Abstract
Surface cross linking is a post-treatment process for superabsorbent polymers (SAPs) leading to an increase in the absorbency under load (AUL). This process is typically carried out through conventional heating method. In the current study, for the first time, microwave method was used for surface treatment process of SAPs based on poly(sodium acrylate). Diglycidyl materials such as 1,4-butanediol diglycidyl ether (BDDGE), polyethylene diglycidyl ether (PEGDGE), and ethylene glycol diglycidyl ether (EGDGE) were utilized as surface cross-linking agents. Also, N,N-Dimethylaniline was used as a catalyst for surface treatment of poly(sodium acrylate) SAP with diglycidyl materials as the external cross linkers. The results showed that surface treatment time can be reduced from 1 to 3 h in the conventional heating to a few minutes by microwave method. The use of catalyst in surface treatment solution resulted in higher AULs. The AULs of SAPs were increased from 14 g/g for unmodified SAP to 17.5, 19 and 20.7 g/g after surface treatment for surface-treated SAPs with BDDGE, PEGDGE and EGDGE, respectively. These results present the microwave method as an effective alternative candidate for thermal surface treatment of SAP which can have economic benefits from the viewpoint of time and energy consumption industrially.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Superabsorbent polymer hydrogels (SAPs) are lightly cross-linked networks that have the ability to absorb and retain large amounts of water and physiological solutions. Superabsorbent is widely used in various products such as water-absorbing materials in feminine napkins, disposable diapers, adult incontinence products and agricultural applications [1,2,3]. Most commercial SAPs are made of polyacrylates derivatives. Since polyacrylates are non-biodegradable, their waste accumulation may give rise to some environmental concerns.
The swelling properties of SAPs under practical conditions are tremendously vital for applications such as feminine napkin or agriculture [4]. Absorbency under load (AUL) is an important characteristic which shows a real estimation of swelling and efficiency of SAPs [5].
The AUL is completely dependent on the nature of an SAP and its synthetic method. For instance, the simultaneous presence of an interpenetrating polymer network and nanoparticles can improve the AUL [6, 7]. Incorporation of an appropriate hydrophobic comonomer can improve the AUL. In this case, incorporation of an optimum amount of comonomer such as styrene has enhanced the swelling capacity and the swelling rate according to a previous report [8].
As it is mentioned above, the AUL can be varied by the synthetic conditions and methods. For example, the type and amount of cross linker can change the amount of AUL. Ramazani-Harandi et al. observed that with the same concentrations, polyethylene glycol diacrylate takes part in formation of an SAP gel with greater storage modulus and AUL, compared to triethylene glycol dimethacrylate as cross linker. On the other hand, the initiator type and concentration can also affect the mechanical and swelling properties of the SAP by changing the gelation rate. Indeed, more defects as a result of fast reactions cause a reduction in AUL and mechanical properties. Addition of an optimum amount of filler enhances the modulus and AUL. Porosity declines the AUL and drying a gel in oven at a proper temperature gives an improvement to the mechanical properties of SAP [4].
The surface treatment is a post-process in SAPs preparation to improve SAP characteristics such as AUL [9, 10], salt resistance [11], reducing gel blockage [12] or inducing antibacterial activity [13]. Surface cross linking is particularly carried out with the aim of AUL enhancement. SAPs with carboxylic acid in their structures can react with an external cross linker such as diglycidyl materials, i.e., by bisphenol A diethylene glycidyl ether (BADGE) and 3,4-epoxycyclohexylmethyl 3,4-epoxycyclohexanecarboxylate (CAE) resins, diols [5] and epoxy silanes [9].
Some research works on surface modification of SAPs were carried out to increase the salt absorbency, i.e., aluminum sulfate [11], divinyl benzene [14] and bis(methacryloylamino)-azobenzene [15] were used as surface modifiers of SAPs to increase the saline absorbency of the modified SAP. The use of cetyltrimethylammonium bromide as a surface modifier showed a multifunctional role in SAP surface modification: (a) cation exchange reaction in the SAP surface to induce antibacterial activity and (b) reducing the gel blockage and increasing the AUL [13]. Gel blockage reduction and AUL enhancement simultaneously occurred in SAPs which were modified with 1,2-dichlroethane [12].
