Abstract
In the present contribution, the development of an high performance liquid chromatography–diode array detector and gas chromatography–flame-ionisation detectors method for the quantification of 53 active substances, 15 co-formulants and 8 impurities in plant protection products is described. The confirmation of the results was performed by comparison of UV spectra or MS spectra of calibration solutions and sample solutions. The method is universally applicable, simple, reliable and fast. The method validation showed sufficient accuracy, linearity, repeatability and specificity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
According to Article 68 of Regulation (EC) No 1107/2009 (European Parliament and Council 2009), the Member States of the European Union have the task to carry out controls on the trade and the use of plant protection products. An important component of the German Plant Protection Control Program is the analysis of the composition of plant protection products, in order to monitor the compliance with the requirements laid down by the authorisation with regard to the contents of active substances and co-formulants (Besinger-Riedel et al. 2008). Moreover, the maximum limits for impurities of active substances and undesired substances contained in co-formulants, which will be referred to as ‘impurities’ in this publication, can be monitored. To perform these controls efficiently, a method allowing the determination of as many active substances, co-formulants and impurities as possible is required due to the ever-changing questions concerning suspicious samples to be dealt with and the great variety of substances to be analysed. Such a method should avoid to apply a large variety of columns, chromatographic conditions and other instrumentations.
The handbook of the Collaborative International Analytical Council (CIPAC) lists methods for numerous substances evaluated by collaborative tests (CIPAC 2014). These methods have been developed and optimised for individual active substances. In view of the multitude of parameters to be controlled, a limitation to CIPAC methods leads to a very frequent switching of the measurement conditions. Moreover, the CIPAC handbook does not describe methods for co-formulants, and methods for impurities are described in exceptional cases only. The documents submitted in the course of the authorisation procedure for plant protection products are another source of test methods for the determination of active substances and relevant impurities in plant protection products (BVL 2013a). Like the CIPAC methods, the use of these methods in the Plant Protection Control Program is subject to the above-mentioned restrictions and therefore their practical use is limited.
The aim of this publication is to introduce an universal and simple method for the qualitative and quantitative determination of components of plant protection products. So far, no requirements have been developed on an EU level with regard to methods for the analysis of formulations to control the trade with plant protection products. Therefore, for the assessment of the validation results, the Working Document SANCO/3030/99 (European Commission 2000), which, however, only interprets data requirements for the authorisation of plant protections products, and a guideline published by the World Health Organisation (WHO 2005) were used for orientation.
2 Materials and methods
2.1 Reagents
Acetonitrile, methanol and acetone were of high performance liquid chromatography (HPLC) grade. Ultrapure water was prepared with the help of an Arium 611 VF Water Purifier (Sartorius, Germany). Phosphoric acid (pure, 85 %), sulphuric acid (pure, 85 %), potassium hydroxide (analytical grade) and potassium dihydrogen orthophosphate (analytical grade) were obtained from Merck (Germany), acetic acid (analytical grade) from Riedel-de-Haen (Germany) and hydrochloric acid (pure, 37 %) from Roth (Germany).
Calibration solutions: Standard substances were purchased from Dr. Ehrenstorfer, Fluka, Roth or Merck (Germany), if possible via certified suppliers, or obtained from applicants.
50 mg of analyte (absolute, considering purity) were weighed into a volumetric flask (50 ml). 30 ml of solvent were added to each flask and the flask was placed in an ultrasonic bath for 15 min. The solvent for HPLC was acetonitrile or methanol as described in Table 1 and acetone for gas chromatography (GC). The flask was removed and allowed to cool to ambient temperature. The solution was diluted to the mark with solvent and mixed well. The resulting stock solution (1 mg/ml) was diluted to calibration solutions of 0.1 and 0.5 mg/ml using electronic pipettes. Deviating concentrations of the calibration solutions of some impurities are presented in Tables 1 and 2.
2.2 Apparatus
The high performance liquid chromatographs, 1200 Series, Agilent (Germany), were equipped with a constant-flow pump, a constant-temperature column compartment, a sample injector capable of injecting 1–10 μl aliquots, a diode array detector (DAD) and a data-handling appliance.
The capillary gas chromatographs, HP 6890 Series, Agilent (Germany), were equipped with split/splitless or PTV cold-injection systems KAS, Gerstel (Germany), as well as with flame-ionisation detectors (FID) or mass-selective detectors (5973 or 7975C, Agilent, Germany) and a data-handling appliance.
