Abstract
Large year-to-year variability in different fish species recruitment has been confirmed by previous studies while diurnal patterns of occupation in two basic reservoir habitats (pelagic and littoral) by different age-0 fish species in late summer are still unclear. Data collected over an 11-year period regarding late-summer age-0 fish assemblages in pelagic and littoral habitats of a reservoir were used to test the recruitment instability and to investigate diurnal habitat use. Trawling was conducted in the pelagic habitat at night while beach seining was conducted in the littoral habitat during day and night. Fluctuations in age-0 fish abundance and species composition were observed with both sampling methods; however, the following spatio-temporal patterns were relatively stable in most investigated years: (1) pelagic species (pikeperch; Sander lucioperca, small perch; Perca fluviatilis, bream; Abramis brama at night), (2) littoral species (large perch, asp; Leuciscus aspius, dace; Leuciscus leuciscus), (3) migratory species likely performing diel horizontal migrations (bleak; Alburnus alburnus), (4) species abundant in the littoral habitat both during day and night and also in pelagic habitat at night (roach; Rutilus rutilus) and (5) species detected in both habitats exclusively at night (ruffe; Gymnocephalus cernuus).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Large year-to-year fluctuations in the recruitment of fish have been commonly observed in freshwater ecosystems (Anderson et al., 1998; Irwin et al., 2009), and numerous biotic and abiotic factors have been associated with this variability. The most important factors influencing the year-class strength in fish are (1) abiotic—water temperature (Lappalainen & Lehtonen, 1995; Grenouillet et al., 2001), water level fluctuations (Summerfelt & Shirley, 1978), wind velocity (Lappalainen & Lehtonen, 1995) and water turbidity (Summerfelt & Shirley, 1978; Neuman et al., 1996); and (2) biotic—food availability (zooplankton density; Bremigan & Stein, 1997), egg quality and predation pressure including cannibalism (Neuman et al., 1996). Research conducted in various water bodies has revealed unequal significance of these factors in year-class strength formation (see e.g. Summerfelt & Shirley, 1978; Lappalainen & Lehtonen, 1995; Quist et al., 2004; Jůza et al., 2009). The strength and structure of a year-class is frequently assumed to be a result of complex interactions among abiotic and biotic variables influencing the growth and mortality of the cohort (Neuman et al., 1996). This fact makes the prediction of species composition and its density extremely difficult, even if worked with reliable biotic and abiotic data.
To estimate the annual recruitment of different species correctly, the knowledge about their late-summer spatio-temporal distribution in lakes or reservoirs is also very important as it can influence fish migrations and affect sampling schedules. In European lakes and reservoirs, the change in spatio-temporal distribution of fish juveniles was investigated in two basic dimensions. Diurnal vertical migrations in the pelagic areas are better described and clearly explained. Age-0 fish utilize these large volumes of deep temperate lakes and reservoirs mostly during the night preferring warm surface and well-oxygenated epipelagic layers (Bohl, 1980; Vašek et al., 2006; Jůza et al., 2009). Diurnal vertical migrations in European freshwaters especially for juvenile smelt (Osmerus eperlanus, Gliwicz & Jachner, 1992) and also for coregonids (Mehner et al., 2007) have been well described. Similar migrations were described for early stages of percid fish in reservoirs. Percids prefer surface layers at night but during the day some percids migrate vertically to deeper layers below the thermocline (Čech et al., 2005).
Diel horizontal migrations, which are usually explained by a trade-off between increased use of sheltered littoral sites in daylight to reduce predation from day-active predators and the opportunity to feed in not only the more exposed but also the more profitable pelagic habitats during twilight periods and at night, are more complex and by far less understood (Gliwicz & Jachner, 1992; Jacobsen & Perrow, 1998; Okun et al., 2005). Studies focused on diel horizontal distribution of fish in European freshwaters frequently failed to distinguish behavioural patterns of different species because hydroacoustics was used as a sampling tool (Bohl, 1980) or focused only on the behaviour of the most abundant age-0 fish species in lakes or reservoirs such as roach (Rutilus rutilus) or perch (Perca fluviatilis, Imbrock et al., 1996; Gliwicz & Jachner, 1992; Borcherding et al., 2002; Hölker et al., 2002). Nonetheless the use of littoral daytime refuges may differ through the ontogenetic stages and among different species (Gliwicz et al., 2006).
Detailed information about spatio-temporal horizontal distribution of different age-0 fish species in European lakes and reservoirs in late summer is rare, and for some species missing in the literature. However, such information would be extremely important in order to understand migration patterns of different species including diurnal horizontal migrations. Knowledge of temporal habitat preferences is also extremely important for monitoring purposes because fisheries scientists and water authorities would know, when and which habitat must be sampled for the most effective and exact monitoring of different age-0 fish species. The aim of this study is to fill the knowledge gap about spatio-temporal habitat preferences of different species of age-0 fish.
Materials and methods
Study area
The study was conducted in the Římov Reservoir, a deep, narrow canyon-shaped artificial lake in southern part of the Czech Republic (48°50′N, 14°30′E, 170 km south of Prague, Fig. 1). The reservoir was built in 1978 on the Malše River and serves as the drinking water storage for the South Bohemian region. The surface area is 2.1 km2, the volume is 33.1 × 106 m3, the flooded river course length is 12 km, the maximum depth is 45 m and the mean depth is 16 m. The average water retention time varies from 80 to 180 days (Seďa & Kubečka, 1997). The reservoir is dimictic with a thermocline depth of about 5 m in summer. The oxygen concentrations vary between 7 and 9 mg l−1 in epilimnion and are lower than 4 mg l−1 under the thermocline (Draštík et al., 2008). The trophic status of the reservoir is moderately eutrophic. The shoreline of the reservoir is composed of rubble slopes (55%), beaches (23%), rocks (14%) and stumps (8%, Kratochvíl et al., 2012). Terrestrial vegetation is sporadically flooded in years with high water levels. The reservoir is in a stable cyprinid phase with common bream (Abramis brama), roach and bleak (Alburnus alburnus) dominating age-1 and older fish community (Říha et al., 2009, 2012).
