Abstract
This research introduces a novel single-step approach to improve the absorbency under load (AUL) of agricultural superabsorbent polymers (SAPs) made of acrylamide. Crosslinked (acrylamide–potassium acrylate–acrylic acid) terpolymers were successfully prepared and modified via a transamidation (amide exchange) reaction. The surface of the polymer particles was treated using polyamine modifiers (i.e., diethylenetriamine and polyethyleneimine) in the presence or absence of AlCl3 as a catalyst. The modification reaction was confirmed via spectral, morphological, and rheological studies. The process variables including time and temperature, the modifier type and amount, and the catalyst concentration were found to affect the polymer swelling properties. The swelling capacity of the control and treated SAPs were determined in deionized water and saline. The AUL in saline, as a key swelling property of SAPs, was also determined. The AUL of the PEI-treated samples (19.82–24.8 g/g) was higher than those of the control (17.7 g/g) and the DETA-treated SAPs (18.3–23.5 g/g). In conclusion, the transamidation effectively improved the AUL of the terpolymer superabsorbents by about 25%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
I. INTRODUCTION
Superabsorbent polymers (SAPs) are lightly crosslinked hydrophilic polymers, which can absorb and retain large amounts of water and physiological solutions. These highly swellable hydrogels are primarily used in hygiene products and agriculture,1–3 and to a lesser extent are used for variety of other purposes including modification of industrial fibers.4
The absorbency under load (AUL) in saline, as one of the most critical properties of SAPs, reflects the actual performance and usability of SAPs in real service. In the case of SAPs used in hygienic products, the main approach to enhance AUL is by reacting (crosslinking) the functional groups, particularly carboxyl groups located at the surface of the SAP particles, with diglycidyl compounds.1 Although there are few reports on surface modification of hygienic SAPs,3–10 we have previously reported surface crosslinking of poly(sodium acrylate–acrylic acid) SAPs using cycloaliphatic epoxy,5 bisphenol A diglycidyl ether,7 diethyleneglycol diglycidyl ether,8 and divinylbenzene.9 Ma et al.11,12 reported a two-step approach for surface modification of SAPs, which includes treatment with ethylene glycol diglycidylether followed by treatment with KH2PO4 and NaAlO2.
Using surface modification, we induced antibacterial properties to poly(sodium acrylate–acrylic acid) SAPs by employing a simple ion-exchange process to achieve hygienic SAPs with greater swelling rate, lower gel-blockage, and greater salt resistance.13 We also utilized surface modification using benzyl chloride and 1,2-dichloroethane13,14 as well as epoxy silane8 to effectively reduce the gel-blocking and improve the AUL properties of our SAPs. Very recently, we used epoxy silane modifiers to enhance the AUL of hygienic SAPs, where the surface modification occurred via the reaction of epoxy groups with carboxyl groups that resulted in the formation of a polysiloxane network at the SAP surface.8
Acrylamide-based SAPs, such as crosslinked poly(sodium acrylate–acrylamide), have also been surface treated using an external crosslinker. In an inverse suspension reaction, hydrophobic divinyl benzene reported improving the swelling properties of the SAP in the presence of salts.9 Another hydrophobic crosslinker, bismethacryloyl azobenzene, has also been used for the same purpose.10 The SAPs treated using hydrophobic crosslinkers possessed a highly porous structure with a core-shell-like morphology, faster swelling rate, and lower soluble fraction.
To our knowledge,1–8,13–15 there is no report on transamidation as an approach to improve SAP properties. Transamidation, a process for transforming one amide to another amide, is conducted via amide–amide or amide–amine reactions. Different catalysts such as AlCl3,16 hydroxylamine hydrochloride,17 and L-proline18 have been used for this process. In agricultural SAPs based on acrylamide (∼60 mol%), the primary amide groups can react with aliphatic amines; therefore, we studied the effect of a small polyamine (i.e., diethylenetriamine) and a macromolecular one (i.e., branched polyethyleneimine) on SAP swelling properties.