The surface cross-linking reactions are mostly carried out through conventional heating; the required heat and time for the reaction are usually between 120–140 °C and 1–3 h, respectively [5]. The other method for surface treatment is photo-induced surface cross linking by UV irradiation (200–300 nm) with (NH4)2S2O8 photoactivated cross-linking agent [16].
This study has two novel perspectives, first, the use of microwave method for surface treatment of SAPs instead of the conventional thermal method, which to the best of our knowledge is not reported in the past. Second, the use of N,N-Dimethylaniline as a catalyst which promotes the chemical reaction between SAPs carboxylic acid groups and diglycidyl groups of external cross linker was investigated for the first time. Different diglycidyl materials such as 1,4-butanediol diglycidyl ether (BDDGE), polyethylene diglycidyl ether (PEDGE), and ethylene glycol diether (EGDGE) were used as external cross linkers for surface treatment. The effects of surface treatment conditions including microwave power, surface treatment time, external cross-linker type and content and catalyst content on the swelling properties of surface-treated samples were investigated. ATR-FTIR spectroscopy and rheological measurements were also used for characterization of microwave surface-treated SAPs.
Experimental
Materials
Cross-linked poly(sodium acrylate), code SAP200 (IPPI, Iran) (particle size 150–200 μm) as superabsorbent, 1,4-butanediol diglycidyl ether (BDDGE, 99%), polyethylene diglycidyl ether (PEGDGE, 99%) and ethylene glycol diglycidyl ether (EGDGE, 99%) as surface cross-linking agents were supplied by Shenzhen Nexconn Pharmatechs (China). N,N-Dimethylaniline (DMA, 99%) as a catalyst, acetone as the solvent and sodium chloride were purchased from Merck (Germany) and were used as received.
Post-treatment (surface cross linking)
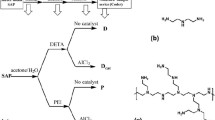
The surface modification was performed according to the previous reports [5]. In brief, optimum amount of the solvent, i.e., a mixture of acetone (90 wt%) and water (10 wt%), was used to dissolve different amounts of BDDGE, EGDGE and PEGDGE. The sample codes based on the amount of surface cross linkers are represented in Table 1. Next, 5.0 g SAP was added into the solution. So, primarily they remained soaking at room temperature for 30 min. Next, the filtered particles were cured in the microwave with three modes of power (250 W, 400 W and 1000 W).
The sample codes (Table 1) are displayed according to AXCYZ symbols. Sign A designates the cross-linker type and is denoted as PE, BD, and EG for PEGDGE, BDDGE, and EGDGE, respectively. The amount of cross linker by weight is shown with index X. The amount of the catalyst (C) by weight is shown with index Y. Power of microwave, i.e., 250, 400 and 1000 W, is represented by Z (2, 4, 10).
Characterization
Free swelling measurements
Swelling capacity of the hydrogel samples was measured using a tea bag method. Dried SAP sample (0.10 g) was put into an excess amount of distilled water or saline solution (0.9 wt% NaCl) to reach equilibrium swelling at ambient condition for 1 h. Then, it was dewatered carefully and the swelling capacity was calculated as g/g according to Eq. 1:
where Ws and Wd are weights of the swollen SAP and initial dry samples, respectively. Swelling measurements were repeated five times.
Absorbency under load (AUL)
The weighed dried SAP sample (0.5 g) was uniformly dispersed on the surface of polyester gauze located on a macro-porous sintered glass filter plate in a Petri dish. A cylindrical solid load (Teflon, D¼ 60 mm) was put on the dry SAP particles while it could be freely slipped into a glass cylinder. Desired load (applied pressure: 0.3 psi) was placed on the sample. Then, 0.9% saline solution was added into the Petri dish. After 30 min, the maximally swollen particles were removed and reweighed. The AUL value was calculated using Eq. 1. AUL measurements were repeated five times.
ATR-FTIR characterization
The FTIR spectra were recorded on Bruker instruments (ABB Bomem MB-100, Germany). The ATR-FTIR (Specac, Golden Gate, UK) were worked to probe the surface of the superabsorbent particles. They were obtained at wavenumber range of 600–4000 cm−1 and resolution of 1 cm−1.