Data collection
General sampling schedule
Age-0 fish were sampled every mid-August in both pelagic and littoral habitats of the Římov Reservoir between 1999 and 2010 (sampling in 2002 was not conducted because of an unusually high flooding event). Sampling in the pelagic area was performed using a fry trawl during the night while fry beach seining was used in the littoral habitat during both day and night. The trawling during the day was tested and found to be inefficient (Jůza & Kubečka, 2007). Considering different species-specific distribution of age-0 fish along the longitudinal profile of the reservoir (Jůza et al., 2009), six localities were determined (Fig. 1) in order to compare species composition and abundance exactly at the same locality of the reservoir.
Detailed description of sampling in the littoral and pelagic habitats
For age-0 fish sampling in littoral habitats a 10 × 2-m fry beach seine with a 1.7-mm mesh size was deployed in areas with sandy, gravel beaches, which are characterized by the highest abundance and species diversity in August (Kratochvíl et al., 2012). The seine net was set in parallel (by its entire length) to the shoreline at a distance of 10 m from the bank and was subsequently drawn to the shore by means of ropes attached on the net ends. Since the slope of the beach banks was approximately 10°, the 2-m depth required to accommodate the height of the net was 10 m from the shore. The water area sampled by each haul was, therefore, approximately 100 m2 and the mean depth of water column sampled by the 2-m high net was 1 m; therefore, the abundance of fish captured was expressed as catch-per-unit of effort (CPUE)—catch per 100 m3 of water area. Three parallel hauls were usually performed at each locality in the particular year.
The pelagic habitat was sampled by a 3 × 3-m fixed-frame fry trawl, which is a quantitative method for pelagic fry night sampling (Jůza & Kubečka, 2007). The trawl body was 10.5-m long and the mesh size was 6.5 mm in the main belly and 4 mm in the cod end (for details see Jůza & Kubečka, 2007). The trawl was towed by either a flat-bottomed boat powered by a 25-hp outboard engine in 1999, 2000, and 2001, or by the research vessel, Ota Oliva (64-hp diesel engine, Kubečka et al., 2003), from 2003 to 2010, usually for 10 min approximately 100 m behind the boat at the speed of 1 ms−1. The CPUE of the trawl was expressed as catch per 100 m3 of water sampled. Sampling was focused especially on the surface water layer (0–3 m) with the highest density of the pelagic age-0 fish (Vašek et al., 2006; Jůza et al., 2009). The deeper water layer (3–6 m) can be characterized by strong percid fry dominance (Jůza et al., 2009), and, therefore, this layer was also sampled in middle and dam parts of the reservoir with sufficient depth (localities 3, 4, 5 and 6). Usually one tow was done in each reservoir locality in particular years and particular depth layers (Fig. 1).
Samples obtained by both sampling techniques were immediately anaesthetized, and subsequently preserved in 4% formaldehyde solution. In the laboratory, fish were identified, counted, and measured for standard length (SL) to the nearest mm.
Statistical analysis
The nonparametric Kruskall–Wallis test (Statistica software, version 10) was performed with the year of sampling entered as the independent variable and CPUE entered as the dependent variable to test differences of CPUE reached by both sampling methods in different years. This was done because our data were not normally distributed. To compare the body lengths of individual fish species, which simultaneously occurred in littoral and pelagic habitats at night, the nonparametric Mann–Whitney U test was used with affiliation to habitat entered as the independent variable and body length entered as the dependent variable. CPUEs of bleak (the only species indicating clear diurnal horizontal migration) reached in different years in pelagic habitats at night and in littoral habitats during the day were correlated by multiple regression (Statistica software, version 10).
Direct gradient redundancy analyses (RDA; Canoco for Windows 4.5 software; Lepš & Šmilauer, 2003) were used to evaluate (1) differences in species composition reached in different years by both sampling techniques and to investigate the preference of different age-0 fish species to (2) pelagic or littoral habitats during (3) day and night. Numbers of fish caught by each individual seine or tow (from 0 to 3 m depth only) were entered into the analyses. During the analyses, scaling was focused on inter-species correlations, species scores were divided by standard deviation and samples were standardized by norm. The tested environmental variables, habitat (pelagic, littoral) and diel period (day, night), were assigned as dummy variables in these analyses. To compare the between-years species composition the influence of year (1999–2009) was tested. To show the affinity of different species to pelagic and littoral habitats during day and night, the untested environmental variables, year (1999–2010) and locality (1–6), were used as covariates because we did not want to interpret them and simultaneously take their effect into account. Statistical significances of all analyses were tested by Monte Carlo permutation tests (999 permutations). The effects of habitat (pelagic–littoral) and diel period (day–night) were tested separately. In all statistical comparisons the level of significance was set to α = 0.05.
To quantify the preference of particular age-0 fish species to the pelagic habitat at night (for 0–3- or 3–6-m-depth layer) or to unstructured littoral habitat (during day or night) and to demonstrate the between-years stability of this preference, the percentage proportion of dominant species in each habitat was expressed for each year (we did not perform any sophisticated statistical test to prove the stability because the differences between years could be caused by different proportions of some species in preferred habitats during day and night). For this purpose the total number of age-0 fish of different species was estimated for all the habitats investigated each year (based on the reached CPUE). The water volumes of the particular layers of littoral (0–2 m) and pelagic (0–3 and 3–6 m) habitats were calculated for each locality from the digital three-dimensional bathymetric model in ArcMap 10.0. (ESRI Inc. 2010, see Table 1 for the ratios). Using this method we obtained total age-0 fish numbers in both pelagic habitats during night and in unstructured littoral habitat during day and night (the sum is not an estimate of total abundance of a species in reservoir because both day and night littoral samples are included in this number). The proportion of different species in different habitats was expressed in percent. For all species this comparison was omitted for the year 2000, because of low fry density. For cyprinids the year 2010 was also omitted because of extreme density and extraordinary size distribution of cyprinid fry, causing an unusual spatio-temporal distribution (see in the “Discussion” section).
Results
Comparison of species composition and CPUE of age-0 fish between years
In total 50,731 age-0 fish were captured in the Římov Reservoir (8,237 individuals by trawling and 42,494 individuals by seining). Species composition of the age-0 fish community significantly differed between years in both habitats sampled (Table 2). Trawl catches (0–3 m) were alternately dominated by roach, bream or bleak, whereas roach, ruffe or perch were alternately abundant at night, and roach or bleak during the day, in seine catches.
