Weiner A, Rodriguez-Cuevas S, Rosenberg M, Hess J, Stewart C, Maruvka YE, Stojanov P, Cortes ML, Seepo S, Cibulskis C, Tracy A, Pugh TJ, Lee J, Zheng Z, Ellisen LW, Iafrate AJ, Boehm JS, Gabriel SB, Meyerson M, Golub TR, Baselga J, Hidalgo-Miranda A, Shioda T, Bernards A, Lander ES, Getz G (2017) Recurrent and functional regulatory mutations in breast cancer. Nature 547(7661):55–60. https://doi.org/10.1038/nature22992
(2017) A convergence of genetics and epigenetics in cancer. Cell 168(4):561–563. https://doi.org/10.1016/j.cell.2017.01.035
Rizzolo P, Silvestri V, Tommasi S, Pinto R, Danza K, Falchetti M, Gulino M, Frati P, Ottini L (2013) Male breast cancer: genetics, epigenetics, and ethical aspects. Ann Oncol 24(Suppl 8):viii75–viii82. https://doi.org/10.1093/annonc/mdt316
You JS, Jones PA (2012) Cancer genetics and epigenetics: two sides of the same coin? Cancer Cell 22(1):9–20. https://doi.org/10.1016/j.ccr.2012.06.008
Acknowledgements
The work of the authors was supported by the National Natural Science Foundation of China (Grant No. 91649107), the Natural Science Foundation of Tianjin City of China (Grant No. 17JCYBJC24100).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, J., Wang, Q., Wang, Q. et al. Mechanisms of resistance to estrogen receptor modulators in ER+/HER2− advanced breast cancer. Cell. Mol. Life Sci. 77, 559–572 (2020). https://doi.org/10.1007/s00018-019-03281-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03281-4