Abstract
Spatial distribution of young-of-the-year (YOY) and older roach, rudd, perch and ruffe was compared in two artificial lakes with macrophytes present and absent, and a valley reservoir, using gillnets. Almost all species of interest and both age categories preferred benthic habitats. The depth distribution in benthic habitats was relatively consistent across water bodies with the highest fish densities found in the shallowest depths. In the macrophyte-rich lake, YOY roach and perch utilize the 3–6 m benthic layer the most, whereas the fish preferred the 0–3 m benthic layer in the macrophyte-poor lake and reservoir. No differences were found in the depth distribution in pelagic habitats sampled by pelagic gillnets for YOY fish between the water bodies. Older fish usually utilized the surface water layer. Macrophytes influenced the depth distribution of YOY fish in benthic habitats, where their density maximum shifted deeper in the macrophyte-rich lake when fewer macrophytes were present in the shallowest benthic depth. In lakes, YOY fish utilized a wider depth spectrum due to the deeper thermocline when compared to the reservoir. Oxygen and temperature stratification are the main factors influencing fish distribution, whereas macrophyte presence particularly influences the depth distribution of YOY fish in benthic habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fish distribution in lakes and reservoirs is not homogeneous (Fischer and Eckmann 1997; Prchalová et al. 2009; Muška et al. 2012). It is influenced by biotic factors such as food availability, predation risk and competition (the “to eat and not to be eaten” rule; Gliwitz et al. 2006; Prchalová et al. 2009) and also abiotic factors such as oxygen concentration and water temperature (“to be or not to be” rule; Järvalt et al. 2005, Prchalová et al. 2009). Fish distribution is also influenced by the ontogenetic stage. During the day YOY fish utilize littoral areas, to find protection from predators. During the night, when they are not so vulnerable in the unstructured habitat, some species feed in pelagial (Bohl 1980; Jůza et al. 2014). For older fish, the distribution pattern is often the opposite. They usually prefer open water during the day and migrate to the littoral for the night (Muška et al. 2013; Říha et al. 2011).
Aquatic macrophyte communities, especially emerged and submerged plants are of a great importance to the dynamics of aquatic ecosystems, affecting both abiotic and biotic processes (Abdel-Tawwab 2005) including fish distribution (Sánches-Botero et al. 2008; Lopes et al. 2015). In aquatic systems, vegetation distribution patterns produce considerable structural variation in both pelagic and littoral zones and fish usually seek macrophytes for refuge. The physical structure provided by plants reduces the chance of being seen by active predators, and consequently, fish are less likely to be predated (Savino and Stein 1982; Lopes et al. 2015). Fish may also be attracted by the high food availability, since the structure provided by plants commonly host high densities of potential food resources such as invertebrates. Considering such conditions of safety and food availability, fish can grow, survive and reproduce successfully in macrophyte stands (Lopes et al. 2015). Nevertheless, negative effects of the presence of macrophytes has been also described in the literature. Studies of European fish species have shown for example that differences in stem densities of aquatic macrophytes result in different foraging rates: the foraging rate of rudd (Scardinius erythrophthalmus Linnaeus, 1758) on Daphnia sp. decreased at stem densities greater than 200 m−2 and that of juvenile perch (Perca fluviatilis Linnaeus, 1758) did not decrease even in the highest stem density of 600 stems m−2 (Winfield 1986). The foraging efficiency of roach (Rutilus rutilus Linnaeus, 1758) decreased substantially even in the lowest stem density (Winfield 1986). The significance of macrophytes can be amplified for small fish, which are more vulnerable to predation (Sogard 1997). The influence of the presence of macrophytes on older fish can therefore differ to that on YOY fish. Vegetated sites in the Great Lakes had higher densities of fish, smaller fish and greater species richness than unvegetated sites (Randall et al. 1996). On the other hand, growth and food resources utilization of Nile tilapia (Oreochromis niloticus Linnaeus, 1758) fry were significantly reduced by dense vegetation (Abdel-Tawwab 2005). Therefore, macrophyte cover may influence the distribution of fish species and these distribution patterns may differ between lakes with and without macrophytes.
The aim of this study is to compare fish distribution in horizontal (littoral-pelagial) and vertical (surface-bottom) dimensions in a lake with developed macrophytes and a lake practically lacking macrophytes. A canyon shaped reservoir, which was deep and thermally stratified with no macrophytes and relatively poor littoral due to water level fluctuations, also was used as a reference water body. The two lakes share similarities in their fish stock composition with roach, perch (terminal mouth position), rudd (superior mouth position) and ruffe (Gymnocephalus cernua Linnaeus, 1758, inferior mouth position) being the dominant species (Vejříková et al. 2016), and oxygen deficits occurring in deeper layers below 15 m. Similarly, roach, ruffe and perch are the abundant species in the investigated valley reservoir (Jůza et al. 2014), and in this water body strong temperature and oxygen stratification occur during the summer (Jůza et al. 2009). Comparison of different types of water bodies with differing characteristics (macrophytes absence/presence, with/without oxygen depletion in deeper layers) with similar fish stocks reveals, which characteristics shape the spatial distribution of fish in temperate artificial water bodies. It can be assumed that due to any oxygen limitations in the deeper layers of both lakes, fish can utilize a wider depth range in these lakes in comparison with the valley reservoir. The horizontal and vertical distribution of YOY fish in particular in the macrophyte-rich lake can be significantly different compared to the macrophyte-poor lake.