The age-0 fish abundance (CPUE) significantly differed between years in all four spatio-temporal habitats (Table 3).
Diurnal habitat preference of different fish species
The Monte Carlo permutation test revealed that habitat type had a significant effect on the species composition of age-0 fish (P = 0.001) and the same significant effect was also revealed for the diel period (P = 0.001, Fig. 2). Habitat type and diel period together accounted for 14.6% of the explained variability in species composition data. Higher variability was explained by the first axis (pelagic–littoral, 9%) than by the second axis (day–night, 5.6%).
Species mostly found in the pelagic habitat
RDA analysis revealed age-0 bream to be the first species with a strong affinity for the pelagic habitat at night (Fig. 2). After recalculating the direct catch for the total volume of sampled habitats, on average 84% of the bream captured during the study were noticed in the surface pelagic habitat at night (Table 4). The trend with the dominance of bream in surface pelagic habitats at night was similar for all years except 2009, when the lowest number of age-0 bream was estimated in the reservoir (Table 4). The mean bream CPUE in pelagic habitat at night over all years sampled in surface layer was 1.1 ind./100 m3; however, the mean CPUE reached in littoral habitats was very low (within the frame of littoral catches) both during day and night (day: CPUE of seine—4.6 ind./100 m3; night: CPUE of seine—3.2 ind./100 m3; Fig. 3). The only exception was the year 2010, when extremely small bream was abundant in pelagic habitat at night and in littoral habitat both during day and night (Table 5; Fig. 3).
Pikeperch (Sander lucioperca) was also usually found to prefer the pelagic habitat during the night (Fig. 2). This preference was not evident in 2001, 2003 and 2005 when the majority of age-0 pikeperch occupied littoral habitat during the night (Table 4). However, it should also be noted that the CPUE was very low in this habitat relative to other species (pikeperch fry was almost missing in these years, Fig. 4). After recalculating the direct catch for the total volume of sampled habitats, on average 80% of the pikeperch captured during the study were noticed in pelagic habitats (0–3 and 3–6 m layers, Table 4). The occurrence of pikeperch in the littoral habitat was relatively low at night in comparison to other species (mean CPUE of seine—1.2 ind./100 m2) or completely missing during the day (Fig. 4). The highest littoral CPUE of pikeperch was found at night in 2004, which is in agreement with the highest CPUE in pelagic habitats at night that year (Fig. 4).
Species likely performing diel horizontal migrations
The bleak arrow was perpendicular to the first (pelagic–littoral) axis, which means nearly no preference for pelagic or littoral habitats (Fig. 2). After recalculating the direct catch for the total volume of sampled habitats, on average 71% of bleak captured during the study were noticed in the littoral habitat during the day (Table 4). During the night bleak was virtually missing in the littoral habitat (only 6% of bleak estimated on basis of CPUE were noticed in this habitat during the night). In surface pelagic habitats (0–3 m) at night, on average, 21% of bleak captured during this study were noticed. The mean littoral CPUE during the day was 7.7 ind./100 m3; however, at night it was 0.6 ind/100 m3 only. The common pattern of age-0 bleak distribution was invalid in 2010, when CPUE of bleak fry was extraordinarily high in all habitats sampled (Fig. 5). When bleak is numerous in the reservoir, it appears that it is abundant in littoral areas during day and in the pelagic areas at night (Fig. 5) suggesting offshore horizontal migration at night. The diurnal horizontal migration of bleak was also indicated by strong correlation between night-time CPUE in pelagic habitats and daytime CPUE in littoral habitats (all years pooled together, P = 0.006, R 2 = 0.68). The night-time offshore migration was not proven in 1999, 2004 and 2009, when bleak disappeared from littoral habitat but did not move to pelagic habitats.
Species detected in littoral habitats both during day and night and likely performing a partial migration to pelagic habitats at night
Roach was the only species whose occurrence was not influenced by habitat or diel period (short arrow indicating any correlation; Fig. 2). After recalculating direct catch for the total volume of sampled habitats, on average 40% of roach captured during this study occupied surface pelagic habitats (0–3 m) during the night, 35% occupied the littoral habitat during the day and 22% occupied the littoral habitat during the night (Table 4). In the deeper pelagic layer (3–6 m) occurrence of roach was rare (2% of all roach captured). This pattern of occurrence of age-0 roach in littoral habitats during both day and night and in the surface pelagic layer at night was consistent between years. In 1999, the CPUE of roach from littoral habitats during day and night was similar to other seasons, while the CPUE of roach in the pelagic habitats during the night was extremely high (Fig. 6). Roach was found to simultaneously inhabit the pelagic and littoral habitats during the night (Fig. 6) and so we compared the body lengths of roach in both habitats at night and also in the littoral habitat during day. Table 5 shows that in nearly all years, when roach was one of the dominant species and there were enough data for statistical comparisons, the body length was not significantly different in both habitats and during day and night. The only significant differences were found in body lengths of roach between pelagic habitats and littoral habitats at night in 2003 and between pelagic habitats at night and littoral habitats during the day in 2010 (Table 5).
Species mostly detected in the littoral habitat
Perch was found to have a strong affinity to the littoral habitat but the ordination diagram showed no correlation with the day–night axis (Fig. 2). During the years sampled, perch showed regular occurrence in the littoral habitat during day and night in addition to the night-time presence in pelagic areas in some years (Fig. 7). After recalculating direct catch for the total volume of sampled habitats, on average 40% of perch captured during this study occupied littoral habitats at night and 18% of them occupied littoral habitats during the day. In pelagic habitats perch preferred the deeper layer (3–6 m) and on average 34% of captured perch occupied this habitat at night (Table 4). Since in some years perch occurred simultaneously in littoral and pelagic habitats at night, body lengths of fish between pelagic and littoral habitats and also between day and night were compared. In all years in which perch was abundant in pelagic habitats during the night, this pelagic perch was significantly smaller in comparison with perch inhabiting the littoral habitat both during day and night (Table 5). The body lengths of perch in the littoral habitat between day and night were similar without significant differences (Table 5).
