Abstract
Background
The impact of changes in skeletal muscle and sarcopenia on outcomes during neoadjuvant chemoradiotherapy (NACR) for patients with esophageal cancer remains controversial.
Patients and Methods
We retrospectively analyzed the data of patients with locally advanced esophageal squamous cell cancer who received NACR followed by esophagectomy between June 2013 and December 2021. The images at third lumbar vertebra were analyzed to measure the cross-sectional area and calculate skeletal muscle index (SMI) before and after NACR. SMI less than 52.4 cm2/m2 for men and less than 38.5 cm2/m2 for women were defined as sarcopenia. The nonlinearity of the effect of percent changes in SMI (ΔSMI%) to survival outcomes was assessed by restricted cubic splines.
Results
Overall, data of 367 patients were analyzed. The survival outcomes between sarcopenia and non-sarcopenia groups had no significant differences before NACR. However, patients in post-NACR sarcopenia group showed poor overall survival (OS) benefit (P = 0.016) and poor disease-free survival (DFS) (P = 0.043). Severe postoperative complication rates were 11.9% in post-NACR sarcopenia group and 5.0% in post-NACR non-sarcopenia group (P = 0.019). There was a significant non-linear relationship between ΔSMI% and survival outcomes (P < 0.05 for non-linear). On the multivariable analysis of OS, ΔSMI% > 12% was the independent prognostic factor (HR 1.76, 95% CI 1.03–2.99, P = 0.039) and significant difference was also found on DFS analysis (P = 0.025).
Conclusions
Patients with post-neoadjuvant chemoradiotherapy sarcopenia have worse survival and adverse short-term outcomes. Moreover, greater loss in SMI is associated with increased risks of death and disease progression during neoadjuvant chemoradiotherapy, with maximum impact noted with SMI loss greater than 12%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In recent years, multimodal therapy based on surgery has shown many significant improvements in perioperative and long-term survival outcomes for patients with locally advanced esophageal cancer.1 Neoadjuvant chemoradiotherapy (NACR) followed by esophagectomy is strongly recommended,2 with the newly published CROSS trial reporting a 10-year survival of 38% in patients treated with NACR.3 Notwithstanding this welcome progress, esophageal cancer still is associated with higher disease recurrence rates, as well as with challenges to physical recovery from neoadjuvant therapy and enhancing long-term quality of life. Many studies reporting nutrition-related indicators such as the Naples prognostic score (NPS), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) have emphasized the clinical influence of nutrition and patients’ physical condition on oncologic and operative outcomes in esophageal cancer.4,5
Sarcopenia, defined by an unintentional loss of skeletal muscle mass, is now commonly described in oncology patients, especially those with gastrointestinal cancer, and is indicated with poor survival benefit.6,7 For patients with esophageal cancer, sarcopenia is reported in a range of 14–80%.7 Progressing to sarcopenia is multivariable with dysphagia, less nutrient intake, higher metabolic rate, and dietary changes all contributory. Among patients with locally advanced esophageal cancer, the addition of neoadjuvant therapy may influence patients’ nutritional status. Notwithstanding, many studies only involved time-specific body compositions, few involved it dynamically during neoadjuvant therapy.8,9,10,11,12,13 In recent years, many studies indicated that skeletal muscle index (SMI) loss after neoadjuvant therapy might affect the patients’ survival benefit, however, the results are varied.14,15,16,17,18,19
This study aimed, first, to investigate whether sarcopenia affected survival outcomes and postoperative outcomes among patients with locally advanced esophageal squamous cell cancer treated with neoadjuvant chemoradiation in a high-volume center. Second, this study aimed to systematically exam changes in SMI including loss and increase after neoadjuvant chemoradiation, and to evaluate the impact of these changes on outcomes.
Patients and Methods
Patients
Study Design
This was a single-center retrospective cohort study. The Institutional Review Board (IRB) of West China hospital approved our study (IRB no. 2022-853), and the writing of informed consent was waived. The data were retrieved from the hospital information system (HIS) and picture archiving and communication system (PACS). This study was followed with the STROBE statement.20
Inclusion and Exclusion Criteria
We retrospectively enrolled all consecutive patients with esophageal cancer who underwent neoadjuvant therapy followed by esophagectomy between June 2013 and December 2021. The inclusion criteria were locally advanced esophageal squamous cell cancer with resectable tumor, clinical stages T2-4aN0-2M0, patient age 18–80 years old with ability to undergo surgery, completed 2 cycles of neoadjuvant chemotherapy, completed a total radiation dose of 40–50.4 Gy, and undergoing minimally invasive McKeown surgery.