Material and methods
Study sites
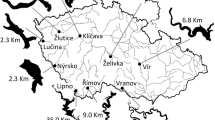
The study took place in two post mining lakes created by flooding of former coal mine and one deep valley reservoir in the Czech Republic. The restoration of Milada Lake (50°39’N, 13°58′E, surface area 252 ha, max. depth 24.7 m, average depth 15.5 m, Fig. 1) was performed from 2001 to 2010 and several species of macrophytes are present in high biomasses to a depth of 12 m. In 2014, scuba divers conducted detailed summer monitoring of species composition and coverage of macrophytes at different depths for the whole lake. Potamogeton pectinatus L. is an important species down to 6 m, Myriophylum spicatum L. down to 8 m and finally Chara sp. L. and Vaucheria De Candolle are dominant in depths down to 12 m. In September dense coverage of submerged macrophytes reached 91% at 2–3 m of depth (Vejříková et al. 2016) and 90% or greater coverage down to a depth of 8 m. At the 10–12 m depth layer the macrophyte coverage was approximately 60%. The only macrophytes-poor zone is the stony embankment in the shallowest littoral up to a 2 m depth.
The restoration of oligotrophic Most Lake (50°32’N, 13°32′E, surface area 310 ha, max.depth 75 m, average depth 22 m, Fig. 1) was performed between 2008 and 2014 and in comparison to mesotrophic Milada Lake, very scarce vegetation coverage developed during the sampling season. Until 2013 Most Lake was virtually lacking macrophytes. In 2014 macrophytes started to occur in low densities (coverage was approximately 18% in 0–4 m and less than 5% in 4–6 m). Dominant species were Potamogeton pectinatus and Potamogeton crispus L. The usual depth of the thermocline in both lakes is about 10 m and the majority of water column has sufficient amounts of oxygen (Fig. 2a, b).
The Římov Reservoir is a meso-eutrophic valley reservoir created by damming the Malše River in 1978 (48°85’N, 14°49′E, surface area 210 ha, max. Depth 45 m, average depth 16 m, Fig. 1). Very few to no macrophytes are present in the reservoir due mostly to its steep banks and water level fluctuations throughout the year. The usual depth of the thermocline in summer is about 4 m and below this depth oxygen concentration decreases sharply to almost zero in depths between 4 and 9 m (Fig. 2c).
Fish sampling
All water bodies were sampled in active vegetation period (July–September) repeatedly during several seasons using benthic (laid on the bottom) and pelagic (floating) gillnets (hereafter BG and PG, respectively). Milada Lake was sampled in 2009, 2010, 2012, 2013 and 2014; Most Lake was sampled every year from 2011 to 2014 and the Římov Reservoir also was sampled yearly from 2010 to 2014. Depth-stratified sampling, total effort based on reservoir area, and maximum sampled depth followed the European sampling protocol (CEN 2005). Because of the longitudinal depth gradient in the Římov Reservoir, shallow tributary parts were omitted.
Benthic gillnets (BG) were used to sample the following depth layers: 0–3, 3–6, 6–9, 9–12, 12–18 and > 18 m. The exception being the >18 m depth that was not sampled in Most Lake in 2011, and was not sampled in Milada Lake in any years. Pelagic gillnets (PG) sampled the depth layers 0–5 m, 5–10 m and > 10 m in all years before 2014 and depth layers 0–3 m, 3–6 m, 6–9 m and > 9 m in 2014. To make sampling before 2014 and in 2014 comparable, catches from 0 to 3 m and 3–6 m from the year 2014 were averaged with regard to the changed net area. Both the BG and the PG consisted of 12 mesh sizes (5, 6.25, 8, 10, 12.5, 15.5, 19.5, 24, 29, 35, 43 and 55 mm, knot to knot) as recommended by European sampling protocol (CEN 2005). The total length of each net was 30 m (12 × 2.5 m panels). The height of the BG was 1.5 m and that of the PG was either 4.5 m or 3 m (in 2014). Usually three localities were sampled in each water body in each year and three nets were deployed in each locality in every depth layer. All nets were set approximately 2 h before sunset and lifted after sunrise to cover maximal peaks of fish activity (Prchalová et al. 2010). All fish captured were counted, identified to the species and separated into young-of-the-year (YOY) and older. Density was expressed as number of fish per 1000 m−2 of netting per night. Roach, rudd, perch and ruffe are the most abundant species in the surveyed water bodies, therefore these four species were target species in our study (separately for YOY and older fish). Depth distribution of ruffe was investigated for benthic habitat only and comparison of horizontal distribution was not performed for this species because of the absence of this benthic species in the pelagic habitat.