Less important, but relatively common species like asp (Leuciscus aspius), gudgeon (Gobio gobio), dace (Leuciscus leuciscus) and chub (Squalius cephalus) were revealed to be rigorously littoral species by RDA analysis (Fig. 2). They were more abundant especially in day littoral samples. Only a few individuals of gudgeon and dace were captured in pelagic habitats at night.
Species detected in both habitats only at night
Ruffe (Gymnocephalus cernuus) was found to have no correlation with the pelagic–littoral axis but strong correlation with night (Fig. 2). Ruffe strongly preferred littoral areas during the night but during the day it’s catch was very rare in this habitat (Fig. 8). In some years, ruffe was also relatively abundant in pelagic habitat at night. After recalculating direct catch for the total volume of sampled habitats, on average 76% of ruffe captured in this study occupied littoral habitats at night and 16% of them occupied the surface pelagic layer at night (Table 4). Dominance of ruffe in littoral habitats at night was consistent between all years sampled (Table 4). Ruffe inhabiting pelagic habitats were significantly smaller than ruffe in littoral habitats in years with simultaneous occurrence of ruffe fry in both habitats at night (Table 5).
Discussion
This study demonstrated how variable the age-0 fish community can be within a reservoir between years and diel periods. Despite this chaos, relatively stable species-specific patterns of spatio-temporal distribution were proved.
Earlier studies conducted in the Římov Reservoir failed to find any correlation between recruitment of different fish species and basic biotic and abiotic parameters (water temperature, water level and zooplankton density) in different years (Jůza et al., 2009). It is extremely difficult to discern factors behind fish recruitment. Thus, a scarcely identifiable complex of biological and environmental characteristics may influence fish density at the end of the first growing season.
During the eleven years of observation, we found much higher densities of age-0 roach and perch in 1999 and bleak and bream in 2010 than in the interim years. While we do not have explanation for the high density of fry in 1999 (Jůza et al., 2009), the reason for high density in 2010 was likely the unusually high water level during spring and summer which flooded terrestrial vegetation and prolonged the spawning period (see bellow in “Discussion” section). Another explanation of high age-0 fish density in 2010 could be intensive trawling in summer 2009, when 17% of the pelagic biomass of especially adult bream was removed from reservoir (M. Říha, unpublished data). This reduction of adult fish could have opened the niche for age-0 fish in the following year.
For many lakes, habitat and resource partitioning are regarded as key factors in the coexistence of species within the entire ecosystem (Lobb & Orth, 1991). The preference of pelagic or littoral habitats by different adult fish species during day and night was studied in lakes and reservoirs (Kubečka, 1993; Muška et al., 2013) and also in rivers (Wolter & Freyhof, 2004) while similar detailed studies were also conducted for age-0 fish in rivers (Copp & Jurajda, 1993). Our 11-year study of both the important habitats indicated the diurnal horizontal distribution of age-0 fish in deep artificial reservoirs and revealed five basic and relatively stable patterns of species-specific spatio-temporal occurrence.
The first species is bream and we questioned where the fish were located during the day since its catch in unstructured littoral areas was sporadic and we did not expect its occurrence in pelagic habitats during the day (see further in “Discussion” section). The occurrence of age-0 bream in structured littoral areas was also extremely rare during point abundance sampling by electrofishing during the day (Kratochvíl et al., 2012). Fischer & Eckmann (1997) found age-0 bream inhabiting the shallowest littoral areas (0–0.5 m depth) during the day in spring but they occurred in the deepest littoral area (1.5–3 m depth) during the day in August. This shift is explained by the fact, that in contrast to juveniles of other cyprinids, juvenile bream become laterally compressed and high backed during their first summer. This change in body shape may lead to much greater susceptibility to turbulence. When the amount of energy spent on adjusting body position in shallow turbulent water increases, deeper area may be the best alternative habitat (Fischer & Eckmann, 1997). Since we only used a 2-m-high beach seine, it is probable that we did not sample the area with the highest bream density in deeper littoral areas. This may be the reason why we lacked the daytime occurrence of bream. The only year in which bream occurred in littoral habitats both during day and night to a greater extent was 2010. The year was characterized by extreme fry density due to unusually small bream (Table 5; Fig. 9a) coming from later spawning events, which is relatively common for cyprinids (Mackay & Mann, 1969; Kestemont et al., 2001). Abnormally high density of age-0 bream in combination with unusually small bream fry in 2010 is the reason for the unusual distribution pattern in this year and also for the occurrence of bream in shallow littoral habitats both during day and night.
Age-0 pikeperch was the second species found to have a strong affinity for the pelagic habitat during the night, and it was rarely found in shallow littoral areas at night. It was not detected in unstructured and also structured littoral during the day (Kratochvíl et al., 2012). The only years in which pikeperch avoided pelagic habitats and stayed in littoral habitats at night were not only in 2001 and 2005 but also in 2003 when the estimated number of age-0 pikeperch in reservoir was very low (Table 4). In the shallow Sulejów Reservoir in Poland two groups of age-0 pikeperch were distinguished (Frankiewicz et al., 1996). The first group inhabited littoral habitats during the night (piscivorous and rapidly growing) and the second group occupied pelagic habitats during the night (planktivorous and slowly growing). The SL of littoral piscivorous pikeperch was approximately 80 mm in August (Frankiewicz et al., 1996). In our study, no such large age-0 pikeperch were captured and their SL was 40–50 mm. Frankiewicz et al. (1996) also note that the length around 60 mm corresponds to when pikeperch switch from pelagic- to littoral-dwelling. In our study, pikeperch captured in the littoral habitats at night were larger (48 mm) than pikeperch captured in pelagic habitats (41 mm, mean of years with pikeperch occurrence in both habitats—2004, 2007, 2009, 2010). This comparison supports the hypothesis of size-dependent habitat use but according to our results, the growth of pikeperch in deep canyon-shaped reservoirs is slower in comparison with shallow reservoirs. Furthermore in August the body length is too small for the occurrence of piscivorous littoral pikeperch fry. This is also the reason for the scarce occurrence of pikeperch in littoral areas at night. Pikeperch completely avoided shallow littoral areas sampled by beach seining during the day. In shallow mixed reservoirs, age-0 pikeperch were observed to be resting very close to the bottom in the daytime during early ontogeny stages (Kratochvíl et al., 2010) and also later in the season (Frankiewicz et al., 1999). It is, therefore, very likely that the deeper pelagic zones (often close to the bottom in shallow reservoirs) with sufficient water temperature and oxygen concentration are the places where age-0 pikeperch spend the daytime in August before evening dispersion into upper pelagic. According to our day trawling with the 6 × 6 m trawl in 2008, pikeperch fry were sporadic but the only species captured (T. Jůza, unpublished data). Like other percid fry, small pikeperch may be part of the bathypelagic fry community creating shoals during the day and performing evening vertical migrations to layers above thermocline in August (Čech & Kubečka, 2006). During the night pikeperch fry is also homogeneously distributed in pelagic and, therefore, more accessible for trawling.