Moreover, the following equipments were used: analytical balance with a sensitivity of ±0.1 mg, Sartorius (Germany), ultrasonic bath, Bandelin (Germany), HandyStep electronic pipettes, Brand (Germany), mechanical shakers, RM 500 S, Olbrich (Germany), and filtering apparatus equipped with 0.2 μm solvent-resistant filters, Spartan 30/0.2RC, Schleicher&Schuell (Germany). The density of liquid samples was determined with the help of a density meter, Densito 30PX, Mettler Toledo (Germany).
2.3 Chromatographic conditions
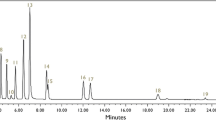
High-performance liquid chromatographic conditions: A 4 mm × 4 mm pre-column packed with Zorbax XDB C18, particle size 5 μm, and a 250 × 4 mm analytical column packed with Lichrospher 100 RP 18, particle size 5 μm, were used. For the impurities atrazine, captan and propazine a 50 mm × 4.6 mm analytical column packed with Zorbax Eclipse XDB C18, particle size 1.8 μm, was employed. The column temperature was 30 °C. For injection volumes, flow rates, mobile phases, detector wavelengths and retention times please see Table 1.
Gas chromatographic conditions: A Zebron 30 m × 0.32 mm column coated with cross-linked ZB 1701, film thickness 0.25 μm (Phenomenex, Germany), was used. The injection volume was 1 μl, the split ratio 50:1, the temperature of the injection port 250 °C and of the flame-ionisation detector (FID) 250 °C. Oven temperature program: 35 °C, 0.5 min hold, rate 35 °C/min to 280 °C, 6 min hold. The carrier gas was helium with a constant flow of 2 ml/min. The detector gases were hydrogen with 40 ml/min, synthetic air with 240 ml/min and helium with 25 ml/min as make-up gas. For the retention times please see Table 2. For the analysis of dimethoate, the following conditions were applied: 1 μl splitless; PTV temperature program: 45 °C, 0.1 min hold, rate 12 °C/s to 260 °C, 3 min hold; PTV purge gas flow: 71 ml/min of helium, gas saver after 1.5 min: 25 ml/min; oven temperature program: 60 °C, 0.5 min hold, rate 25 °C/min to 280 °C, 2 min hold. The carrier gas was helium with a constant flow of 1.4 ml/min. The retention time was 7.8 min.
Mass spectrometric conditions: Analytical column, injection, carrier gas and oven temperature as described under GC conditions. The ionisation was performed using EI, positive mode, and 70 eV ionisation energy. The source, the quadrupole and the transfer line were heated up to 230, 150 and 280 °C, respectively. Full scan spectra of the analytes were recorded.
2.4 Sample preparation and determination
For each sample, the solutions were prepared in duplicate. A part of the well homogenised sample (a quantity sufficient to contain approximately 25 mg of analyte) was transferred into a volumetric flask (50 ml). After the exact weighing of the sample, about 30 ml of solvent (HPLC: acetonitrile or methanol as described in Table 1; GC: acetone) were added and the solution was mixed thoroughly. The flask was placed in an ultrasonic bath for 15 min (for chlorothalonil: 60 min), removed and allowed to cool to room temperature. Then the solution was filled up to the mark with solvent and was mixed. When the solution was cloudy, it was filtered through a 0.2 μm filter prior to analysis.
The analytical HPLC or GC column was equilibrated until a stable baseline was obtained. The solvent blank and portions of two sample solutions in duplicate were injected, bracketing them by injection of calibration solutions.
For identification, the retention times were recorded, and for quantification the relevant peak areas were measured.
2.5 Identification, calibration and calculation
Identification was done by comparison of the retention times of samples and standards. The retention times corresponded to the calibration standards with an acceptable tolerance, normally of ±1 % both for HPLC and for GC.
A linear calibration curve was obtained from duplicate measurements at three concentration levels, using the method of least squares.
The content (c in g/l for liquids or g/kg for solids) of the analyte is calculated using the area of the analyte peak in the chromatogram of the sample (Y), the mass of the sample taken (E in mg), the volume of the sample solution (V in ml), the density of the sample in case of liquid formulations (r in g/ml), the intercept of the calibration curve (b) and the slope of the calibration curve (m). The calculation was performed with the help of the software of the Agilent ChemStation.
2.6 Validation
To evaluate the accuracy of the analytical method, recovery tests using standard addition were applied. The recoveries of product samples fortified with an appropriate amount of analyte (normally 100 % of the initial amount of the analyte) ranged from 97.8 to 103.2 % for analyte concentrations higher than 10 g/kg or g/l and from 83.7 to 106.7 % for impurities.
