Abstract
Objective
Asthma, a well-known disease with high morbidity, is characterized by chronic airway inflammation. However, the allergic inflammation mechanisms of follistatin-like protein 1 (FSTL1) have not been elucidated. This study aims to investigate the effects of FSTL1 in ovalbumin (OVA)-induced mice and macrophages on nucleotide-binding domain and leucine-rich repeat protein 3 (NLRP3)/interleukin-1β (IL-1β) signaling pathway.
Methods
Mice were randomly divided into control-WT, OVA-WT, control-Fstl1±, OVA-Fstl1±. Histological changes were assessed by HE and PAS staining. The protein levels of Muc-5AC, FSTL1, NLRP3, and IL-1β in lung tissue were detected by immunohistochemistry and ELISA. The bronchoalveolar lavage fluid (BALF) in mice and human serum samples were detected by ELISA. Then, mice were grouped into control, FSTL1, MCC950 + FSTL1 to further investigate the relationship between FSTL1 and NLRP3/IL-1β. Alveolar macrophage cells (MH-S cells) were separated into control, OVA, FSTL1, OVA + FSTL1, OVA + siNC, OVA + siFSTL1, MCC950, and FSTL1 + MCC950 groups to explore the effect of FSTL1 on the NLRP3/IL-1β signaling. The protein expression of NLRP3 and IL-1β in MH-S cells was detected by Western blot analysis.
Results
The present results uncovered that Fstl1± significantly ameliorated OVA-induced Muc-5AC production and mucus hypersecretion. Fstl1± was also found to decrease the production of inflammatory cytokines and inflammatory cell infiltration in OVA-induced asthmatic mice. Meanwhile, the serum concentrations of FSTL1 and IL-1β were higher in asthma subjects than the health subjects, and Fstl1± ameliorated the production of NLRP3 and IL-1β in OVA-induced asthmatic mice. Furthermore, mice by injected FSTL1 substantially stimulated the expression of NLRP3 and IL-1β, while pretreatment with MCC950 in mice significantly weakened the production of NLRP3 and IL-1β induced by injection FSTL1. Pretreatment with siFSTL1 or MCC950 significantly reduced the production of NLRP3 and IL-1β induced by OVA or FSTL1 in MH-S cells.
Conclusions
The study results showed that FSTL1 played an important role in allergic airway inflammation by activating NLRP3/IL-1β. Hence, inhibition FSTL1 could be applied as a therapeutic agent against asthma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Asthma, a common respiratory disease affecting 1%–18% of the population in different countries, is characterized by airway inflammatory infiltration and airway hyper-responsiveness [1, 2]. Although current therapeutic strategies such as beta agonists and corticosteroids are commonly used, the morbidity and mortality rates among patients remain high. Therefore, novel therapeutic targets and strategies are being developed.
Follistatin-like protein 1 (FSTL1), also referred as FRP or TSC-36, is a TGF-β1-inducible secreted glycoprotein belonging to the SPARC family and is widely generated in non-hematopoietic cells especially in the mesenchymal lineage [3]. FSTL1 is a cardioprotective factor [4], pro-fibrotic agent [5], and a bi-directional regulator of tumor based upon the origin of the tumorigenic cell line [6]. Asthmatic patients express high levels of FSTL1 in plasma and bronchoalveolar lavage fluid (BALF) compared with health subjects [7]. Thus, FSTL1 participates in the pathologic process of asthmatic airway inflammation.
The NLRP3 inflammasome complex, composed by NLRP3, adaptor molecule apoptosis-associated speck-like protein containing a caspase recruitment domain, and pro-caspase 1, is a multiprotein oligomer that plays a key role in innate immunity [7]. After the pathogen-associated molecular patterns or damage-associated molecular patterns are recognized, inflammasome components assemble, self-oligomerize, then cleave precursor pro-IL-1β into its mature bioactive form IL-1β [8]. IL-1β participates in airway inflammatory infiltration, differentiating and activating Th2 cells that lead to Th2 cytokines release [9], recruitment of dendritic cell into lymph nodes [10], promotion of Th17 inflammatory responses correlating with the severity of asthma [11], and initiation of the airway smooth muscle hyper-reactivity [12].