The second group includes species performing (based on our sampling schedule) diel horizontal migrations occupying shallow littoral habitats during the day and migrating to pelagic habitats during twilight to remain there for the night. The only species in the fry community of the Římov Reservoir fitting into this group was bleak. In almost all years, in which bleak was an important component of the fry community, it was very abundant in pelagic areas but was virtually missing in shallow littoral areas during the night. Density of bleak in littoral during the day was many times higher than during the night. The only years in which bleak did not migrate to pelagic habitats at night were 1999 and 2004, when extremely low numbers of bleak were estimated in the reservoir and we did not manage to catch any by trawling. The only year with high estimated numbers of bleak without offshore migration was 2009; however, we do not have any explanation for this exceptional occurrence. Age-0 bleak was also found to be a typical night-time epipelagic species by using gillnets in the Římov Reservoir (Prchalová et al., 2009) and it was the dominant species in littoral beach seine day catches in the Mušov Reservoir in the Czech Republic (Jurajda & Regenda, 2004). As in the case of bream, an unusually high density of bleak resulted in an extraordinary spatio-temporal distribution of this species in 2010. In this year, bleak was present in littoral areas both during day and night and was also very abundant in pelagic areas at night. In 2010 multiple spawning events resulted in a wide size range of age-0 bleak (9–48 mm, Fig. 9b), when most of them were smaller than their expected size in August (SL <20 mm, Table 5). The extremely high density of bleak and its associated length distribution shifted towards smaller sized fry is likely the reason for the unusual spatial distribution with high night abundance of bleak in littoral areas in 2010.
The only species without a sharply defined spatio-temporal horizontal distribution was roach representing thus the third group of ubiquitous species. According to our results, roach did not show clear affinity to either pelagic or littoral habitats during the night utilizing littoral habitats both during day and night in almost all years investigated. On contrary age-0 roach was often found to be a common species performing diel horizontal migrations in lakes (Bohl, 1980; Gliwicz & Jachner, 1992), but considering our observations, the pelagic night-time migrations of roach were not as well pronounced as in the case of bleak and these shifts were only partial. Gliwicz et al. (2006) investigated the night-time pelagic occurrence of roach and discovered that all roach did not move far from the littoral areas each night and in small stratified lakes juvenile roach was caught in both pelagic and littoral areas over a 24-h period (Järvalt et al., 2005). No significant differences in body length of pelagic and littoral roach meant that roach migrating to pelagic areas at dusk or staying in the littoral at night were not size-segregated. The partial migration of roach can be considered as a result of two condition-dependent alternative strategies: either stay in the original habitat or migrate to an alternative habitat for a limited period of time (Brodersen et al., 2008). It was discovered for roach that individuals in better condition migrated from the lake to connected streams with lower food supply, but lower predation risk in winter, whereas individuals in weaker condition did not migrate, staying in the lake with higher food supply but higher predation risk (Brodersen et al., 2008). According to this theory, age-0 roach in better condition should stay in safer but less profitable littoral areas at night and fish in worse condition should migrate to more profitable pelagic habitat even with a higher risk of predation. Future research is necessary to discern if physical condition is responsible for age-0 roach’s habitat partitioning or if there are some other mechanisms influencing it. Also the ubiquity of roach in the reservoir demonstrates the high plasticity of this species, which is the most common cyprinid in most Europe (Kottelat & Freyhof, 2007).
The fourth group includes species with a strong affinity to littoral habitats and whose occurrence in pelagic habitats was infrequent or rare. Perch was the species that preferred littoral habitats during both day and night and was found in pelagic habitats in some years. It’s density in littoral areas was usually higher at night which corresponds with the results of Lewin et al. (2004). Increased estimated numbers of perch in pelagic habitats at night were found in 1999, 2001, 2005, 2007 and 2010. Higher numbers of perch were estimated (with regards to the density reached by trawl) in the deeper pelagic layer (3–6 m) more often than in the surface layer, which is in agreement with pelagic distribution of percids in the Římov Reservoir (Jůza et al., 2009). The predominance of age-0 perch in littoral areas is the result of the timing of the study as it coincides with the peak of age-0 perch of littoral phase in August. After spring hatching in the littoral habitat the perch larvae move to the pelagic areas and remain there for 1–2 months before returning back to the littoral (Treasurer, 1988). By the end of July perch juveniles could only be caught in littoral habitats in deep Lake Constance and there was no evidence for offshore migration at night (Wang & Eckmann, 1994). Age-0 perch left the littoral zone definitely and moved into deep waters when autumnal mixing occurred in late October (Wang & Eckmann, 1994). Comparison of body sizes revealed that perch occupying pelagic habitat during the night were significantly smaller than perch in the littoral zone both during day and night. Since perch migration from pelagic to littoral habitats is size-dependent (Wang & Eckmann, 1994), it is very likely that perch found in pelagic habitats during the night in some years were the smallest individuals and had not undergone the shift to littoral yet. Using hydroacoustics perch fry were recorded forming shoals in the bathypelagic layers of the Římov Reservoir during the day in August (Čech & Kubečka, 2006) but in the evening these shoals disintegrated and migrated to the surface water layers (M. Čech pers. com.). Daytime perch shoals are not accessible for trawling so the pelagic habitat appears empty during the day. It is, therefore, evident that two spatially segregated groups of perch can occur in late summer when large perch fry use littoral habitats and smaller perch fry use pelagic habitats during both day and night performing diurnal vertical migrations.