Data analyses
The inter-water body differences in fish distribution were analysed by a factorial ANOVA model with interactions up to the second degree using Depth, Habitat and Water body factors. Since the data contains a lot of zeros, a zero inflated model was used, where zeros and non zeros are treated separately in the model (the model is the mixture of the binomial logistic model and the model for non zeros, the details can be found in Zuur et al. (2009)). Since the overdispersion appeared also in the non-zeros, a negative binomial model was used for them. The statistical inference was made in the following way. The model with two-way interactions was computed for model 1 with let say 3 factors. Then the model with two-way interactions was computed for model 2 with 2 factors. Then the likelihood ratio test comparing these two models was computed in order to reveal the input of the single factor missing in the model 2. Note here that we do not study the interactions but we include the possible effect of interactions into the comparison of the models.
In 2014, for which macrophyte coverage at different depth layers of the macrophyte-rich Milada Lake is available, we explored the dependencies of fish densities on the macrophytes density (expressed as the percentage coverage exactly in the place of gillnet installation), temperature and oxygen concentration by the regression model with negative binomial distribution of exploratory variables. This distribution enables the modelling of overdispersed fish counts. Because this model was not applicable for older roach and older rudd, a regression model with quasi-poisson distribution was performed for these two groups. The regression model was not performed for YOY rudd and YOY ruffe because of low occurrence of these two groups.
Statistical analyses were conducted using R 3.3.0 (R Foundation for Statistical Computing, R Core Team 2016), especially the R package pscl was used to compute the zero inflated model (Jackman 2017).
Results
Habitat associations in different water bodies
Benthic and pelagic densities of different YOY and older fish species in the three water bodies are shown in Figs. 3 and 4. For YOY roach and perch, no differences in preferred habitat between water bodies were found (p = 0.36, Df = 4, Chi square = 4.36 and p = 0.48, Df = 4, Chi square = 3.48, respectively). YOY roach preferred benthic over pelagic habitat in Milada Lake and the Římov Reservoir, whereas in Most Lake YOY roach prevailed in the pelagic habitat (Fig. 3a). YOY perch preferred benthic habitat in all water bodies (Fig. 3b). For YOY rudd the analysis was not applicable because of the low number of YOY rudd captured in the Římov Reservoir. Significant differences in habitat utilization between water bodies were found for older roach and older rudd (p = 0.003, Df = 4, Chi square = 16.21 and p = 0.02, Df = 4, Chi square = 12.16, respectively). The highest proportion of benthic older roach was found in the Římov Reservoir (87%, for exact values of densities see Fig. 4a), the lowest in the Milada Lake (65%, Fig. 4a). Older rudd preferred the pelagic habitat in the Římov Reservoir (93% of older rudd were pelagic), its highest affinity to benthic habitat was found in the Milada Lake (84% of older rudd were benthic, Fig. 4b). Habitat preference of older perch was insignificant between water bodies (p = 0.22, Df = 4, Chi square = 5.68) and older perch strongly preferred benthic habitats (Fig. 4c).
Density of older roach (a), rudd (b) and perch (c) in benthic and pelagic habitats of Milada Lake, Most Lake and Římov Reservoir. Median values (thick lines), average (asterisk), upper and lower quartiles (boxes), maximum and minimum values (whiskers) and outliers (empty circles) are shown. Created in R 3.3.0
Benthic depth preference in different water bodies
Benthic densities of different YOY and older fish species in different depth layers of each water body are shown in Figs. 5 and 6. The only significant differences between water bodies for YOY fish were found for roach (p = 0.002, Df = 4, Chi square = 13.31). In Most Lake and the Římov Reservoir, YOY roach preferred the shallowest benthic layer (0–3 m, 56% Most, 83% Římov Reservoir), whereas in the macrophyte-rich Milada Lake they preferred the deeper benthic layer (3–6 m, 45%, Fig. 5a). Similarly, YOY perch preferred the shallowest benthic layer in Most Lake and the Římov Reservoir (46 and 84%, respectively), however in Milada Lake, perch preferred the deeper benthic layer (3–6 m, 50%, Fig. 5b). The inter-water body differences were not statistically significant for YOY perch (p = 0.37, Df = 4, Chi square = 5.65). YOY ruffe preferred the 3–6 m benthic depth layer in Most Lake and the Římov Reservoir (52 and 54% respectively), but dominated the shallowest benthic layer in Milada Lake (57%, Fig. 5c). The inter-water body differences were not statistically significant for YOY ruffe (p = 0.34, Df = 4, Chi square = 6.21). In both lakes YOY rudd preferred the shallowest benthic habitat (0–3 m, Fig. 5d). In the benthic habitat significant inter-water body differences were found for older ruffe (p = 0.001, Df = 4, Chi square = 14.25), while roach was on the border of statistical significance (p = 0.05, Df = 4, Chi square = 9.14). Older ruffe preferred the second shallowest benthic habitat (3–6 m) in Most Lake and Římov Reservoir (37 and 43%, respectively), while they dominated in the second deepest benthic depth (9–12 m, 34%, Fig. 6c) in Milada Lake. In comparison, older roach preferred the second shallowest (3–6 m) benthic layer in Milada and Most Lakes and the shallowest benthic layer (0–3 m) in the Římov Reservoir (Fig. 6a). The inter-water body differences in benthic depth distribution were not significant for older perch (p = 0.42, Df = 4, Chi square = 1.92). Differences in depth preference of YOY and older rudd were impossible to analyze due to the absence of rudd in benthic habitats of the Římov Reservoir, however older rudd preferred shallowest benthic layer (0–3 m) in both lakes (Fig. 6d).