MCC950, a small molecule and selective cytokine release inhibitor, can specifically block NLRP3 activation and reduce IL-1β production [13]. Hence, MCC950 is a potential therapeutic agent for NLRP3-associated syndromes and may alleviate IL-1β-induced airway inflammation. In this study, we conducted an in-depth analysis of the effects and mechanisms of FSTL1 on NLRP3/IL-1β secretion in mice and macrophages. Overall, the results of this study may provide a pharmacological basis for asthma treatment.
Materials and methods
Subjects
Asthma subjects were diagnosed with asthma according to the Global Initiative for Asthma (2019 edition), and age-matched health subjects were collected at Qilu Hospital of Shandong University (Jinan, China). The characteristics of asthma and health subjects are shown in Table 1. The study was approved by the Ethics Review Committee for Human Studies at Qilu Hospital of Shandong University (Grant NO. KYLL-2017[ks]-112).
Cell culture and treatment
MH-S cells were purchased from Cell Line Bank (Shanghai, China). MH-S cells were cultured in complete culture medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 0.1 mg/ml streptomycin in an incubator with a humidified atmosphere of 95% air and 5% CO2 at 37 °C. MH-S cells were seeded in six-well culture plates at a density of 1 × 105 cells per well, and then used for all assays.
MH-S cells were stimulated with different doses of FSTL1 for 24 h to choose the best stimulus time and concentration. MH-S cells were then divided into control, OVA, FSTL1, OVA + FSTL1, OVA + siNC, OVA + siFSTL1, MCC950, FSTL1 + MCC950 groups. MH-S cells in the control group were treated in DMEM alone. MH-S cells in the OVA group were treated in DMEM with OVA (40 μM) for stimulation 8 h. MH-S cells in the FSTL1 group were treated in DMEM with FSTL1 (100 ng/mL) for stimulation 8 h. MH-S cells in the OVA + FSTL1 group were treated in DMEM with FSTL1 (100 ng/mL) and OVA (40 µM) for stimulation 8 h. MH-S cells in the OVA + siNC group were transfected with siNC for 48 h and then in DMEM with OVA (40 μM) for stimulation 8 h. MH-S cells in the OVA + siFSTL1 group were transfected with siFSTL1 (FSTL1 small interfering RNA) using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol for 48 h and then in DMEM with OVA (40 μM) for stimulation 8 h. MH-S cells in the MCC950 group were treated in DMEM with MCC950 (50 μM). MH-S cells in the FSTL1 + MCC950 group were pretreated with MCC950 (50 μM) for 24 h and then in DMEM with FSTL1 (100 ng/mL) for stimulation 8 h. Finally, cell supernatant was used for detection of NLRP3 and IL-1β.
Model and grouping
Fstl1± mice were a generous gift from Xiang Gao (Nanjing University, Nanjing, China) and Xu Zhang (Institute of Neuroscience, Shanghai Institute for Biological Sciences, Chinese Academy of Sciences, Shanghai, China). Fstl1± mice were generated as described previously [14]. The wild type (WT) female C57BL/6 mice, 6–8 weeks, were attained from the Animal Experimental Center of Jinan. All protocols were approved by the Institutional Animal Care and Use Committee of Shandong University.