The affinity of typically rheophilic species to shallow littoral zones is not surprising. Age-0 asp, chub and gudgeon were captured in littoral habitats in larger numbers during the day. For age-0 dace the affinity to littoral areas particularly in day time was also observed and only a few individuals of gudgeon and dace were captured in pelagic habitat at night. In Lake Constance chub and dace juveniles also preferred shallow (<50 cm) littoral habitats during the day (Fischer & Eckmann, 1997) and juvenile asp was the dominant species in day beach seine catches in the lowland reservoir system of Nové Mlýny (Jurajda et al., 1997). According to our results age-0 dace, chub, asp and gudgeon are species with strong affinities to littoral habitats and were more numerous during the day. Their night-time migration to pelagic habitat was not found during our study.
The fifth behavioural pattern includes species with exclusive occurrence in both habitats at night. Ruffe regularly occurred in shallow littoral habitats during the night and also in pelagic habitats at night. During the daytime ruffe was practically undetectable by our sampling techniques. Using electrofishing and trammel nets age-0 ruffe was found to prefer the deepest littoral habitat between 1.5 and 3 m depth during the day (Fischer & Eckmann, 1997) and in the Římov Reservoir ruffe were observed lying on the bottom in depths of about 10 m by scuba divers (M. Říha pers. com.). It is, therefore, probable that ruffe was inaccessible by seining used in the shallow littoral areas during the day. In 2005, 2009 and 2010, ruffe was relatively abundant in pelagic habitats at night and these fish were significantly smaller than those caught in littoral habitats during the same period. Since ruffe, as well as perch, undergo the obligatory pelagic phase during early ontogeny (Čech et al., 2005) and its return back to littoral habitat is size-dependent (Matěna, 1995), it is likely that the age-0 ruffe cohort did not shift completely to the littoral habitat in these years of sampling.
Pelagic and littoral habitats are two contrasting zones in lentic freshwater systems. While vertical gradients of light, temperature and other factors are the main structuring forces in pelagic habitats, littoral habitats are characterized by a high structural complexity (Hölker et al., 2002). The complexity, which is connected with the protection against visually hunting predators, is the reason why littoral habitats are strongly preferred by age-0 fish (Bohl, 1980; Gliwicz & Jachner, 1992) and also by older individuals of tiny species (Phoxinus eos, Naud & Magnan, 1988) during the day. To investigate the horizontal distribution of different age-0 fish species in the Římov Reservoir, the littoral habitat was sampled both during day and night but the pelagic habitat was sampled by trawl during the night only. The reason why we did not use trawling during the day was because we were able to catch very few age-0 fish in the pelagic habitat during the day although we used a trawl that was four times larger (6 × 6 m) trawl (T. Jůza, unpublished data). Even in 1999, when the age-0 fish density was extremely high in the Římov Reservoir the catch of age-0 fish in pelagic habitats during the day was a very rare event (Vašek et al., 2006). Using a 15 × 8 m adult trawl in Římov Reservoir, age-0 fish were a regular component of the catch during the night, while fish smaller than 90 mm were completely missing during the day (Říha et al., pers.com.). The failure of day trawling can be attributed to better avoidance of trawls during the day because fish can see it (North & Murray, 1992) or it could be caused by the aggregated distribution of age-0 fish during daytime compared to a more even distribution at night (Masson et al., 2001) and lower probability to hit the aggregation. Alternatively, pelagic habitats of lakes remain practically unoccupied by small fish during the day (Bohl, 1980; Gliwicz & Jachner, 1992) and with regards to the inability of small and large trawls to catch age-0 fish during the day this was also the case in the Římov Reservoir.
Another debatable fact connected with inshore sampling is that we only sampled the unstructured littoral habitats (beaches) using beach seine and, therefore, species inhabiting the structured littoral areas were beyond our scope. According to the results of Kratochvíl et al. (2012) beaches are habitats with the highest abundance and species diversity in August in the Římov Reservoir. Rubble slopes and rocks were found to be strongly preferred by perch and shorelines with tree stumps were dominated by perch and roach. The only inaccuracy we have done by sampling in unstructured littoral areas only is that we have likely significantly underestimated the occurrence of littoral perch because this species was found to have a strong affinity to structured littoral areas (especially rubble slopes, Kratochvíl et al., 2012). The shoreline character has, beyond all doubt, a strong influence on diurnal fish distribution. The gentle sloped beaches make up only 23% of shoreline length in the Římov Reservoir and the majority of its shoreline constitutes rubble slopes (Kratochvíl et al., 2012). These steep and structured areas, which are extremely difficult to be sampled, probably represents the ideal day refuges for species present in pelagic areas at night and usually rare in shallow unstructured littoral areas during the day (bream and pikeperch).
Our results also clearly showed that although age-0 fish density is usually relatively low in pelagic habitats at night, the large total volume makes it to have the same importance as littoral habitats (Table 4). It is, therefore, very important to sample both habitats for an accurate assessment of age-0 fish communities in lakes and reservoirs although the pelagic age-0 fish density can appear negligible in most years and reservoirs according to relative catches. When a specific species is targeted, this study provides valuable information about the timing of sampling and type of habitat that should be sampled for its accurate assessment using common fry sampling techniques.
References
Anderson, M. R., S. J. Fisher & D. W. Willis, 1998. Relationship between larval and juvenile yellow perch abundance in Eastern South Dakota glacial lakes. North American Journal of Fisheries Management 18: 989–991.
Bohl, E., 1980. Diel pattern of pelagic distribution and feeding in planktivorous fish. Oecologia 44: 368–375.
Borcherding, J., M. Bauerfeld, D. Hintzen & D. Neumann, 2002. Lateral migrations of fishes between floodplain lakes and their drainage channels at the Lower Rhine: diel and seasonal aspects. Journal of Fish Biology 61: 1154–1170.
Bremigan, M. T. & R. A. Stein, 1997. Experimental assessment of the influence of zooplankton size and density on Gizzard Shad recruitment. Transactions of the American Fisheries Society 126: 622–637.