II. EXPERIMENTAL
A. Materials
Crosslinked acrylamide–potassium acrylate–acrylic acid terpolymer (particle size 150–200 µm), SUPER AB A200, was purchased from NanoAb Iranian Co. (Tehran, Iran). According to the manufacturer, the SAP sample was not surface-modified, and its chemical composition was 60, 30, and 10 mol% of acrylamide, potassium acrylate, and acrylic acid, respectively. Anhydrous aluminum chloride (AlCl3, 98%), diethylenetriamine (DETA, 98%), sodium chloride (NaCl, 99.5%), and solvents were purchased from Merck (Darmstadt, Germany). Polyethyleneimine (PEI, branched) was purchased from Shenzhen Nexconn Pharmatechs Ltd., Shenzhen, China. All chemicals were used as received.
B. SAP surface treatment
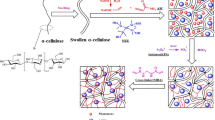
Surface modification was performed according to the previous reports after some appropriate modifications.5,6,16 In brief, SAP particles (5.0 g) were soaked in 20 mL of treatment solution (acetone/water, 90/10 v/v) containing polyamine modifiers (DETA or PEI) and AlCl3. After 30 min, the modified SAP particles were filtered and placed in an air-forced oven. The dried particles were placed in a desiccator. The effect of time and temperature of the surface treatment on free-swelling properties (g/g absorption capacity in either deionized water or in saline) and AUL in saline were investigated. Figure 1 shows the surface treatment process, sample codes, and the chemical structures of the polyamine crosslinking agents.
C. Characterization
The central chemistry of this research is to react primary amide groups of the SAP structure with amine groups of an external agent at the very surface (micron-sized) of the SAP particles. Therefore, ATR-FTIR (Specac, Golden-gate, U.K.) spectroscopy was used to confirm the surface treatment. The spectra were obtained at a wave number range of 600–4000 cm−1 and a resolution of 1 cm−1.
A scanning electron microscope (SEM; Vegall, Tescan Co, Brno, Czech Republic) equipped with an EDX detector (INCA model, Ofordinst) was used to perform a microstructural study on the SAP particles. The samples dried at 100 °C for 2 h were sputter coated with gold and imaged in the secondary electron mode with an accelerating voltage of 20 kV.
The rheological measurements were performed on the swollen samples (1.00 g dry SAP in 10.0 g distilled water) using a para-physical vibrational device (model MCR 300, Anton Paar Co., Ostfildern, Germany) at 25 °C, and no special trap system was used due to negligible dehydration. The plate diameter was 25 mm and the distance between the plates was set at 3 mm. The strains were selected to be in the linear viscoelastic range. The effect of shear strain on G′ at a constant frequency (1 rad/s) was investigated. Since G′ (ω) was independent of the strain at deformations below 0.2%, the strain was set at 0.2%. The frequency sweep test was carried out between 0.1 and 100 rad/s.19 Relative standard deviation (RSD) for the rheometric method was found about ±3–5%.6
Swelling capacity of the hydrogel samples was measured using a tea bag method. The SAP samples (0.20 g) were left in 100 mL of distilled water at room temperature until they reached their equilibrium swelling capacities. The swelling capacity was calculated according to the following equation:
where ws and wd are the weights of the swollen and dried gels, respectively.6QDW and QS stand for the swelling capacity in distilled water and saline (NaCl aqueous solution, 0.90 wt%), respectively.
To determine the AUL,19 0.5 g of a dried SAP sample was uniformly dispersed on the surface of a polyester gauze sited on a macroporous sintered glass filter plate in a Petri dish. A cylindrical solid load (Teflon, d = 60 mm) was placed on the dry SAP particles that could freely slip in a glass cylinder. The sample was loaded (0.3 psi) and saline was then added to the Petri dish. After 90 min, the swollen particles were removed, reweighed, and the AUL value was calculated using Eq. (1). Swelling measurements were repeated five times, and the RSD for QDW, QS, and, AUL were ±8, ±2, and ±4%, respectively.