Mice were randomly divided into control-WT, OVA-WT, control-Fstl1±, OVA-Fstl1± groups (n = 8/group). The OVA-induced allergic airway inflammatory model was established as previously reported [15]. Mice were sensitized by i.p. injection of 50 µg OVA (grade V, Sigma, USA) on days (0, 7, and 14), followed by intranasal administration of 10 µg OVA on days 21–28. Mice in the control-WT or control-Fstl1± groups were treated in corresponding manner with isometric PBS alone. The wild-type mice were grouped into control, FSTL1, MCC950 + FSTL1 to further investigate the relationship between FSTL1 and NLRP3/IL-1β. Mice in the FSTL1 group were intranasally administered with 10 µg FSTL1 (Sino Biological, Beijing) daily for 15 days [16], and PBS intranasal mice were treated with PBS. Mice in the FSTL1 + MCC950 group were i.p. injected with 200 µg MCC950 (Target Molecule, USA) 2 h before each FSTL1 administration.
BALF collection and analysis
BALF was collected to analyze cellular components and cytokines in supernatants. After ligating one side of the bronchus, we flushed the other side of lungs with 0.5 mL of ice-cold PBS for three times via tracheal catheter [17, 18]. Approximately, over 80% of the instilled volume was recovered. Then, the BALF samples were centrifuged, and the supernatant was collected and stored at − 80 ℃ for the following measurement of cytokines.
The cell pellets were resuspended in 1 mL of PBS, and 0.1 mL of suspension was obtained to count the total number of nucleated cells under a cytometer. The differential cell counts were smeared on slides and processed by Wright–Giemsa staining (Sigma-Aldrich, St. Louis, MO, USA). At least 200 cells were classified and counted for each slide by using the brightfield microscope according to cell morphology.
Histological analysis
The lung tissues of mice were fixed in 10% formalin for more than 24 h and then dehydrated, paraffin-embedded, and cut into 5-μm-thick sections. The sections were dewaxed, rehydrated, and stained via hematoxylin–eosin (HE) and periodic acid–schiff (PAS) staining. According to previously described methods [19], the severity of peribronchial and perivascular inflammation was graded using lung inflammatory scores of 0–4, and the goblet cell hyperplasia was evaluated using numerical scores of 0–4 as determined by the PAS-positive cells in each airway.
Immunohistochemistry
Sections were deparaffinized, rehydrated, pretreated with 10 mM sodium citrate for antigen recovery, prevented from the endogenous peroxidase by 3% H2O2, and blocked with 5% bovine serum. Next, the slides were incubated with primary antibodies of anti-FSTL1, anti-NLRP3, and anti-Muc-5AC at 4 °C. After extensive washing, the sections were incubated with the corresponding HRP-conjugated secondary antigens for 1 h at room temperature and then developed with DAB solution (Boster, China). Finally, the slides were counterstained with hematoxylin. The positive area of the target protein was measured using the Image-Pro Plus 6.0 software (Media Cybernetics, USA).
Levels of inflammatory cytokines in BALF
The levels of IL-4, IL-5, IL-13, and IL-1β in the BALF supernatant of mice were detected using ELISA kits (CUSABIO, China) according to the manufacturer’s instructions. The FSTL1 and IL-1β levels in human serum were also measured by ELISA (Abcam, USA). All calibrations and analyses were performed in duplicate.
Levels of IL-1β in lung tissues
The levels of IL-1β in lung tissues were detected according to the manufacturer’s instructions (Abcam, USA). Lung tissue was minced and thoroughly rinsed in PBS to remove blood. Homogenize 100 mg of wet tissue in 500 µL extraction buffer. The mixture of tissue and extraction buffer was incubated on ice for 20 min, and then centrifuged at 18,000g for 20 min at 4 °C. The supernatants were transferred into clean tubes. The samples were immediately aliquoted and stored at − 80 °C. The unit of IL-1β concentration was ng/mL.
Western blot analysis
Lung supernatants were obtained for protein concentration determination using the BCA protein assay kit (Boster, China). Then, equal amounts of protein samples were separated by SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride (PVDF) membranes. PVDF membranes were blocked with 5% non-fat milk for 1 h at room temperature, and then incubated with diluted primary antibodies of NLRP3, IL-1β, and GAPDH. Then, PVDF membranes were incubated with HRP-conjugated secondary antibody for 2 h at room temperature. The binding of all antibodies was detected using an enhanced chemiluminescence.