Rheological characterization
The rheological measurements of samples were performed using a Paar-physical oscillatory rheometer (MCR 300, Germany) at 25 °C with parallel plate geometry (plate diameter of 25 mm, with a gap of 3 mm). The storage modulus (G′) and loss modulus (G″) were recorded as a function of angular frequency at constant shear strain of 0.2% (for being in the linear viscoelastic zone). All samples were weighed (0.2 g) and 5.0 g of de-ionized water was added over them.
Results and discussion
Reaction mechanism
Different amounts of BDDGE, PEGDGE and EGDGE as epoxy cross linkers and catalyst (DMA) were used for surface cross linking of poly(sodium acrylate) SAPs. The mechanism of reactions is displayed in Scheme 1. As it is obvious from Scheme 1, the carboxylic acid groups of SAP could react with oxirane rings in the epoxy cross linkers [17] leading to ring opening of the epoxy groups and to joining the polymer chains (Scheme 1a).
Acid groups of the SAP can react with oxirane rings of cross linkers through four possible mechanisms according to Scheme 1b. In reaction 1, the epoxy ring is opened by an acid group of SAP and this leads to the formation of the network [9]. The presence of DMA catalyst can trigger reactions 2 and 3. Reaction 4 occurs due to the presence of water in the system.
FTIR spectroscopy
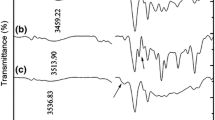
FTIR spectroscopy technique was applied to detect the ester group’s formation as a result of a reaction between sodium acrylate –COOH groups and BDDGE. Figure 1 displays the FTIR spectra of linear sodium acrylate and its cross-linked surface modified by DMA catalyst through microwave method.
According to Fig. 1, the absorption peak of 3376 cm−1 is related to stretching vibrations of OH bonds, which shifts to 3445 cm−1 after modification, due to the formation of less strong hydrogen bond, as compared to that of carboxylic acid. The absorption peak at 1101 cm−1 attributed to C–O which is another group in the network structure and does not appear in the base sample. According to the shift in OH peak and the presence of a sharp C–O peak in the spectrum of the modified SAP, it can be concluded that the reactions 2 and 3 (Scheme 1b) are the main reactions taken place in this modification condition. Also, the characteristic absorption peak at 1257 cm−1 is related to C–O ester groups of the network structure [18].
The peaks related to the carboxylic acid and carboxylate are represented at 1691 and 1554 cm−1, respectively, for the base sample [18, 19]. As it is mentioned before, the glycidyl groups of epoxy resin have an affinity to react with the carboxylic pendant groups of the superabsorbent. After the modification reaction, the C=O peak of carboxylic acid shifts to a higher wavenumber (1712 cm−1). It should be noted that due to the high polarity, carboxyl group has the ability to form stronger hydrogen bonds than the amines and alcohols [20].
Swelling studies
Figure 2a shows water and saline absorbency (QDW and QS, respectively) for microwave surface cross-linked SAPs containing different amounts of cross linker. The swelling capacity of the unmodified sample in distilled water is decreased from 365 to 318 g/g and 290 g/g for SAPs which are modified with 100 and 200 g/L BDDGE (100 and 200 g of BDDGE in 1 L of solvent mixture) as surface cross-linking agent, respectively. Indeed, the reaction between SAP’s carboxylic acid groups and the diglycidyl groups of surface cross-linking agent promotes an increase in cross-link density of the SAPs surface which lowers the swelling capacity in microwave method.
Figure 2b shows AUL of BDDGE surface-treated samples by microwave irradiation. The AUL has an ascending trend with the increase of surface cross-linker content, i.e., the AUL of unmodified SAP is 14 g/g which is increased to 15.3 and 16.5 g/g at surface cross-linker contents of 100 and 200 g/L, respectively. AUL enhancement in modified SAP is attributed to the increase of cross-link density on the SAP surface and creating a core–shell structure [9]. Cross-link density in the core is dependent on internal cross-linker content during SAP synthesis, and the external cross-link density is related to the surface modification. The increase of external cross linker in the surface treatment solution increases the probability of reaction between diglycidyl groups of surface cross linker and SAP’s carboxylic acid groups leading to an increase in cross-link density on the SAP surface.