III. RESULTS AND DISCUSSION
As outlined in Fig. 1, two different polyamines (i.e., DETA and PEI) were used for the surface treatment in the absence and presence of AlCl3 as a catalyst. The modified samples were then spectrally and morphologically characterized, and their swelling behavior was investigated.
Figure 2(a) shows the possible reaction between the amide groups of the SAP with the amine groups of the polyamine modifier.
A. Spectral study
Figure 3 shows the ATR-FTIR spectra of the intact and DETA-modified SAPs. With the intact SAP, the wide peak appeared at 3186–3300 cm−1 belongs to the stretching vibration of the N–H peak of the primary amide groups, overlapping with the carboxylic acid O–H groups. Moreover, the stretching vibration of the C=O carbonyl groups and the C–H groups are appeared at 1658 and 2945 cm−1, respectively. The peak at ∼1552 cm−1 is assigned to the N–H bending in all the samples.
In general, the amide I band is appeared at 1670–1650 cm−1 as a very strong band, for primary amides in the solid phase. Secondary amides have a characteristic, strong absorption at 1570–1515 cm−1.20 Primary amines have a medium-to-strong absorption band in the 1650–1580 cm−1 region, and secondary amines have a weaker band at 1580–1490 cm−1. The C–N stretching absorption of primary aliphatic amines is weak and generally occurs in the 1090–1020 cm−1 range. Secondary aliphatic amines have two bands of medium intensity at 1190–1170 cm−1 and 1145–1130 cm−1.20 On the other hand, in the modified samples, some of the primary amide groups of SAP and some of the amine groups of the modifiers have been remained unreacted. Since various vibrational modes of N–H, C–N, and C=O bonds of the amides and amines appear in similar ranges (in the fingerprint region of 1660–1100 cm−1), all the absorption bands are similarly observed in the spectra of the modified samples.
Meanwhile, a weak peak is appeared at ∼3250 cm−1 for the treated SAPs, which is assigned to the nonreacted N–H stretching vibration of the polyamine modifiers. More importantly, the transamidation reaction primarily decreases the intensity of the initial amide peak that appears at 3186 cm−1 (the N–H stretching of the primary amide group). This is the most acceptable spectral evidence for the conversion of the primary amides at the SAP surface.
B. Morphological study
Morphologies of the intact and surface-treated SAPs in the absence and presence of AlCl3 using DETA as a surface crosslinking agent are shown in Fig. 4. As clearly shown in Figs. 4(a)–4(c), the surface of the intact SAP is more uniform and smoother than that of the treated SAP. This indicates that the surface morphology was influenced by the surface treatment.
Figures 4(d) and 4(e) show SEM images of the samples treated with PEI in the presence or absence of AlCl3 as the transamidation catalyst. The smooth surface of the SAP was changed into a rough surface following the noncatalyzed treatment [Fig. 4(d)] and become lumpy following the treatment in the presence of catalyst [Fig. 4(e)]. With the latter, a slightly porous morphology was also observed.
The effect of catalyst was found to be more significant with DETA than with PEI as the surface modifier. As shown in Fig. 4(b), in some areas, the morphology of the DETA-treated SAP in the absence of AlCl3 was found similar to that of the intact SAP, while these regions were disappeared in the presence of the catalyst. On the other hand, such intact regions were not noticeable in the case of the PEI-treated SAP in the absence of AlCl3 [Fig. 4(d)]. This confirmed that the catalyst assisted the completion of the surface treatment by the DETA.