Statistical analysis
The data are expressed as mean ± SEM. Two groups were evaluated by Student’s ttest. Comparisons among multiple groups were performed using one-way and two-way ANOVA analysis. Differences between the groups were considered to be significant when P < 0.05.
Results
Fstl1 ± attenuated inflammatory cells and pathological changes after OVA exposure
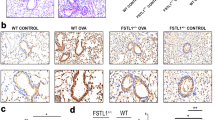
As shown in Fig. 1A–F, mice in the OVA-WT group exhibited irregular lung tissue structure, thickened airway epithelium, and infiltration of inflammatory cells around the bronchus compared with the control and Fstl1± group. PAS staining showed excessive secretion of mucus and Muc-5AC in the OVA-WT group compared with the control and Fstl1± group. However, Fstl1± treatment significantly improved the lung tissue damage and inflammatory cell infiltration caused by OVA, and significantly inhibited the secretion of mucus and and Muc-5AC caused by OVA.
Mice in OVA-WT and OVA-Fstl1± groups were sensitized with OVA by i.p. injection at days 0, 7, and 14, and an OVA challenge was performed by intranasal administration every day at days 21–28. The control mice were treated only with PBS. Histologic examination was stained with HE (A, magnification 400 ×). PAS staining (B, magnification 400 ×) and immunohistochemistry (C, magnification 400 ×) stained with Muc-5AC were used to assess mucin production. D bronchial inflammation score of A. E mucus intensity of B. F Muc-5AC intensity of C. Data shown are mean ± SEM. # p < 0.05 versus the control-WT group. #*p < 0.05 versus the OVA-WT and Control-Fstl1± groups
Fstl1 ± inhibited the production of inflammatory cells and cytokines in BALF
As shown in Fig. 2A–I, results showed the infiltration of inflammatory cells in to BALF, mainly including eosinophils, neutrophils and macrophages, leukocytes and lymphocytes were significantly elevated after OVA treatment. In addition, results also showed the infiltration of inflammatory cytokines (IL-4, IL-5, IL-13, and IL-1β) into BALF were significantly elevated after OVA treatment (Fig. 2A–I). However, Fstl1± treatment in mice significantly improved the infiltration of inflammatory cells and cytokines into BALF caused by OVA compared with control (Fig. 2A–I).
Total leucocytes and the percentage of different cells (eosinophils, lymphocytes, neutrophils, and macrophages) were observed within the BALF (A–E). The cytokine levels of IL-4 (G), IL-5 (H), IL-13 (I), and IL-1β (F) in BALF were detected by ELISA. Data shown are mean ± SEM. #p < 0.05 versus the control-WT group. #*p < 0.05 versus the OVA-WT and Control-Fstl1± groups
Fstl1 ± inhibited OVA-induced NLRP3/IL-1β signaling pathway
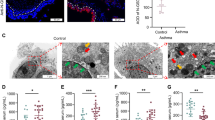
The serum FSTL1 and IL-1β levels in asthma patients were assessed. The characteristics of patients with asthmatic and healthy are shown in Table 1. As shown in Fig. 3B, C, the levels of FSTL1 and IL-1β in serum were elevated in patients with asthma compared with the control. In addition, the expressions of FSTL1 and NLRP3 and IL-1β in bronchi were dramatically increased compared the control group (Figs. 3A, D, and 4A–D). However, Fstl1± significantly inhibited the expression of FSTL1 in bronchi, and further inhibited the expression of NLRP3 and IL-1β in bronchi (Figs. 3A, D, and 4A–D). In conclusion, Fstl1± reduced the expression of NLRP3 and IL-1β by inhibiting FSTL1 expression in bronchi.