Effect of microwave power on the swelling properties
The intensity of microwave irradiation is an important factor in microwave-assisted reaction [21]. The effect of three microwave modes on the swelling properties of modified SAPs was investigated (Table 2). Microwave irradiation time was 40 s in high mode and the higher irradiation time in high mode was not used due to the color changes in SAPs and probable degradations. On the other hand, microwave irradiation time can be increased with the decrease in microwave power. The irradiation times for medium and medium–low modes were 2 and 4 min, respectively. AUL enhancement is observed for microwave-assisted surface-modified SAP in all modes. According to Table 2, the appropriate condition for microwave-assisted surface treatment of SAP is lower microwave power and higher microwave irradiation time (AUL 16.7 g/g).
Effect of catalyst content
For the first time, the effect of a catalyst on surface cross linking of SAPs with diglycidyl materials through microwave irradiation was investigated. In this regard, DMA was used as the catalyst to promote the chemical reaction between carboxylic groups of SAP and diglycidyl groups of the surface cross-linking agent. Figure 3 shows the effect of catalyst content on swelling properties (Fig. 3a) and AUL (Fig. 3b) of microwave-assisted surface-treated SAPs. Swelling capacity in distilled water is decreased in surface-treated SAP in the presence of the catalyst (Fig. 3a). The results indicate that the use of catalyst can improve the chemical reaction between diglycidyl surface cross linker and SAPs. Hence, cross-link density in modified SAP in the presence of the catalyst is higher leading to swelling capacity reduction. A trend of swelling capacity reduction with the increase of catalyst content is observed up to catalyst content of 100 g/L and the higher catalyst content does not have a considerable effect on the swelling capacity of the modified SAP.
Figure 3b shows the AUL of microwave-assisted surface-treated SAP versus catalyst content. The AUL has an ascending trend with the increase of catalyst content up to 100 g/L, i.e., the AUL of surface-treated SAP is increased from 14 in the absence of catalyst to 15 and 16 g/g in the presence of 50 and 100 g/L DMA, respectively. The increase of cross-link density in the presence of catalyst may increase cross-link density in the modified SAP which causes to AUL enhancement. Higher catalyst content, 150 and 200 g/L, causes lower AUL. Therefore, the optimum catalyst content in microwave surface treatment of SAPs with the diglycidyl surface cross-linking agent is 100 g/L.
Effect of cross-linker type and concentration
In all previous experiments, in SAP microwave-assisted surface treatment, BDDGE was used as a surface cross-linking agent. In this section, the effect of two other diglycidyl materials on swelling properties of the microwave-assisted surface is investigated. Table 1 shows the reaction conditions for each experiment. Swelling capacity in distilled water is considerably dropped in EGDGE- and PEGDGE-modified SAP in comparison with intact SAP, i.e., the swelling capacity of intact SAP is decreased from 365 to 208 g/g and 198 g/g for modified SAP with 150 g/L EGDGE or PEGDGE, respectively (Fig. 4a). This significant reduction in swelling capacity is attributed to high cross-link density enhancement as a result of surface cross linking with these two materials.
On the other hand, QS is decreased with the increase in surface cross-linker content. Therefore, the saline absorbency of intact SAP is dropped from 59 to 53 g/g and 51 g/g for 150 g/L of external cross linker, EGDGE and PEGDGE, respectively. Similar to QDW, the lowering QS of surface modified SAP compared to intact SAP can be attributed to the increase in cross-link density after surface modification and creation of a core–shell structure in SAP.
Figure 4b shows the effect of EGDGE and PEGDGE contents on the AUL of the surface-treated samples. The AUL of intact SAP has an ascending trend with the increase in surface cross-linker content for both EGDGE and PEGDGE in microwave-assisted surface treatment process. It is increased from 14 to 19 g/g and 20.7 g/g for 150 g/L surface cross linkers, respectively. These two surface cross linkers have had superior effects on AUL than BDDGE which were formerly used for microwave-assisted surface cross linking. The highest level in AUL enhancement using BDDGE was only 17% while they were 35 and 47% for EGDGE and PEGDGE, respectively. These higher AUL amounts could be related to higher hydrophilicity of EGDGE and PEGDGE compared to BDDGE. The AUL enhancement by these cross linkers showed that microwave surface treatment can increase cross-link density in SAP surface in a short time through EGDGE and PEGDGE reactions with SAP’s carboxylic acid groups. The efficiency of these surface cross linkers promotes a high increase in cross-link density on the SAP surface which leads to highly enhanced AUL.