Density of YOY roach (a), perch (b), ruffe (c) and rudd (d) in different depths of benthic habitat in Milada Lake, Most Lake and Římov Reservoir. Median values (thick lines), average (asterisk), upper and lower quartiles (boxes), maximum and minimum values (whiskers) and outliers (empty circles) are shown. Created in R 3.3.0
Density of older roach (a), perch (b), ruffe (c) and rudd (d) in different depths of benthic habitat in Milada Lake, Most Lake and Římov Reservoir. Median values (thick lines), average (asterisk), upper and lower quartiles (boxes), maximum and minimum values (whiskers) and outliers (empty circles) are shown. Created in R 3.3.0
Pelagic depth preference in different water bodies
From YOY fish in the pelagic habitat only roach and perch were abundant enough in all water bodies to perform the analysis. The inter-water body differences in pelagic depth distribution were insignificant (p = 0.9 both for roach and perch). YOY of both species preferred the surface pelagic layer (0–5 m) in all water bodies (Fig. 7a, b). For older fish, significant differences in pelagic depth distribution between water bodies were found for roach and perch (p = 0.003, DF = 4, Chi square = 17.31 and p = 0.006, Df = 4, Chi square = 15.28, respectively). Older roach preferred the surface pelagic layer (0–5 m) in the macrophyte-rich Milada Lake and also in the Římov Reservoir (70 and 95% respectively, Fig. 7c). In the the macrophyte-poor Most Lake older roach preferred the deeper pelagic layer (5–10 m, 70%). Older perch dominated in surface pelagic layer (0–5 m) in the Římov Reservoir only (78%), whereas in both lakes it preferred deeper pelagic layer (5–10 m, Fig. 7d, 87 and 80% in Most and Milada Lakes respectively). Pelagic depth distribution of older rudd was not significantly different between water bodies (p = 0.15, Df = 4, Chi square = 8.33) and it always preferred the surface pelagic layer (0–5 m).
Density of YOY roach (a), YOY perch (b), older roach (c) and older perch (d) in different depths of pelagic habitat in Milada Lake, Most Lake and Římov Reservoir. Median values (thick lines), average (asterisk), upper and lower quartiles (boxes), maximum and minimum values (whiskers) and outliers (empty circles) are shown. Created in R 3.3.0
Factors driving distribution of fish in the macrophyte-rich Milada Lake in 2014
Regression models revealed that the presence of macrophytes positively influenced the density of YOY roach and also perch (Table 1) in particular. For the older fish the presence of macrophytes only positively influenced older roach significantly (Table 1). Temperature significantly positively influenced the distribution of older roach, older perch, YOY roach and YOY perch (Table 1) and oxygen concentration significantly influenced the density of YOY perch, older perch and older ruffe (Table 1). Oxygen concentration had no significant effect on other fish group due to the near constant concentration levels down to a depth of 12 m (Fig. 2). Below 12 m, where the oxygen concentration decreases rapidly, no fish were captured.
Discussion
In our study we observed relatively marginal differences in fish distribution in lakes with and without macrophytes and also in comparison with a deep valley reservoir. These differences were pronounced especially in the benthic distribution of YOY fish and older ruffe. Solely YOY roach and older rudd preferred pelagic over benthic habitats in the macrophyte-poor lake and valley reservoir respectively. The rest of the species and age groups reached higher densities in benthic rather than pelagic habitats in all water bodies. Any differences found in YOY fish depth distribution in benthic habitats were generally that the highest densities were deeper in the macrophyte-rich Milada Lake in comparison with the macrophyte-poor Most Lake and Římov Reservoir. If there were inter-water body differences in depth preference in the pelagic habitat, the highest older fish density in the macrophyte-rich Milada Lake and in Římov Reservoir was found in the surface layer, however in the macrophyte-poor Most Lake fish preferred the deeper pelagic layer. In the macrophyte-rich Milada Lake we found a positive effect of macrophytes on the distribution of YOY roach and perch especially.