Lung sections of mice were prepared and stained with immunohistochemistry to evaluate the protein expression of FSTL1 (A) in lung tissues. FSTL1 (B) and IL-1β (C) levels in the serum of control and asthma were detected by ELISA. The correlation between serum FSTL1 and IL-1β levels is shown in D. E FSTL1 intensity of A. Data shown are mean ± SEM. #p < 0.05 versus the health group. #*p < 0.05 versus the control-WT group. #**p < 0.05 versus the OVA-WT and Control-Fstl1± groups
Pretreatment with MCC950 attenuated the activation of NLRP3/ IL-1β signaling pathway induced by FSTL1 in mice
The results showed that the inflammation degree of lung tissues induced by FSTL1 was increased (Fig. 5A, C). In addition, the expression levels of NLRP3 and IL-1β ascended after inhalation with FSTL1 through the nose compared with control (Fig. 5B, D, E). However, this airway inflammation and the expression levels of NLRP3 and IL-1β induced by FSTL1 were significantly inhibited by pretreatment with MCC950 (Fig. 5A–E). Therefore, the results further demonstrated that FSTL1 promoted the activation of NLRP3 and IL-1β signaling.
Mice in the FSTL1 group were intranasally administered with 10 µg FSTL1 daily for 15 days. Mice in the FSTL1 + MCC950 group were i.p. injected with 200 µg MCC950 2 h before each FSTL1 administration. Histopathologic changes were stained with HE (A, magnification 400 ×). The protein of NLRP3 (B, magnification 400 ×) was stained with immunohistochemistry. The level of IL-1β (E) in lungs was detected by ELISA. C bronchial inflammation score of A. D NLRP3 intensity of B. Data shown are mean ± SEM. #p < 0.05 versus the control group. #*p < 0.05 versus the OVA group
FSTL1 promoted the activation of NLRP3/ IL-1β signaling in MH-S cells.
As shown in Fig. 6A–D, the expression of NLRP3 increased in a dose-dependent manner when MH-S cells were treated by FSTL1. We detected the components at different time points, showing that the level of NLRP3 significantly elevated after FSTL1 stimulation for 12 h. Therefore, the above results proved that FSTL1 (100 ng/μl) stimulation MH-S cells for 12 h was the best choice.
MH-S cells were stimulated with different doses and period of FSTL1 for 12 h. Protein expression of NLRP3 (A and B) were detected by Western blot analysis. Then, MH-S cells were treated with OVA, FSTL1, siFSTL1, and MCC950 to determine the expression of NLRP3 (E) and IL-β (E). F and G Protein intensity of E. Data shown are mean ± SEM. #p < 0.05 versus the control group. $p < 0.05 versus the control group. &p < 0.05 versus the control and FSTL1 and OVA groups. #*p < 0.05 versus the OVA + FSTL1 group. ^p < 0.05 versus the control group. $*p < 0.05 versus the FSTL1 group
In addition, the expression levels of NLRP3 and IL-1β induced by FSTL1 or OVA or OVA + FSTL1 ascended compared with control (Fig. 6E–G). Results also showed that the expression levels of NLRP3 and IL-1β induced by OVA + FSTL1 was significantly higher than OVA or FSTL1 group (Fig. 6E–G). Transfection with siFSTL1 significantly inhibited the expression levels of NLRP3 and IL-1β induced by OVA compared with OVA + siNC group (Fig. 6E–G). Finally, the expressions of NLRP3 and IL-1β induced by FSTL1 were significantly inhibited by pretreatment with MCC950 (Fig. 6E–G).
Discussion
Asthma is a heterogeneous disease, which is usually characterized by chronic airway inflammation. FSTL1 plays an important role in airway remodeling, and we further investigated the role of FSTL1 in airway inflammation induced by asthma. The present study results supported that targeting FSTL1 may be a potential treatment strategy for patients with asthma.