C. Swelling studies
1. DETA as surface modifier
a. Effect of DETA and the catalyst concentration
The effect of surface crosslinker concentration in the absence of transamidation catalyst on swelling properties of SAP (samples D1–D4) is presented in Table I. Free-swelling capacity (QDW values) of the intact SAP was 296 ± 23.7 g/g and decreased as the DETA concentration increased. For instance, the swelling capacity of the D2 sample in distilled water was 237.8 ± 19.0 g/g, showing a 20% lower swelling capacity than that of the intact SAP. Nevertheless, the QS values indicate that the change in swelling was insignificant in saline. Due to the presence of salt and load in real applications, the free-swelling data (i.e., QDW and QS) are not practically useful, therefore we primarily relied on the saline AUL values as explained below.
As shown in Table I, the DETA treatment led to ∼30% increase in AUL for D2 (23.04 ± 0.92 versus 17.71 ± 0.71 g/g). The anticipated reduced free-swelling and increased AUL values can be attributed to DETA reacting with the SAP amide groups. This reaction increases the crosslink density of the SAP particles at their surfaces.
Since crosslinking density has often a direct relationship with AUL, the results showed that DETA was a suitable surface crosslinking agent for acrylamide-based superabsorbents. The high content of amide groups (∼60%) facilitated the transamidation reaction between amide and amines, leading to an increased crosslink density and hence an improved AUL. Although a successful amide–amine reaction requires using a proper catalyst,16–18,21 our data suggested that the transamidation reaction between DETA and amide groups at the SAP surface did not necessarily need to be catalyzed.
The effect of DETA concentration on the swelling properties of the treated SAP in the presence of AlCl3 was investigated, and the results are shown in Table I (samples Dcat1, Dcat2, and Dcat3). The swelling capacity of the SAPs treated with DETA (Dcat3) in the presence of AlCl3 resulted in 152.9 ± 12.2 g/g swelling capacity in distilled water (49% loss), while loss in swelling was found around 20% in the absence of the catalyst.
Due to the presence of water in the reaction medium, the anhydrous AlCl3 reacts with water and generates aluminum hydroxide, Al(OH)3. Given the known effect of the multivalent cations in reducing the SAP swelling capacity,2,11 the SAP surface can potentially be crosslinked with Al(OH)3 resulting in a lower swelling capacity. The same aluminum compound can also catalyze the transamidation reaction as proposed in Fig. 2(b).
In general, the swelling capacity of ionic hydrogels is highly sensitive to the presence of metal cations in the swelling media.10 Therefore, carboxyl groups of the SAP surface react with three-valent cations of the aluminum compound. This irreversible reaction leads to the formation of new ionic crosslinks at the surface of the SAP particles, resulting in significant reduction in swelling, particularly in distilled water.
The saline-absorbency of the unmodified SAP was 35.62 ± 0.7 g/g and did not change with the amounts of DETA in the treatment solution (Table I, QS of D1 to D4 samples). Similarly, the presence of AlCl3 had no substantial effect on saline absorbency of the treated SAP. This can be attributed to the fact that the saline absorbency is not highly dependent on the crosslink density,22 and the key parameter affecting saline absorbency remains to be the main structure of the polymer chain (including the core of particles) that remains unchanged in the course of the surface treatment with or without the catalyst. This outcome is different from what we previously concluded for saline absorbency of nonacrylamide-based SAPs (i.e., sodium acrylate-co-acrylic acid polymers), where surface crosslinking using a diglycidylether led to a lower saline absorbency.2,3
The AUL of the intact SAP was 17.71 ± 0.71 g/g and increased with the amounts of DETA in the treatment solution (Table I). For instance, the AUL of the DETA-treated sample (Dcat3) was increased up to 23.46 ± 0.94 g/g (∼33%). As presented in Fig. 2, the transamidation reaction presumably increased the surface crosslink density of the SAP particles, and these additional crosslinks increased the AUL. The AUL improvement is nearly identical for transamidation with and without the Al compound used as the catalyst and ionic crosslinker.
2. PEI as surface modifier
a. Effect of PEI and the catalyst concentration
Swelling capacities of the PEI-treated SAPs are shown in Table II. Except for the sample Pcat2, PEI (with or without the catalyst) had no considerable effect on the SAP swelling capacity. For instance, the SAP treated with 3.0 g of PEI lost only 6% of its swelling capacity after treatment.