Macrophytes play an important role in structuring fish assemblages because they provide higher productivity and higher carrying capacity for food resources due to the availability of substrates for prey (Agostinho et al. 2007). Moreover, they affect the balance of the forage efficiency of predators with the refuge needs for prey (Miranda and Hodges 2000). Aquatic plants, in contrast, affect physical and chemical conditions of the water, as respiration by plants and associated biota can reduce dissolved oxygen concentrations, particularly during warmer months and at night, producing conditions often intolerable for fish (Miranda and Hodges 2000). The oxygen deficits also occur during organic (including macrophytes) decomposition (Chapman et al. 1996). The vegetation coverage therefore also influences fish densities (Miranda and Hodges 2000). Theoretically, presence of macrophytes could result in higher attractiveness for fish due to protection and food supply but an excess in density of macrophyte cover can vice versa mean that fish avoid such zones due to oxygen depletion (Miranda and Hodges 2000) or simply excessively dense plant cover poses mechanical obstacles narrowing the space that fish can actually use (Abdel-Tawwab 2005).
Usually, we did not find differences in habitat associations between water bodies. With the exception of YOY roach in the macrophyte-poor Most Lake and older rudd in the canyon shaped Římov Reservoir, the remaining species and age categories reached higher densities in benthic rather than pelagic habitat in all water bodies. Fish usually migrate diurnally between pelagic and littoral habitats (Bohl 1980; Jůza et al. 2014; Muška et al. 2013) and pelagic gillnets sample fish within the pelagic habitat only, whereas benthic gillnets cross the migration track between littoral and pelagial habitats, making it more likely for fish to encounter benthic gillnets during horizontal migration. Higher pelagic density of YOY roach in the macrophyte-poor Most Lake indicates that the absence of macrophytes in the benthic habitat reduces the amount of horizontal migrations in YOY roach resulting in higher utilization of the pelagic habitat. However, this theory is not supported by results from the Římov Reservoir where higher YOY roach densities were observed in the macrophyte-less benthic habitat as compared to Milada Lake where macrophytes are an important refuge for YOY fish. The reason for the different horizontal distribution of YOY roach in Most Lake and the Římov Reservoir could be the predation pressure in the littoral because pike (Esox lucius Linnaeus, 1758) and wels (Silurus glanis Linnaeus, 1758) have been intensively stocked in Most Lake. In comparison with the Římov Reservoir, high densities of older perch are present in the littoral habitat of both lakes (see Fig. 3c) and it is safer for dominant YOY roach to utilize the pelagic habitat of Most Lake rather than staying in the dangerous, macrophyte poor littoral. In a small stratified lake, YOY roach were caught both in pelagic and benthic habitats over a 24 h period (Järvalt et al. 2005) and due to its plasticity, this species is the most common cyprinid in the most of Europe (Kottelat and Freyhof 2007). YOY roach was a species that performed partial migrations to the pelagic habitat at night, where some YOY roach stay in the littoral and some migrate to the pelagic habitat (Jůza et al. 2014). Our data showed that the behavior of YOY roach is different from the behavior of YOY perch, which was completely missing in the catch of pelagic gillnets and whose migration intensity can be influenced by the presence of macrophytes. Roach tended to utilize the pelagic habitat in gillnet catches as well in the Želivka Reservoir, whereas YOY perch preferred the benthic habitat (Prchalová et al. 2008). It seems that each YOY fish species keeps its typical horizontal distribution and the presence of macrophytes can be important for the protection of YOY roach especially under enhanced predation pressure in the littoral. In the case of older fish there were significant differences in the utilization of benthic and pelagic habitats in each water body for roach and rudd. In the remaining cases, the benthic habitat was preferred and we did not observe clear differences in habitat utilization between lakes with and without macrophytes. Older perch was captured almost exclusively in the benthic habitat in all water bodies. Older perch, roach and of course ruffe also dominated the benthic habitat in the Želivka Reservoir (Prchalová et al. 2008).