The repeatability, determined as relative standard deviation of independent determinations, varied generally from 0.01 to 1.73 % for analyte concentrations between 1 and 800 g/l or g/kg and for impurities between 0.14 and 4.78 % for analyte concentrations between 0.1 to 10 g/l or g/kg. Detailed validation data are presented in Tables 1 and 2.
In order to ensure the specificity of the method, UV spectra of peaks resulting from the high-performance chromatographic measurement of calibration solution and sample solution were compared. The UV spectra were recorded in the range of 190 to 400 nm. For analytes determined by GC, the specificity was confirmed by comparing the mass fragments in full-scan mass spectra of calibration solution and sample solution. Additionally library mass spectra (Wiley and NIST, supplied by Agilent, USA) were used for clarification.
The linearity of the analytical calibration was checked by calculating the correlation coefficient, which was better than 0.999 for nearly all analytes.
3 Discussion
The method is based on several years of analyses of plant protection products, which were mainly carried out in the framework of market monitoring activities. During this time, efforts were made to develop a uniform method for numerous active substances, co-formulants and impurities. For this purpose, individual measurement conditions were optimised, e.g. for HPLC measurements the column temperature was increased from 20 to 30 °C and the calibration range for active substances and co-formulants was aligned. In Tables 1 and 2 the validation data for 53 active substances, 15 co-formulants and 8 impurities can be found. The data are exemplary only, since for some analytes several sets of data were elaborated over time. For the establishment of the data, the requirements of standard EN ISO/IEC 17025 (CEN 2000) were observed.
The accuracy of the method was characterised by determining the recovery rates. Working Document SANCO/3030/99 (European Commission 2000) lays down 98–102 % as acceptable range for recovery rates in case of analyte concentrations above 100 g/kg or g/l, 97–103 % in case of concentrations of 10 to 100 g/kg or g/l, 95 % - 105 % in case of <10 g/kg or g/l and 90–110 % in case of 0.1–1 g/kg or g/l. Also for the assessment of the recovery rates of co-formulants, the requirements of SANCO/3030/99 for active substances were applied. For impurities <1 g/kg or g/l, SANCO/3030/99 lays down recoveries between 75 and 125 %.
Apart from some slightly deviating values for the active substances 2,4-D, flusilazole and prosulfuron, the determined recoveries meet the requirements of Working Document SANCO/3030/99.
The repeatability was determined by analysing 3 to 6 test samples taken from one container of plant protection products. For the repeatability, values which are below the limits determined by means of the modified Horwitz equation (European Commission 2000) are considered to be acceptable. The requirements regarding repeatability are met.
The correlation coefficients of the calibration curves, which were in general above 0.999, prove the very good linearity of the measurements.
The specificity of the analytes shown in Tables 1 and 2 could be verified by comparison of UV or MS spectra of calibration solutions and sample solutions.
Some active substances consist of isomers, which are not always separated under the given HPLC conditions. The validation data were determined on the basis of the sum of isomers. The retention times of some isomers are listed in Tables 1 and 2.
For the analysis of some plant protection products by HPLC it turned out to be helpful to apply a rinsing gradient after the elution of the active substance in order to clean the measurement system of disturbing substances and to be able to start the next measurement in due time.
Since most of the plant protection products possess UV-active functional groups, the active substances can in general be determined reliably by means of HPLC–UV. However, when the UV signal of an analyte is too low for a reproducible analysis by HPLC–UV, which is the case e.g. for the active substance dimethoate, it is advisable to apply GC–FID for substances which can be transferred into a gaseous state without decomposing or as reproducible decomposition products. In case of thermally unstable substances, the use of a cold-injection system improves the accuracy and repeatability of the results. The application of this injection technique was described for dimethoate as an example (Table 2, chapter 2.3).
Polar substances like glyphosate and glufosinate can equally be determined with the described method if, by derogation from the some conditions described under chapter 2.1 and 2.3, an analytical separation column packed with Nucleosil NH2 is used. The validation data for glyphosate and glufosinate were satisfactory; nevertheless, it might be possible to increase the specificity of the UV measurement at 195 nm by employing a mass-spectrometric detector.