Treatment with PEI resulted in different degrees of modification that can be attributed to two opposite phenomena involved in the swelling process of the PEI-modified samples: (i) surface crosslinking that reduces the swelling capacity and (ii) increased hydrophilicity that increases the swelling capacity due to the highly branched nature of the PEI compared with the DETA. Due to the significant contribution of the latter, the swelling capacity of the Pcat2 SAP increased from 296.0 ± 23.7 to 333.6 ± 26.7 g/g.
Among the main factors determining the swelling capacity of an ionic hydrogel (i.e., polymer–solvent interaction, crosslinking leading to elastic forces, electrostatic repulsion of similar charges of the network, and ionic mobility leading to osmotic pressure difference between the gel and the swelling medium),14,23 the first factor plays a significant role in determining the ultimate swelling capacity of the PEI-treated SAPs. Branched PEI is a bulky macromolecule containing a large number of primary, secondary, and tertiary amine groups (Fig. 1). Only primary and secondary amine functionalities contribute in the transamidation reaction as shown in Fig. 2(b). All the tertiary amine groups as well as the residual nonreacted primary and secondary amine groups are remained intact after the treatment. Either these amine groups or the newly formed secondary and tertiary amide groups linking on the SAP surface can presumably increase the polymer–solvent interaction through hydrogen bonding, which improves the swelling capacity of the PEI-modified SAPs. As hypothesized in Fig. 5, the aliphatic amine groups at the surface of the PEI-treated SAPs are much more abundant than the amide groups at the surface of their DETA counterparts, favoring the hydrogen bonding with water.
On the other hand, the nitrogen atom of the amide bond of the treated surfaces is electron-deficient and cannot effectively participate in H-bonding.20 Therefore, compared with the PEI-treated SAPs, the hydrogen bonding will be less abundant with the DETA-treated samples prepared under similar conditions.
For the Pcat6 sample, the PEI treatment reduced the swelling capacity in saline by 8% down to 32.65 ± 0.65 g/g. However, it is to be noted that the changes in saline absorbency were not noticeable among the PEI-modified samples.
Table II also shows the changes in AUL as the PEI content of the reaction medium increases. With about 41% increase, the highest AUL of 24.84 ± 0.99 g/g was obtained for the Pcat2 sample, which was significantly higher than what obtained for its DETA counterpart (Table I, sample Dcat3). Despite its superior performance, the PEI-treated SAPs were however lumpy in nature after drying, which requires further investigation and improvement to be used as a desirable final product.
Table II also shows changes in swelling capacity with changes in AlCl3 concentration. In general, AlCl3, as the transamidation catalyst, increased the AUL. While the AUL of the PEI-treated SAP in the absence of the catalyst (i.e., P1) was 20.03 ± 0.80 g/g, the AUL was increased up to 24% when the same sample was treated in the presence of 0.06 g of AlCl3. The reason for the improvement was discussed in Sec. III.C.1.a.
Summing up, both DETA and PEI did not affect the QS of the samples treated with or without the catalyst (Fig. 6). For the DETA-treated samples, however, QDW was decreased particularly for the samples modified in the presence of catalyst (Fig. 6, samples D3 and Dcat3). The adverse effect of PEI on QDW was significantly lower than that of the DETA-treated samples modified either with or without the catalyst. As shown in Fig. 7, while the AUL was improved for all treated SAPs, the catalyst was found to be more effective on AUL when PEI was used for the treatment.
The effect of time and temperature on QDW, QS, and AUL of DETA- and PEI-treated SAPs were also investigated and the results are denoted in Tables SI and SII (Supplementary Material).
D. Rheological study
Transamidation reaction forms new crosslinks at the SAP surface, resulting an increase in the crosslink density of the SAP and hence its stiffness and modulus. Since the storage modulus is directly related to crosslinking density according to the Flory–Rehner correlation,16 such an observation can hence be verified through rheological measurements.