Depth distribution of YOY roach and perch in the benthic habitat showed very similar trends. In the macrophyte-poor Most Lake and Římov Reservoir, juveniles of these two species preferred the shallowest layer between 0 and 3 m, whereas in the macrophyte-rich Milada Lake they preferred deeper layer between 3 and 6 m. Also the benthic depth distribution of YOY ruffe showed similar trends between lakes. Benthic depth distribution was relatively similar in Most Lake and the Římov Reservoir with the highest YOY ruffe density between 3 and 6 m, whereas in the Milada Lake YOY ruffe particularly utilized the shallowest benthic layer between 0 and 3 m. The same trend of YOY roach and perch affinity to the shallowest benthic layer was found in an earlier study of the Římov Reservoir (Prchalová et al. 2009). YOY ruffe also preferred shallowest benthic layer in the study of Prchalová et al. (2009), which is shallower than in our study. YOY rudd preferred shallowest benthic layer in both lakes. If the rudd was captured in the benthic habitat of the Želivka Reservoir, it almost always preferred the shallowest depth layer (Prchalová et al. 2008). The preference of YOY roach and perch for the shallowest benthic layer in the Římov Reservoir is not surprising because of the thermal and oxygen stratification. The usual thermocline depth is about 4 m in summer (Jůza et al. 2009) and below this depth, the temperature and oxygen concentration decrease significantly. Part of the second shallowest benthic layer is obviously oxygen limited in the valley reservoir, which is the reason for lower densities of YOY fish of most species in this layer. YOY ruffe was the only species that slightly preferred the benthic depth between 3 and 6 m, which was probably due to its benthic dwelling lifestyle. Also YOY ruffe was practically missing below 6 m because of the oxygen limitation in the valley reservoir. The presence of macrophytes is probably the reason why the benthic depth distribution of YOY roach, perch and ruffe in Most Lake is more similar to distribution in the Římov Reservoir than to that in Milada Lake. In both water bodies without macrophytes, the shallowest benthic layer between 0 and 3 m is preferred by YOY roach and perch. In the macrophyte-rich Milada Lake there is a stony embankment at this depth zone, where almost no macrophytes occur. Densities of YOY roach and perch were significantly positively correlated with the macrophyte density. Macrophytes occur deeper than 2 m, and YOY roach and perch obviously prefer the shallowest depth with macrophyte presence (3–6 m). The trend of YOY ruffe is opposite to roach and perch and this benthic dwelling species obviously prefers bottoms without macrophytes. Ruffe prefer clean bottom (Hölker and Thiel 1998), which may be the reason why it prefers the shallowest benthic depth in Milada Lake. The only significant differences in the benthic distribution of older fish were found for ruffe. Older ruffe preferred the shallowest benthic depths (0–3 and 3–6 m) in Most Lake and the Římov Reservoir but preferred the 9–12 m depth layer in Milada Lake. Macrophytes are present in depths down to 12 m (Vejříková et al. 2016) but in this relatively deep zone, macrophytes cover is not so dense in comparison with the shallower layers (60% of the bottom surface approximately). It is possible that the difference in macrophyte density is the reason for the dominance of older ruffe in the relatively deep benthic habitat of Milada Lake. Too dense macrophyte cover on the bottom can lower the feeding efficiency of the ruffe, which is a typical benthic feeder (Kangur and Kangur 1996). This is the reason why older ruffe prefer depths between 9 and 12 m in Milada Lake, where it is still enough oxygen but the macrophyte density is lower. Generally, the depth distribution of older fish seems to be less influenced by the presence of macrophytes than YOY fish, but older fish utilize a wider depth spectrum especially in both lakes, where the oxygen conditions allow it. Older roach and perch also utilize the benthic depth between 9 and 12 m in both lakes, which was not observed for YOY fish. Studies showed that older fish live deeper than juveniles, which is connected with the changing of maximal sensitiveness to the wavelength of light during fish ontogeny (Guthrie and Muntz 1993). Due to higher water transparency in both lakes as compared to the valley reservoir light penetrates deeper in the water column. This can be, besides temperature and oxygen conditions, another reason for the utilization of deeper depths in Milada and Most lakes by older fish.
Unlike its influence in the benthic habitat it appears that the presence of macrophytes did not influence YOY fish distribution in the pelagic habitat. Dominant roach preferred the surface pelagic layer of the macrophyte-rich Milada Lake and no inter-water body differences in depth distribution were found for YOY fish. Differences in pelagic depth distribution among the water bodies were found for older roach and perch. Roach dominated in the surface pelagic layer (0–5 m) in Milada Lake and the Římov Reservoir, whereas older roach preferred 5–10 m depth layer in Most Lake. Perch preferred the second pelagic layer (5–10 m) in both lakes and the surface pelagic layer (0–5 m) in the Římov Reservoir. Roach usually prefer surface water layers because the optimal temperature for growth lies between 20 and 27 °C (van Dijk et al. 2002) and it also has a higher feeding efficiency in temperatures exceeding 18 °C (Persson 1986). The reason for the preference for the deeper pelagic layer by older roach in Most Lake remains unspecified but the presence of macrophytes seems unimportant in this case. Perch have a greater feeding efficiency below 18 °C (Persson 1986). Older perch utilized the pelagic depth layer between 5 and 10 m in both lakes but it preferred the surface pelagic layer (0–5 m) in the valley reservoir. In both lakes without oxygen limitations in deeper strata perch clearly preferred the slightly colder pelagic depth layer between 5 and 10 m, whereas in the valley reservoir with a steep vertical oxygen gradient, perch is forced to utilize the warmer surface pelagic layer. The significance of temperature and oxygen gradient for the vertical distribution of fish is obvious in both lakes, where the usual thermocline depth is about 9 m and below this depth temperature decreases significantly. In both lakes fish especially utilized the three shallowest layers (0–9 m) in the benthic habitat and the two shallowest layers (0–10 m) in the pelagic habitat. Only a few fish were captured deeper in the benthic habitat or were completely missing in the pelagic habitat.