Table 3 makes a good comparison between the swelling properties of the microwave surface-treated SAPs of this work with the previous results of thermally surface-treated SAPs. As it is indicated in the table, in thermal method, significant time and temperature are required for the surface treatment process. On the other hand, the microwave method in just 4 min and medium power results in an increase of AULs. So, this method can compete with the traditional thermal method and can economically surpass it. As the surface treatment reaction is carried out faster, the possibility of polymer chain degradations generated by exposure to heat for a long time is also reduced.
In addition, the reduction of swelling capacities in distilled water and saline for surface-treated samples is 45.7 and 13.5% using microwave method. This reduction for QDW and QS in thermal method is 72.6 and 52.3%, respectively [5].
Rheological properties
Figure 5 shows storage modulus versus angular frequency for intact SAP and microwave-assisted surface cross linking using different diglycidyl materials. The intact SAP has the lowest storage modulus and all microwave-assisted surface-treated SAPs show enhanced storage modulus. The highest storage modulus belongs to the surface-treated SAP with PEGDGE, i.e., storage modulus of intact SAP at angular frequency of 1 is 918 Pa which is increased to 1720 Pa after surface treatment. This enhanced storage modulus is attributed to the direct relationship between the storage modulus and cross-link density [22].
The increase in cross-linked density in SAP surface after treatment causes higher storage modulus observed in rheological measurements. The AUL and rheological measurements both show that PEGDGE and EGDGE are superior surface cross-linking agents compared to BDDGE through microwave-assisted surface treatments.
Conclusion
A new method for surface treatment of polyacrylate superabsorbent method was introduced through microwave-assisted surface cross linking. The surface treatment process required diglycidyl materials such as BDDGE, EGDGE and PEGDGE and a catalyst such as DMA. A microwave-assisted surface cross-linking process as a rapid and efficient method was used in surface treatment of SAP which increased its AUL in comparison to the conventional heating process. The optimum conditions for surface treatment process through microwave method were applied as 100 g/L of catalyst, the microwave power of 250 W, the microwave irradiation time of 4 min, and surface cross-linker content of 150 g/L for EGDGE or PEGDGE.
The higher AUL was obtained particularly for EGDGE and PEGDGE which increased by 35% and 47% in comparison to intact SAP.
These results indicate that microwave-assisted surface cross linking is an economic and facile process which significantly reduces surface treatment time and its required surface treatment energy.
References
Zohuriaan-Mehr MJ, Kabiri K (2008) Superabsorbent polymer materials: a review. Iran Polym J 17:451–477
Fang S, Wang G, Xing R, Chen X, Liu S, Qin Y, Li K, Wang X, Li R, Li P (2019) Synthesis of superabsorbent polymers based on chitosan derivative graft acrylic acid-co-acrylamide and its property testing. Int J Biol Macromol 132:575–584
Ashkani M, Bouhendi H, Kabiri K, Rostami MR (2019) Synthesis of poly(2-acrylamido-2-methyl propane sulfonic acid) with high water absorbency and absorption under load (AUL) as concrete grade superabsorbent and its performance. Constr Build Mater 206:540–551
Ramazani-Harandi MJ, Zohuriaan-Mehr MJ, Yousefi AA, Ershad-Langroudi A, Kabiri K (2009) Effects of structural variables on AUL and rheological behavior of SAP gels. J Appl Polym Sci 113:3676–3686