The exclusion criteria were presence of other malignancy or other illness that might influence body composition, distant progression after completed chemoradiotherapy, salvage esophagectomy, gastrostomy before esophagectomy, upfront colonic or jejunal interposition before surgery, cervical esophageal tumor, and missing data on clinicopathological characteristics, complications, computed tomography (CT) images, postoperative follow-up.
Neoadjuvant Chemoradiation Regimen
Briefly, neoadjuvant chemoradiotherapy consisted of two cycles of chemotherapy with concurrent radiation. The chemotherapy regimen was paclitaxel-based platinum [cisplatin (TP) or carboplatin (TC)] or cisplatin plus 5-fluorouracil (FP). Paclitaxel [intravenous (iv) 175 mg/m2] was administered on day 1 with a combination of cisplatin (iv 25 mg/m2/day) on days 1–3 or with a combination of carboplatin (iv AUC 5) on day 1. Cisplatin (iv 25 mg/m2/day) was scheduled on days 1–3 with 5-fluorouracil (iv 500 mg/m2/day) on days 1–3. The radiation regimen, ranging from 40 to 50.4 Gy, was delivered in 23–28 fractions (1.8–2.0 Gy/frac), starting on the first day of the first chemotherapy cycle (week 1).
Surgery and Postoperative Surveillance
Esophagectomy was scheduled at 6–8 weeks after completed neoadjuvant chemoradiation, using McKeown minimally invasive surgery through chest, upper abdomen, and left neck (three incisions), and two-field lymphadenectomy (chest and upper abdomen). The follow-up visits were scheduled at 4 months postoperation and every 6 months thereafter, and contrast-enhanced CT scans were obtained.
Skeletal Muscle Index Assessment and Definition of Sarcopenia
Contrast-enhanced CT scans were routinely performed before and after NACR (all post-NACR CT scans were obtained within 1 week before surgery). The patients’ staging and re-staging were analyzed through those CT scans. The images at third lumbar vertebra (L3) were analyzed by a single blinded investigator to measure the cross-sectional area (cm2) using MATLAB R2020b software (MathWorks Inc., Natick, MA, USA). Skeletal muscle index (SMI) was calculated as the ratio of the pooled cross-sectional area (at L3) to the square of the height (m2). On the basis of previous large population study, SMI less than 52.4 cm2/m2 for men and less than 38.5 cm2/m2 for women were defined as sarcopenia.21,22 The percent changes in SMI (ΔSMI %) before and after NACR was derived as follows: (SMI pre-NACR − SMI post-NACR)/SMI pre-NACR × 100%.
Outcomes Measurement
The primary outcomes were survival benefit including overall survival (OS) and disease-free survival (DFS). The recurrence of local esophagus and locoregional lymph nodes and the metastasis of distant lymph nodes and distant organs were identified as disease-related progression. DFS was calculated from the day of surgery to the date of disease-related progression or death. Staging was performed according to the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) staging system (8th edition).
The secondary outcomes were histological tumor response and postoperative complications defined as the Clavien–Dindo (CD) classification system.23 Pathological complete response (pCR) was defined as no tumor cells in the specimen, and no tumor cells in lymph nodes (ypT0N0). Tumor response score (TRS) was defined as follows: grade 0, grade 1, grade 2, and grade 3.24 TRS 0 and TRS 1 means good response, however, TRS 2 and TRS 3 means partial response and no response, respectively.
Statistical Analysis
Data and outcomes were analyzed using GraphPad Prism version 8.0 (San Diego, CA, USA) and R version 4.2.1 (R Development Core Team, Vienna, Austria). Continuous data were expressed as mean (standard deviation, SD) or median (interquartile range, IQR), and count data were expressed as absolute numbers and percentages. The chi-squared test or Fisher’s exact test were used to analyze categorical data. The Student’s t-test or the Mann–Whitney U test were used to analyze continuous data. Statistical significance was considered when the two-sided probability (p-value) was less than 0.05. Survival curves were drawn using the Kaplan–Meier method and compared using the log-rank test. Hazard ratio (HR) with its 95% confidence interval (CI) was reported. Factors associated with overall survival were analyzed using Cox proportional hazards regression model. The multivariable-adjusted model was used for the analysis of overall survival and adjusted for sex, age, and potential factors with a significant p-value in the univariate analysis.