FSTL1, a secreted glycoprotein, participates in many biological processes and is involved in various inflammatory diseases and conditions [20]. Both anti- and pro-inflammatory effects for FSTL1 have been studied, such as in rheumatoid arthritis [21, 22], osteoarthritis [23, 24], juvenile rheumatoid arthritis [25], acute coronary syndrome [26], intervertebral disc disease, and obeosity [27, 28]. The present study demonstrated that FSTL1 significantly exacerbated airway inflammatory cells and factors infiltration into the BALF. Moreover, the inflammatory damage and mucus secretion were inhibited by Fstl1±.
The mechanism by which FSTL1 exerts its pro-inflammatory effects has not been determined, and various signaling pathways may be involved. Yury et al. pointed that FSTL1 could be consumed by endotoxin-stimulated monocytes and macrophages, thereby enhancing NLRP3 inflammasome-mediated IL-1β secretion from above cells [29]. The present study identified that FSTL1 acted on promoting the activation of NLRP3/IL-1β signaling in OVA-induced asthma. Pretreatment with MCC950 obviously inhibited the expression of NLRP3 and IL-1β in mice and MH-S cells.
Alveolar macrophages (AMs) are important candidates for inducing airway inflammation. AMs express high levels of NLRP3 mRNA and are the major sources of locally produced IL-1β [30]. IL-1β is associated with neutrophil inflammation [31], asthma severity [32], frequent exacerbations [33, 34]. Ken et al. reported that DC-derived IL-1β promotes OVA-specific Th2 cell activation, thus aggravating the allergic airway eosinophilia depending on an IL-4/IL-13-STAT6 pathway [35]. The administration of anti-IL-1β-neutralizing antibody dramatically reduces the increase in IL-4, IL-5, IL-13, and tumor necrosis factor-α (TNF-α) in lungs [36]. The present study showed that pretreatment with MCC950 or siFSTL1 obviously inhibited the expression of NLRP3 and IL-1β induced by FSTL1 in mice and MH-S cells, and further reduced bronchial inflammatory injury. Therefore, the TSTL1-induced activation of NLRP3/IL-1β signaling plays a key role in the progression of asthma inflammation.
Due to some deficiencies in this study, further studies are needed to determine the mechanism of FSTL1 involvement in asthma pathogenesis. In conclusion, the study results have underscored the key role of FSTL1 in promoting allergic airway inflammation by activating NLRP3/IL-1β. This study and our continuing efforts may provide a novel treatment or diagnostic strategy for asthma.
Availability of data and materials
The data generated during the study are available from the corresponding author on reasonable request.
References
Liao Z, Xiao HT, Zhang Y, Tong RS, Zhang LJ, Bian Y, et al. IL-1beta: a key modulator in asthmatic airway smooth muscle hyper-reactivity. Expert Rev Respir Med. 2015;9:429–36.
Liu Y, Liu T, Wu J, Li T, Jiao X, Zhang H, et al. The Correlation between FSTL1 Expression and Airway Remodeling in Asthmatics. Mediators Inflamm. 2017;2017:7918472.
Chaly Y, Hostager B, Smith S, Hirsch R. Follistatin-like protein 1 and its role in inflammation and inflammatory diseases. Immunol Res. 2014;59:266–72.
Ogura Y, Ouchi N, Ohashi K, Shibata R, Kataoka Y, Kambara T, et al. Therapeutic impact of follistatin-like 1 on myocardial ischemic injury in preclinical models. Circulation. 2012;126:1728–38.
Zheng X, Qi C, Zhang S, Fang Y, Ning W. TGF-beta1 induces fstl1 via the smad3-c-jun pathway in lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2017;313:L240–51.
Kudo-Saito C, Ishida A, Shouya Y, Teramoto K, Igarashi T, Kon R, et al. Blocking the FSTL1-DIP2A axis improves anti-tumor immunity. Cell Rep. 2018;24:1790–801.
Johnson VJ, Yucesoy B, Luster MI. Prevention of IL-1 signaling attenuates airway hyperresponsiveness and inflammation in a murine model of toluene diisocyanate-induced asthma. J Allergy Clin Immunol. 2005;116:851–8.