Brodersen, J., P. A. Nilsson, L. Hanson, C. Skov & C. Brönmark, 2008. Condition-dependent individual decision-making determines cyprinid partial migration. Ecology 89: 1195–1200.
Copp, G. H. & P. Jurajda, 1993. Do small riverine fish move inshore at night? Journal of Fish Biology 43: 229–241.
Čech, M. & J. Kubečka, 2006. Ontogenetic changes in the bathypelagic distribution of European perch fry Perca fluviatilis monitored by hydroacoustic methods. Biologia Bratislava 61: 211–219.
Čech, M., M. Kratochvíl, J. Kubečka, V. Draštík & J. Matěna, 2005. Diel vertical migrations of bathypelagic perch fry. Journal of Fish Biology 66: 685–702.
Draštík, V., J. Kubečka, M. Tušer, M. Čech, J. Frouzová, O. Jarolím & M. Prchalová, 2008. The effect of hydropower on fish stock: comparison between cascade and non-cascade reservoirs. Hydrobiologia 609: 25–36.
Fischer, P. & R. Eckmann, 1997. Spatial distribution of littoral fish species in a large European lake, Lake Constance, Germany. Archiv für Hydrobiologie 140: 91–116.
Frankiewicz, P., K. Dabrowski & M. Zalewski, 1996. Mechanism of establishing bimodality in a size distribution of age-0 pikeperch, Stizostedion lucioperca (L.) in the Sulejów Reservoir, Central Poland. Annales Zoologici Fennici 33: 321–327.
Frankiewicz, P., K. Dabrowski, A. Martyniak & M. Zalewski, 1999. Cannibalism as a regulatory force of pikeperch, Stizostedion lucioperca (L.), population dynamics in the lowland Sulejow Reservoir (Central Poland). Hydrobiologia 408/409: 47–55.
Gliwicz, Z. M. & A. Jachner, 1992. Diel migrations of juvenile fish—a ghost of predation past or present. Archiv für Hydrobiologie 124: 385–410.
Gliwicz, Z. M., J. Slon & I. Szynkarczyk, 2006. Trading safety for food: evidence from gut contents in roach and bleak captured at different distances offshore from their daytime littoral refuge. Freshwater Biology 51: 823–839.
Grenouillet, G. B., G. A. Hugueny, J. Carrel, M. Olivier & D. Pont, 2001. Large-scale synchrony and inter-annual variability in roach recruitment in the Rhone River: the relative role of climatic factors and density-dependent process. Freshwater Biology 46: 11–26.
Hölker, F., S. S. Haertel, S. Steiner & T. Mehner, 2002. Effects of piscivore-mediated habitat use on growth, diet and zooplankton consumption of roach: an individual-based modelling approach. Freshwater Biology 47: 2345–2358.
Imbrock, F., A. Appenzeller & R. Eckmann, 1996. Diel and seasonal distribution of perch in Lake Constance: a hydroacoustic study and in situ observations. Journal of Fish Biology 49: 1–13.
Irwin, B. J., L. G. Rudstam, J. R. Jackson, A. J. VanDeValk, J. L. Forney & D. G. Fitzgerald, 2009. Depensatory mortality, density-dependent growth, and delayed compensation: disentangling the interplay of mortality, growth, and density during early life stages of yellow perch. Transactions of the American Fisheries Society 138: 99–110.
Jacobsen, L. & M. R. Perrow, 1998. Predation risk from piscivorous fish influencing the diel use of macrophytes by planktivorous fish in experimental ponds. Ecology of Freshwater Fish 7: 78–86.
Järvalt, A., T. Krause & A. Palm, 2005. Diel migration and spatial distribution of fish in a small stratified lake. Hydrobiologia 547: 197–203.
Jurajda, P. & J. Regenda, 2004. Littoral 0+ fish assemblages in three reservoirs of the Nové Mlýny dam (Czech Republic). Czech Journal of Animal Science 49: 450–457.
Jurajda, P., M. Reichard & E. Hohausová, 1997. A survey of inshore 0+ juvenile fish community in the Nové Mlýny lowland reservoir, Czech Republic. Folia Zoologica 46: 279–285.
Jůza, T. & J. Kubečka, 2007. The efficiency of three fry trawls for sampling the freshwater pelagic fry community. Fisheries Research 85: 285–290.
Jůza, T., M. Vašek, J. Kubečka, J. Seďa, J. Matěna, M. Prchalová, J. Peterka, M. Říha, O. Jarolím, M. Tušer, M. Kratochvíl, M. Čech, V. Draštík, J. Frouzová, E. Hohausová & J. Žaloudík, 2009. Pelagic underyearling communities in canyon-shaped reservoir in late summer. Journal of Limnology 68: 304–314.
Kestemont, P., J. Rinchard, F. Damoiseau & M. Tans, 2001. Seasonal variation in egg and larval quality in two multiple-spawner cyprinid fish, the bleak (Alburnus alburnus) and the white bream (Blicca bjoerkna). Archiv für Hydrobiologie 135(Suppl (Large rivers 12, No. 2–4)): 357–371.
Kottelat, M. & J. Freyhof, 2007. Handbook of European Freshwater Fishes. Kottelat, Cornol, Switzerland and Feyhof, Berlin: 646 pp.
Kratochvíl, M., M. Čech, M. Vašek, J. Kubečka, J. Hejzlar, J. Matěna, J. Peterka, J. Macháček & J. Seďa, 2010. Diel vertical migrations of age 0+ percids in a shallow, well-mixed reservoir. Journal of Limnology 69: 305–310.
Kratochvíl, M., T. Mrkvička, M. Vašek, J. Peterka, M. Čech, V. Draštík, T. Jůza, J. Matěna, M. Muška, J. Seďa, P. Znachor & J. Kubečka, 2012. Littoral age 0+ fish distribution in relation to multi-scale spatial heterogeneity of a deep-valley reservoir. Hydrobiologia 696: 185–198.
Kubečka, J., 1993. Night inshore migration and capture of adult fish by shore seining. Aquaculture Research 24: 685–689.
Kubečka, J., J. Matěna & J. Peterka, 2003. Vzorkování rybích obsádek volné vody údolních nádrží. Water Management 10: 273–275.