Figure 8 shows storage modulus versus angular frequency for the intact SAP, and corresponding samples treated with DETA and PEI. The storage modulus was increased for the surface modified SAPs compared with the unmodified one. At an angular frequency of 1 rad/s, the storage moduli were found to be 1520, 1610, and 2020 Pa for the intact SAP, DETA-treated, and the PEI-treated SAPs, respectively. The results showed that the transamidation reaction utilizing both surface modifiers desirably increased the modulus of the hydrated SAPs. With no surprise, these data are clearly in agreement with those obtained from the AUL studies, as both storage modulus and the AUL values are determined by similar factors.
The moduli differences of the intact and the surface-treated samples are not major but they should be considered. These minor differences are due to the fact that the crosslinking has occurred only at the surface, and the extent of the surface-crosslinking is very low, while the crosslinking degree of the bulk is unchanged. It means that the main polymer network structure [i.e., poly(AM–potassium acrylate–acrylic acid)] having highest impact on the storage modulus24 is rather unchanged after the surface treatment.6,8 Therefore, a significant difference between the modulus of the intact and the surface-modified samples is not expected.
IV. CONCLUSIONS
Through catalytic and noncatalytic transamidation reactions, polyamine structures including DETA and PEI could improve the swelling properties of the acrylamide-based SAPs that are commonly used in agriculture. The saline AUL, a critical swelling factor for agricultural and hygiene applications, was increased with the transamidation time and temperature. Both polyamines resulted in more or less similar swelling behavior and increased the AUL value by about 25% in general. However, the use of PEI resulted in some undesirable outcomes such as lumpy SAP structure and agglomeration of SAP particles, which requires further improvement in the process. Overall, the transamidation process can successfully and feasibly be used to improve saline AUL of the acrylamide-based agricultural SAPs.
SUPPLEMENTARY INFORMATION SUMMARY
The supplementary information includes Tables SI and SII. Table SI represents the effect of transamidation time and temperature on DETA-treated SAP sample free absorbency in distilled water (QDW), saline (QS) and saline absorbency under load (AUL). Also, the effect of transamidation time and temperature on the PEI-treated SAP sample QDW, QS, and AUL are denoted in Table SII.
References
H. Omidian, S. Doroudiani, and K. Kabiri: Advances in non-hygienic applications of superabsorbent hydrogel materials. J. Mater. Sci. 45, 5711 (2010).
M.J. Zohuriaan-Mehr and K. Kabiri: Superabsorbent polymer materials: A review. Iran. Polym. J. 17, 451 (2008).
A. Sabzevari and K. Kabiri: Converting date seed biomass into highly absorbing hydrogel. Iran. Polym. J. 25, 597 (2016).
A. Sabzevari, K. Kabiri, and M. Siahkamari: Induced superabsorbency in polyester fiber. Iran. Polym. J. 25, 635 (2016).
N. Moini and K. Kabiri: Effective parameters in surface cross-linking of acrylic-based water absorbent polymer particles using bisphenol A diethylene glycidyl ether and cycloaliphatic diepoxide. Iran. Polym. J. 24, 977 (2015).
N. Moini, K. Kabiri, M.J. Zohuriaan-Mehr, H. Omidian, and N. Esmaeili: Fine tuning of SAP properties via epoxy-silane surface modification. Polym. Adv. Technol. 28, 1132 (2017).
N. Moini, K. Kabiri, M.J. Zohuriaan-Mehr, and N. Esmaeili: Simple and efficient approach for recycling of fine acrylic-based superabsorbent waste. Polym. Bull. 73, 1119 (2016).
N. Moini, K. Kabiri, and M.J. Zohuriaan-Mehr: Practical improvement of SAP hydrogel properties via facile tunable cross-linking of the particles surface. Polym. Plast. Technol. Eng. 55, 278 (2016).