Besnard AG, Guillou N, Tschopp J, Erard F, Couillin I, Iwakura Y, et al. NLRP3 inflammasome is required in murine asthma in the absence of aluminum adjuvant. Allergy. 2011;66:1047–57.
Simpson JL, Phipps S, Baines KJ, Oreo KM, Gunawardhana L, Gibson PG. Elevated expression of the NLRP3 inflammasome in neutrophilic asthma. Eur Respir J. 2014;43:1067–76.
Li R, Wang J, Li R, Zhu F, Xu W, Zha G, et al. ATP/P2X7-NLRP3 axis of dendritic cells participates in the regulation of airway inflammation and hyper-responsiveness in asthma by mediating HMGB1 expression and secretion. Exp Cell Res. 2018;366:1–15.
Besnard AG, Togbe D, Couillin I, Tan Z, Zheng SG, Erard F, et al. Inflammasome-IL-1-Th17 response in allergic lung inflammation. J Mol Cell Biol. 2012;4:3–10.
Zhang Y, Xu CB, Cardell LO. Long-term exposure to IL-1beta enhances Toll-IL-1 receptor-mediated inflammatory signaling in murine airway hyperresponsiveness. Eur Cytokine Netw. 2009;20:148–56.
Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248–55.
Li KC, Zhang FX, Li CL, Wang F, Yu MY, Zhong YQ, et al. Follistatin-like 1 suppresses sensory afferent transmission by activating Na+, K+-ATPase. Neuron. 2011;69(5):974–87.
Nie H, Wang A, He Q, Yang Q, Liu L, Zhang G, et al. Phenotypic switch in lung interstitial macrophage polarization in an ovalbumin-induced mouse model of asthma. Exp Ther Med. 2017;14:1284–92.
Miller M, Beppu A, Rosenthal P, Pham A, Das S, Karta M, et al. Fstl1 promotes asthmatic airway remodeling by inducing oncostatin M. J Immunol. 2015;195:3546–56.
Chong L, Zhang W, Nie Y, Yu G, Liu L, Lin L, et al. Protective effect of curcumin on acute airway inflammation of allergic asthma in mice through Notch1-GATA3 signaling pathway. Inflammation. 2014;37:1476–85.
Ritter M, Straubinger K, Schmidt S, Busch DH, Hagner S, Garn H, et al. Functional relevance of NLRP3 inflammasome-mediated interleukin (IL)-1beta during acute allergic airway inflammation. Clin Exp Immunol. 2014;178:212–23.
Myou S, Leff AR, Myo S, Boetticher E, Tong J, Meliton AY, et al. Blockade of inflammation and airway hyperresponsiveness in immune-sensitized mice by dominant-negative phosphoinositide 3-kinase-TAT. J Exp Med. 2003;198:1573–82.
Miyamae T, Marinov AD, Sowders D, Wilson DC, Devlin J, Boudreau R, et al. Follistatin-like protein-1 is a novel proinflammatory molecule. J Immunol. 2006;177:4758–62.
Chaly Y, Marinov AD, Oxburgh L, Bushnell DS, Hirsch R. FSTL1 promotes arthritis in mice by enhancing inflammatory cytokine/chemokine expression. Arthritis Rheum. 2012;64:1082–8.
Clutter SD, Wilson DC, Marinov AD, Hirsch R. Follistatin-like protein 1 promotes arthritis by up-regulating IFN-gamma. J Immunol. 2009;182:234–9.
Ni S, Miao K, Zhou X, Xu N, Li C, Zhu R, et al. The involvement of follistatin-like protein 1 in osteoarthritis by elevating NF-kappaB-mediated inflammatory cytokines and enhancing fibroblast like synoviocyte proliferation. Arthritis Res Ther. 2015;17:91.
Wang Y, Li D, Xu N, Tao W, Zhu R, Sun R, et al. Follistatin-like protein 1: a serum biochemical marker reflecting the severity of joint damage in patients with osteoarthritis. Arthritis Res Ther. 2011;13:R193.