Our results clearly showed that there are relatively marginal differences in fish distribution between different types of water bodies. The effect of macrophytes on different habitats and depth utilization was found especially for YOY fish, however the distribution of older fish followed trends typical for each particular species, given their abiotic characteristics.
References
Abdel-Tawwab M (2005) The effect of artificial vegetation density on growth and growth related parameters of Nile Tilapia, Oreochromis niloticus (L.) fry. Turk J Fish Aquat Sci 5:63–68
Agostinho AA, Thomaz SM, Gomes LC, Baltar SLSMA (2007) Influence of the macrophyte Eichhornia azurea on fish assemblages of the upper Paraná River floodplain (Brazil). Aquat Ecol 41:611–619. https://doi.org/10.1007/s10452-007-9122-2
Bohl E (1980) Diel pattern of pelagic distribution and feeding in planktivorous fish. Oecologia 44:368–375. https://doi.org/10.1007/BF00545241
CEN (2005) European standard EN 14 757. Water Quality—Sampling of Fish with Multimesh Gillnets
Chapman LJ, Chapman CA, Chandler M (1996) Wetland ecotones as refugia for endangered fishes. Biol Conserv 78:263–270. https://doi.org/10.1016/S0006-3207(96)00030-4
Fischer P, Eckmann R (1997) Spatial distribution of littoral fish species in a large European Lake, lake Constance, Germany. Arch Hydrobiol 140:91–116. https://doi.org/10.1127/archiv-hydrobiol/140/1997/91
Gliwitz ZM, Slon J, Szynkarczyk I (2006) Trading safety for food: evidence from gut contents in roach and bleak captured at different distances offshore from their daytime littoral refuge. Freshw Biol 51:823–839. https://doi.org/10.1111/j.1365-2427.2006.01530.x
Guthrie DM, Muntz WRA (1993) Role of vision in fish behaviour. In: Pitcher TJ (ed), Behaviour of teleost fishes, 2nd edn. Chapman & Hall, pp 89-128
Hölker F, Thiel R (1998) Biology of ruffe (Gymnocephalus cernuus (L.)) – a review of selected aspects from European literature. J Great Lakes Res 24:186–204. https://doi.org/10.1016/S0380-1330(98)70812-3
Jackman S (2017) Pscl: classes and methods for R developed in the political science computational laboratory. United States studies Centre, University of Sydney. Sydney, new South Wales, Australia. R package version 1.5.2. URL https://github.com/atahk/pscl/
Järvalt A, Krause T, Palm A (2005) Diel migration and spatial distribution of fish in a small stratified lake. Hydrobiologia 547:197–203. https://doi.org/10.1007/s10750-005-4160-z
Jůza T, Vašek M, Kubečka J, Seďa J, Matěna J, Prchalová M, Peterka J, Říha M, Jarolím O, Tušer M, Kratochvíl M, Čech M, Draštík V, Frouzová J, Hohausová E, Žaloudík J (2009) Pelagic underyearling communities in a canyon-shaped reservoir in late summer. J Limnol 68:304–314. https://doi.org/10.3274/JL09-68-2-13
Jůza T, Vašek M, Kratochvíl M, Blabolil P, Čech M, Draštík V, Frouzová J, Muška M, Peterka J, Prchalová M, Říha M, Tušer M, Kubečka J (2014) Chaos and stability of age 0-fish assemblages in a temperate deep reservoir: unpredictable success and stable habitat use. Hydrobiologia 724:217–234. https://doi.org/10.1007/s10750-013-1735-y
Kangur K, Kangur A (1996) Feeding of ruffe (Gymnocephalus cernuus) in relation to the abundance of benthic organisms in Lake Vârtsjärv (Estonia). Ann Zool Fenn 33:473–480
Kottelat M, Freyhof J (2007) Handbook of European freshwater fishes. Kottelat, Cornol, Swizerland and Freyhof, Berlin
Lopes CTMER, Silva JCB, Behrend RDL, Gomes LC (2015) Dense macrophytes influence the horizontal distribution of fish in floodplain lakes. Environ Biol Fish 98:1741–1755. https://doi.org/10.1007/s10641-015-0394-4
Miranda LE, Hodges KB (2000) Role of aquatic vegetation coverage on hypoxia and sunfish abundance in bays of a eutrophic reservoir. Hydrobiologia 427:51–57. https://doi.org/10.1023/A:1003999929094
Muška M, Tušer M, Frouzová J, Draštík V, Čech M, Jůza T, Kratochvíl M, Mrkvička T, Peterka J, Prchalová M, Říha T, Vašek M, Kubečka J (2013) To migrate or not to migrate: partial diel horizontal migration of fish in a temperate reservoir. Hydrobiologia 707:17–28. https://doi.org/10.1007/s10750-012-1401-9
Muška M, Vašek M, Modrý D, Jirků M, Ojwang WO, Malada JO, Kubečka J (2012) The last snapshot of natural pelagic fish assemblage in Lake Turkana, Kenya: a hydroacoustic study. J Great Lakes Res 38:98–106. https://doi.org/10.1016/j.jglr.2011.11.014