Lappalainen, J. & H. Lehtonen, 1995. Year-class strength of pikeperch (Stizostedion lucioperca L.) in relation to environmental factors in a shallow Baltic Bay. Annales Zoologici Fennici 32: 411–419.
Lepš, J. & P. Šmilauer, 2003. Multivariate Analysis of Ecological Data Using CANOCO. Cambridge University Press, Cambridge: 282 pp.
Lewin, W. C., N. Okun & T. Mehner, 2004. Determinants of the distribution of juvenile fish in the littoral area of a shallow lake. Freshwater Biology 49: 410–424.
Lobb, M. D. & D. J. Orth, 1991. Habitat use by an assemblage of fish in a large warmwater stream. Transactions of the American Fisheries Society 120: 65–78.
Mackay, I. & K. H. Mann, 1969. Fecundity of two cyprinid fishes in the River Thames, Reading, England. Journal of Fisheries Research Board of Canada 26: 2795–2805.
Masson, S. N., J. Angeli, J. Guillard & B. Pinel-Alloul, 2001. Diel vertical and horizontal distribution of crustacean zooplankton and young of the year fish in a sub-alpine lake: an approach based on high frequency sampling. Journal of Plankton Research 23: 1041–1060.
Mehner, T., P. Kasprzak & F. Hölker, 2007. Exploring ultimate hypotheses to predict diel vertical migrations in coregonid fish. Canadian Journal of Fisheries and Aquatic Sciences 64: 874–886.
Matěna, J., 1995. Ichthyoplankton and 0+ pelagic fish in the Římov Reservoir (Southern Bohemia). Folia Zoologica 44: 31–43.
Muška, M., M. Tušer, J. Frouzová, V. Draštík, M. Čech, T. Jůza, M. Kratochvíl, T. Mrkvička, J. Peterka, M. Prchalová, M. Říha, M. Vašek & J. Kubečka, 2013. To migrate, or not to migrate: partial diel horizontal migration of fish in a temperate freshwater reservoir. Hydrobiologia 707: 17–28.
Naud, M. & P. Magnan, 1988. Diel onshore-offshore migrations in northern redbelly dace Phoxinus eos (Cope), in relation to prey distribution in a small oligotrophic lake. Canadian Journal of Zoology 66: 1249–1253.
Neuman, E., E. Roseman & H. Lehtonen, 1996. Determination of year-class strength in percid fishes. Annales Zoologici Fennici 33: 315–318.
North, A. W. & A. W. A. Murray, 1992. Abundance and diurnal vertical distribution of fish larvae in early spring and summer in fjord at South Georgia. Antarctic Science 4: 405–412.
Okun, N., R. Mendonca & T. Mehner, 2005. Diel shifts in community composition and feeding of juvenile fishes in the pelagic area of a large shallow lake. Limnologica 35: 70–77.
Prchalová, M., J. Kubečka, M. Čech, J. Frouzová, V. Draštík, E. Hohausová, T. Jůza, M. Kratochvíl, J. Matěna, J. Peterka, M. Říha, M. Tušer & M. Vašek, 2009. The effect of depth, distance from dam and habitat on spatial distribution of fish in an artificial reservoir. Ecology of Freshwater Fish 18: 247–260.
Quist, M. C., C. S. Guy, R. J. Bernot & J. L. Stephen, 2004. Factors related to growth and survival of larval walleyes: implications for recruitment in a southern Great Plains reservoir. Fisheries Research 67: 215–225.
Říha, M., J. Kubečka, M. Vašek, J. Seďa, T. Mrkvička, M. Prchalová, J. Matěna, M. Hladík, M. Čech, V. Draštík, J. Frouzová, E. Hohausová, O. Jarolím, T. Jůza, M. Kratochvíl, J. Peterka & M. Tušer, 2009. Long-term development of fish populations in the Římov Reservoir. Fisheries Management and Ecology 16: 121–129.
Říha, M., T. Jůza, M. Prchalová, T. Mrkvička, M. Čech, V. Draštík, M. Muška, M. Kratochvíl, J. Peterka, M. Tušer, M. Vašek & J. Kubečka, 2012. The size selectivity of the main body of a sampling pelagic trawl in freshwater reservoirs during the night. Fisheries Research 127–128: 56–60.
Seďa, J. & J. Kubečka, 1997. Long-term biomanipulation of Rimov Reservoir (Czech Republic). Hydrobiologia 345: 95–108.
Summerfelt, R. C. & K. E. Shirley, 1978. Enviromental correlates to year-class strength of largemouth bass in Lake Carl Blackwell. Proceedings of the Oklahoma Academy of Science 58: 54–63.
Treasurer, J. W., 1988. The distribution and growth of lacustrine 0+ perch, Perca fluviatilis. Environmental Biology of Fishes 21: 37–44.
Vašek, M., J. Kubečka, J. Matěna & J. Seďa, 2006. Distribution and diet of 0+ fish within a canyon-shaped European reservoir in late summer. International Review of Hydrobiology 91: 178–194.
Wang, N. & R. Eckmann, 1994. Distribution of perch (Perca fluviatilis L.) during their first year of life in Lake Constance. Hydrobiologia 277: 135–143.
Wolter, C. & J. Freyhof, 2004. Diel distribution patterns of fishes in a temperate large lowland river. Journal of Fish Biology 64: 632–642.
Acknowledgments
We would like to thank Zdeněk Prachař and other colleagues who helped us with the field work, Leslie Tse for English correction and anonymous reviewers for helpful suggestions. This study was supported by institutional support RVO: 60077344 and the project CZ.1.07/2.3.00/20.0204 (CEKOPOT) co-financed by the European Social Fund and the state budget of the Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: M. Power
Rights and permissions
About this article
Cite this article
Jůza, T., Vašek, M., Kratochvíl, M. et al. Chaos and stability of age-0 fish assemblages in a temperate deep reservoir: unpredictable success and stable habitat use. Hydrobiologia 724, 217–234 (2014). https://doi.org/10.1007/s10750-013-1735-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-013-1735-y



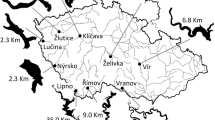

 —trawl tow;
—trawl tow;
 —beach seine)
—beach seine)