Wilson DC, Marinov AD, Blair HC, Bushnell DS, Thompson SD, Chaly Y, et al. Follistatin-like protein 1 is a mesenchyme-derived inflammatory protein and may represent a biomarker for systemic-onset juvenile rheumatoid arthritis. Arthritis Rheum. 2010;62:2510–6.
Widera C, Horn-Wichmann R, Kempf T, Bethmann K, Fiedler B, Sharma S, et al. Circulating concentrations of follistatin-like 1 in healthy individuals and patients with acute coronary syndrome as assessed by an immunoluminometric sandwich assay. Clin Chem. 2009;55:1794–800.
Liu Y, Wei J, Zhao Y, Zhang Y, Han Y, Chen B, et al. Follistatin-like protein 1 promotes inflammatory reactions in nucleus pulposus cells by interacting with the MAPK and NFkappaB signaling pathways. Oncotarget. 2017;8:43023–34.
Fan N, Sun H, Wang Y, Wang Y, Zhang L, Xia Z, et al. Follistatin-like 1: a potential mediator of inflammation in obesity. Mediators Inflamm. 2013; 2013:752519.
Chaly Y, Fu Y, Marinov A, Hostager B, Yan W, Campfield B, et al. Follistatin-like protein 1 enhances NLRP3 inflammasome-mediated IL-1beta secretion from monocytes and macrophages. Eur J Immunol. 2014;44:1467–79.
Lee YG, Jeong JJ, Nyenhuis S, Berdyshev E, Chung S, Ranjan R, et al. Recruited alveolar macrophages, in response to airway epithelial-derived monocyte chemoattractant protein 1/CCl2, regulate airway inflammation and remodeling in allergic asthma. Am J Respir Cell Mol Biol. 2015;52:772–84.
Gwyer Findlay E, Hussell T. Macrophage-mediated inflammation and disease: a focus on the lung. Mediators Inflamm. 2012; 2012:140937.
Kim RY, Pinkerton JW, Essilfie AT, Robertson AAB, Baines KJ, Brown AC, et al. Role for NLRP3 Inflammasome-mediated, IL-1beta-dependent responses in severe, steroid-resistant asthma. Am J Respir Crit Care Med. 2017;196:283–97.
Fu JJ, McDonald VM, Baines KJ, Gibson PG. Airway IL-1beta and systemic inflammation as predictors of future exacerbation risk in asthma and COPD. Chest. 2015;148:618–29.
Mahmutovic Persson I, Menzel M, Ramu S, Cerps S, Akbarshahi H, Uller L. IL-1beta mediates lung neutrophilia and IL-33 expression in a mouse model of viral-induced asthma exacerbation. Respir Res. 2018;19:16.
Arae K, Morita H, Unno H, Motomura K, Toyama S, Okada N, et al. Chitin promotes antigen-specific Th2 cell-mediated murine asthma through induction of IL-33-mediated IL-1beta production by DCs. Sci Rep. 2018;8:11721.
Kim SR, Kim DI, Kim SH, Lee H, Lee KS, Cho SH, et al. NLRP3 inflammasome activation by mitochondrial ROS in bronchial epithelial cells is required for allergic inflammation. Cell Death Dis. 2014; 5:e1498.
Acknowledgements
We would like to thank Xiang Gao and Wen Ning for their generous help in Fstl1+/- mice.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81770029), National Key Research and Development Project (2017YFC1310601).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was approved by the Ethics Review Committee for Human Studies at Qilu Hospital of Shandong University and Institutional Animal Care and Use Committee of Shandong University (Grant NO. KYLL-2017[ks]-112).
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Zhang, D., Liu, T. et al. FSTL1 aggravates OVA-induced inflammatory responses by activating the NLRP3/IL-1β signaling pathway in mice and macrophages. Inflamm. Res. 70, 777–787 (2021). https://doi.org/10.1007/s00011-021-01475-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-021-01475-w