Persson L (1986) Temperature-induced shift in foraging ability in two fish species, roach (Rutilus rutilus) and perch (Perca fluviatilis): implications for coexistence of poikilotherms. J Anim Ecol 55:829–839. https://doi.org/10.2307/4419
Prchalová M, Kubečka J, Čech M, Frouzová J, Draštík V, Hohausová E, Jůza T, Kratochvíl M, Matěna J, Peterka J, Říha M, Tušer M, Vašek M (2009) The effect of depth, distance from dam and habitat on spatial distribution of fish in an artificial reservoir. Ecol Freshw Fish 18:247–260. https://doi.org/10.1111/j.1600-0633.2008.00342.x
Prchalová M, Kubečka J, Vašek M, Peterka J, Seďa J, Jůza T, Říha M, Jarolím O, Tušer M, Kratochvíl M, Čech M, Draštík V, Frouzová J, Hohausová E (2008) Distribution patterns of fishes in a canyon-shaped reservoir. J Fish Biol 73:54–78. https://doi.org/10.1111/j.1095-8649.2008.01906.x
Prchalová M, Mrkvička T, Kubečka J, Peterka J, Čech M, Muška M, Kratochvíl M, Vašek M (2010) Fish activity as determined by gillnet catch: a comparison of two reservoirs of different turbidity. Fish Res 102:291–296. https://doi.org/10.1016/j.fishres.2009.12.011
R Core Team. (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Randall RG, Minns CK, Cairns VW, Moore JE (1996) The relationship between an index of fish production and submerged macrophytes and other habitat features at three littoral areas in the Great Lakes. Can J Fish Aquat Sci 53:35–44. https://doi.org/10.1139/f95-271
Říha M, Kubečka J, Prchalová M, Mrkvička T, Čech M, Draštík V, Frouzová J, Hohausová E, Jůza T, Kratochvíl M, Peterka J, Tušer M, Vašek M (2011) The influence of diel period on fish assemblage in the unstructured littoral of reservoirs. Fisheries Manag Ecol 18:339–347. https://doi.org/10.1111/j.1365-2400.2011.00790.x
Sánches-Botero JI, Araujo-Lima CAMR, Garcez DS (2008) Effects of types of aquatic macrophyte stands and variations of dissolved oxygen and of temperature on the distribution of fishes in lakes of the amazonian floodplain. Acta Limnol Bras 20:45–54
Savino JF, Stein RA (1982) Predator-prey interaction between largemouth bass and bluegills as introduced by simulated, submerged vegetation. T Am Fish Soc 111:255–266. https://doi.org/10.1577/1548-8659(1982)111<255:PIBLBA>2.0.CO;2
Sogard SM (1997) Size-selective mortality in the juvenile stage of teleost fishes: a review. B Mar Sci 60:1129–1157
Van Dijk PLM, Staaks G, Hardewig I (2002) The effect of fasting and refeeding on temperature preference, activity and growth of roach, Rutilus rutilus. Oekologia 130:496–504. https://doi.org/10.1007/s00442-001-0830-3
Vejříková I, Vejřík L, Syväranta J, Kiljunen M, Čech M, Blabolil P, Vašek M, Sajdlová Z, Chung SHT, Šmejkal M, Frouzová J, Peterka J (2016) Distribution of herbivorous fish is frozen by low temperature. Sci Rep - UK 6:39600. https://doi.org/10.1038/srep39600
Winfield IJ (1986) The influence of simulated aquatic macrophytes on the zooplankton consumption rate of juvenile roach, Rutilus rutilus, Rudd, Scardinius erythropthalmus, and perch, Perca fluviatilis. J Fish Biol 29:37–48. https://doi.org/10.1111/j.1095-8649.1986.tb04997.x
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer Science + Business Media
Acknowledgments
We would like to thank Fish Ecology Unit (FishEcU, www.fishecu.cz) for data collection assistance and Leslie Tse for correcting the English of the manuscript. The study was supported by the Norwegian Financial Mechanism 2009-2014 under contract number MSMT-28477/2014 (project number 7F14316), the COST-CZ program under contract number MSMT-LD15021 and by the SoWa Research Infrastructure funded by MEYS CZ grant LM2015075, programme “Projects of Large Infrastructure for Research, Development, and Innovations”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interest exist.
Ethics statement
Fish sampling and treatment was conducted in compliance with guidelines from the Experimental Animal Welfare Commission under the Ministry of Agriculture of the Czech Republic.
Rights and permissions
About this article
Cite this article
Jůza, T., Blabolil, P., Čech, M. et al. Spatial distribution of four freshwater fish species in different types of artificial European water bodies. Biologia 73, 647–658 (2018). https://doi.org/10.2478/s11756-018-0075-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-018-0075-9