J. Huang, Z.M. Huang, Y.Z. Bao, and Z.X. Weng: Synthesis and characterization of reinforced acrylic-based superabsorbents crosslinked with divinylbenzene. J. Appl. Polym. Sci. 100, 1594 (2006).
T.K. Mudiyanselage and D.C. Neckers: Highly absorbing superabsorbent polymer. J. Polym. Sci., Part A: Polym. Chem. 46, 1357 (2008).
S. Ma, M. Liu, and Z. Chen: Preparation and properties of a salt-resistant superabsorbent polymer. J. Appl. Polym. Sci. 93, 2532 (2004).
Z. Chen, M. Liu, and S. Ma: Synthesis and modification of salt-resistant superabsorbent polymers. React. Funct. Polym. 62, 85 (2005).
S. Shahi and M.J. Zohuriaan-mehr: Antibacterial superabsorbing hydrogels with high saline-swelling properties without gel blockage: Toward ideal superabsorbents for hygienic applications. J. Bioact. Compact. Polym. 32, 1 (2016).
S. Shahi, H.R. Motasadizadeh, and M.J. Zohuriaan-Mehr: Surface modification of superabsorbing hydrogels via a feasible esterification. Int. J. Polym. Mater. Polym. Biomater. 66, 544 (2017).
N. Moini, K. Kabiri, and M.J. Zohuriaan-Mehr: Surface treatment of superabsorbents. U.S. Patent No. US 2018/0008960 A1, 2018.
E. Ban, D.C.H. Bigg, and G. Bertrand: Aluminum chloride-promoted transamidation reactions. J. Org. Chem. 59, 4035 (1994).
C.L. Allen, N. Atkinson, and B. Williams: Transamidation of primary amides with amines using hydroxylamine hydrochloride as an inorganic catalyst. Angew. Chem. Int. Ed. 51, 1383 (2012).
S.N. Rao, D.C. Mohan, and S. Adimurthy: An efficient catalyst for transamidation of carboxamides with amines L-proline. Org. Lett. 8, 8 (2013).
M.J. Ramazani-Harandi, M.J. Zohuriaan-Mehr, A.A. Yousefi, A. Ershad-Langroudi, and K. Kabiri: Rheological determination of the swollen gel strength of superabsorbent polymer hydrogels. Polym. Test. 25, 470 (2006).
G. Socrates: Infrared and Raman Characteristic Group Frequencies, 3rd ed. (Wiley, New York, 2001); pp. 107 and 144.
S.L. Yedage, D.S. D’silva, and B.M. Bhanage: MnO2 catalyzed formylation of amines and transamidation of amides under solvent-free conditions. RSC Adv. 5, 80441 (2015).
K. Kabiri, H. Omidian, S.A. Hashemi, and M.J. Zohuriaan-Mehr: Synthesis of fast-swelling superabsorbent hydrogels: Effect of crosslinker type and concentration on porosity and absorption rat. Eur. Polym. J. 39, 1341 (2003).
H. Omidian, S.A. Hashemia, P.G. Sammesb, and I. Meldrumb: A model for the swelling of superabsorbent polymers. Polymer 39, 6697 (1998).
M.J. Ramazani-Harandi, M.J. Zohuriaan-Mehr, A.A. Yousefi, A. Ershad-Langroudi, and K. Kabiri: Effects of structural variables on AUL and rheological behavior of SAP gels. J. Appl. Polym. Sci. 113, 3676 (2009).
Author information
Authors and Affiliations
Corresponding author
Supplementary Material
43578_2018_33162327_MOESM1_ESM.docx
Supporting Information: Degradation kinetics in different polymer-fullerene blends investigated by electron spin resonance (approximately 3.13 MB)
Rights and permissions
About this article
Cite this article
Azizi, A., Kabiri, K., Zohuriaan-Mehr, M.J. et al. Transamidation: A feasible approach of surface modification to improve absorbency under load of agricultural superabsorbent materials. Journal of Materials Research 33, 2327–2335 (2018). https://doi.org/10.1557/jmr.2018.240
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2018.240