Co-formulants in plant protection products can have various different structures and functions (Steer et al. 2007; Vinke 2014). Especially low-molecular compounds from the group of solvents and anti-freezing agents are suitable for analysis, while the determination of polymers and oligomers is not practical for market monitoring purposes (Vinke 2014). The analysed co-formulants are listed in Table 2. The validation data were determined on the basis of individual plant protection products. Since the same gas chromatographic measurement conditions were applied, it is theoretically possible to determine all the compounds listed in Table 2 in one run. A multi method for selected compounds is under development. As the retention times of the individual compounds are similar, a mass-spectrometric detection can be recommended.
The impurities mostly originate from the production process of the active substance. The limits for relevant impurities are laid down in the Regulation (EU) No 540/2011 (Commission 2011). This Regulation also specifies substances like atrazine, folpet and propazine as relevant impurities in the actual active substances. Non-specified impurities like captan in folpet or metalaxyl-M in tebuconazol must be determined in a range of 0.1 g/l or g/kg, since they are to be classified as so-called foreign substances (BVL 2013b). Not only active substances, but also co-formulants can be contaminated with undesired substances. For some of these impurities legal maximum limits exist (Chemikalien-Verbotsverordnung 2012). Since impurities may occur in very low concentrations, the calibration range has to encompass lower concentrations than for active substances and co-formulants. The present method includes the determination of the above-mentioned impurities. For the analysis of these impurities, the use of the ultra high performance liquid chromatography (UPLC) or rapid-resolution-technique proved to be beneficial.
The present method allows the qualitative and quantitative determination of numerous ingredients of plant protection products. So far, validation data for 53 active substances, 15 co-formulants and 8 impurities have been elaborated. This work is being continued. The method is reliable, fast and easy. It allows the analysis of active substances and co-formulants with only one HPLC or GC column. Thanks to its universality, the method is well suited to efficiently deal with many questions arising in the framework of market control.
References
Besinger-Riedel A, Vinke C, Corsten K, Siebers J (2008) Untersuchung der Zusammensetzung und der Eigenschaften von Pflanzenschutzmitteln—Ein Baustein des Pflanzenschutz-Kontrollprogramms. J Verbr Lebensm 3:265–271
BVL (2013a) www.bvl.bund.de/DE/04_Pflanzenschutzmittel/01_Aufgaben/08_Produktchemie/03_Analytik/psm_analytik_node.html
CEN (2000) General requirements for the competence of testing and calibration laboratories (ISO/IEC 17025:1999)
Chemikalien-Verbotsverordnung (2012) Verordnung über Verbote und Beschränkungen des Inverkehrbringens gefährlicher Stoffe, Zubereitungen und Erzeugnisse nach dem Chemikaliengesetz, Chemikalien-Verbotsverordnung in der Fassung der Bekanntmachung vom 13. Juni 2003 (BGBl I S. 867) zuletzt geändert durch die Verordnung vom 21. Juli 2008 (BGBl I S. 1328)
CIPAC (2014) www.cipac.org
Commission (2011) Commission implementing regulation (EU) No 540/2011 of 25 May 2011 implementing regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. Off J Eur Union L 153:1–185 16.11.2011
European commission (2000) Technical material and preparations: Guidance for generating and reporting methods in support of pre- and post-registration data requirements for Annex II (part A, section 4) and Annex III (part A, section 5) of Directive 91/414, Working document SANCO/3030/99 rev.4, 11/07/00
European Parliament and Council (2009) Regulation (EC) No 1107/2009 of the European Parliament and the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing council directives 79/117/EEC and 91/414/EEC Off J Eur Union L 309:1–50 24.11.2009
Steer A, Alt V, Goebel D (2007) Bewertung der Produktqualität von Pflanzenschutzmitteln. J Verbr Lebensm 2:83–88
Vinke C (2014) Bewertung von Untersuchungen an Pflanzenschutzmitteln aus der Marktkontrolle. J Verbr Lebensm (doi:10.1007/s00003-013-0856-6)
WHO (2005) CIPAC, WHO, FAO: Quality control of pesticide products—guideline for national laboratories, WHO/CDS/GCDPP/2005.15, World Health Organization, Rom
Acknowledgments
The authors thank Astrid Bräuer, Klaus Goldmann, Nuray Tezel and Cerry Walther for their excellent technical assistance and Monika Jüsgen and Ralf Hänel for proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siebers, J., Besinger-Riedel, A. & Vinke, C. Determination of active substances, co-formulants and impurities in plant protection products using high performance liquid chromatography and gas chromatography. J. Verbr. Lebensm. 9, 137–144 (2014). https://doi.org/10.1007/s00003-014-0873-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00003-014-0873-0