Moini N, Kabiri K (2015) Effective parameters in surface cross-linking of acrylic-based water absorbent polymer particles using bisphenol A diethylene glycidyl ether and cycloaliphatic diepoxide. Iran Polym J 24:977–987
Kheirabadi M, Bagheri R, Kabiri K (2015) Structure, swelling and mechanical behavior of a cationic full-IPN hydrogel reinforced with modified nanoclay. Iran Polym J 24:379–388
Kheirabadi M, Bagheri R (2015) Swelling and mechanical behavior of nanoclay reinforced hydrogel: single network vs. full interpenetrating polymer network. Polym Bull 72:1663–1681
Shi X, Wang W, Wang A (2011) Synthesis and enhanced swelling properties of a guar gum-based superabsorbent composite by the simultaneous introduction of styrene and attapulgite. J Polym Res 18:1705–1713
Moini N, Kabiri K, Zohuriaan-Mehr MJ, Omidian H, Esmaeili N (2017) Fine tuning of SAP properties via epoxy-silane surface modification. Polym Adv Technol 28:1132–1147
Azizi A, Kabiri K, Zohuriaan-Mehr MJ, Bouhendi H, Karami Z (2018) Transamidation: a feasible approach of surface modification to improve absorbency under load of agricultural superabsorbent materials. J Mater Res 33:2327–2335
Ma S, Liu M, Chen Z (2004) Preparation and properties of a salt-resistant superabsorbent polymer. J Appl Polym Sci 93:2532–2541
Shahi S, Motasadizadeh HR, Zohuriaan-Mehr MJ (2017) Surface modification of superabsorbing hydrogels via a feasible esterification reaction: toward tunable superabsorbent for hygienic applications. Int J Polym Mater Polym Biomater 66:544–557
Shahi S, Zohuriaan-Mehr MJ, Omidian H (2016) Antibacterial superabsorbing hydrogels with high saline-swelling properties without gel blockage: toward ideal superabsorbents for hygienic applications. J Bioact Compat Polym 32:128–145
Huang J, Huang ZM, Bao YZ, Weng ZX (2006) Synthesis and characterization of reinforced acrylic-based superabsorbents crosslinked with divinylbenzene. J Appl Polym Sci 100:1594–1600
Mudiyanselage TK, Neckers DC (2008) Highly absorbing superabsorbent polymer. J Polym Sci Part A Polym Chem 46:1357–1364
Jockusch S, Turro NJ, Mitsukami Y, Matsumoto M, Iwamura T, Lindner T, Flohr A, Massimo G (2009) Photoinduced surface crosslinking of superabsorbent polymer particles. J Appl Polym Sci 111:2163–2170
Moini N, Kabiri K, Zohuriaan-Mehr MJ (2016) Practical Improvement of SAP hydrogel properties via facile tunable cross-linking of the particles surface. Polym Plast Technol Eng 55:278–290
Pavia D, Lampman GM, Kriz GS (2001) Introduction to spectroscopy Chap 2, 4th edn. Cole, Cengage Learning, Washington DC
Max JJ, Chapados C (2004) Infrared spectroscopy of aqueous carboxylic acids: comparison between different acids and their salts. J Phys Chem A 108:3324–3337
Dong J, Ozaki Y, Nakashima K (1997) Infrared, raman, and near-infrared spectroscopic evidence for the coexistence of various hydrogen-bond forms in poly(acrylic acid). Macromolecules 30:1111–1117
Charde MS, Shukla A, Bukhariya V, Chakole RD (2012) A review on: a significance of microwave assist technique in green chemistry. Int J Phytopharm 2:39–50
Ramazani-Harandi MJ, Zohuriaan-Mehr MJ, Yousefi AA, Ershad-Langroudi A, Kabiri K (2006) Rheological determination of the swollen gel strength of superabsorbent polymer hydrogels. Polym Test 25:470–474
Ghasri M, Jahandideh A, Kabiri K, Bouhendi H, Zohuriaan-Mehr MJ, Moini N (2019) Glycerol-lactic acid star-shaped oligomers as efficient biobased surface-modifiers for improving superabsorbent polymer hydrogels. Polym Adv Technol 30:390–399
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghasri, M., Bouhendi, H., Kabiri, K. et al. Superabsorbent polymers achieved by surface cross linking of poly(sodium acrylate) using microwave method. Iran Polym J 28, 539–548 (2019). https://doi.org/10.1007/s13726-019-00722-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-019-00722-6