The nonlinearity of the effect of percent changes in SMI (ΔSMI %) to survival outcomes was assessed by restricted cubic splines (3 knots). To find the optimal cut-off value of ΔSMI% for classifying patients into good or poor survival benefit groups, the Maxstat (R package), a maximal chi-squared statistics method, was used. The patients were first divided into two groups according to the cut-off value of SMIpre-NACR, namely, pre-NACR sarcopenia and pre-NACR non-sarcopenia groups. Similarly, the patients were also classified into two groups according to the cut-off value of SMIpost-NACR, namely, post-NACR sarcopenia and post-NACR non-sarcopenia groups. Second, on the basis of the results of restricted cubic splines (RCS) and maximal chi-squared statistics, we further classified the patients into four groups according to the quartile of ΔSMI%.
Results
Patients’ Characteristics
In total, 671 patients with locally advanced esophageal squamous cell cancer underwent neoadjuvant therapy followed by surgery. After screening, completed data of 367 patients were analyzed according to our inclusion and exclusion criteria (Supplementary Fig.1). Clinical and pathological characteristics of included patients are summarized in Table 1. The majority of all patients were aged 62 years, were men (81.2%), had a normal BMI, and were clinically staged as cT3 (77.11%), cN1 (64.8%), or cIII (57.8%). In terms of neoadjuvant therapy regimen, most patients completed two cycles of paclitaxel-based platinum (94.2%), among whom 77.9% and 16.3% patients received TP and TC regimen, respectively. A total of 256 patients (69.8%) completed neoadjuvant radiation with a dose of 40–45 Gy. Postoperative complications classified as CD I–II and III–IV were reported in 26.2% and 7.1% of patients, respectively. Postoperative 30-day mortality was 3.0%. Pathological staging showed frequent ypT0 and ypN0 stages (40.3% and 67.0%, respectively), and staging I was the most frequent TNM stage (53.4%). The pCR rate was 34.6% in all patients. The microscopic radical resection rate (R0) was 98.2%. Tumor regression score of 0, 1, 2, 3 was found in 39.0%, 15.8%, 34.9%, and 10.4% of patients, respectively. There was a significant linear correlation between changes in weight and changes in SMI after NACR (P = 0.003, Supplementary Fig. 2), and the Δweight increased with the ΔSMI (Supplementary Fig. 3).
Primary Outcomes
Effect of Sarcopenia on Survivorship
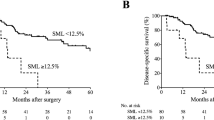
After a mean follow-up of 28 months [95% confidence interval (CI) 26–30 months], 107 (29.2%) patients died, and the 5-year cumulative OS rate was 58.0 % for all included patients. The clinicopathological characteristics of four groups (pre-NACR sarcopenia versus pre-NACR non-sarcopenia and post-NACR sarcopenia versus post-NACR non-sarcopenia) are summarized in Supplementary Table 1. The groups showed significant difference in sex (P = 0.000) and BMI (P = 0.000). On initial survival analysis, the 5-year cumulative OS rate was 56.6% in pre-NACR sarcopenia group and 59.0% in pre-NACR non-sarcopenia group, respectively (log-rank P = 0.439). Of note, patients in post-NACR sarcopenia group compared with those in post-NACR non-sarcopenia group showed poor survival benefit (5-yr cumulative OS 44.3% versus 64.6%; HR 1.67, 95% CI 1.05–2.43, P = 0.016). Similarly, significant difference also was found in the DFS (5-year cumulative DFS 43.9% versus 61.0%; HR 1.48, 95% CI 1.01–2.16, P = 0.043). The survival curves using Kaplan–Meier estimate are shown in Fig. 1.
Effect of the Changes of SMI during NACR on Survivorship
According to the results of restricted cubic splines, the association of ΔSMI% with OS and DFS was non-linear (P = 0.047, P = 0.001, respectively) (Fig. 2). Moreover, the percent changes in SMI during NACR showed a strong U-shaped association with cancer progression. Notably, additional SMI loss after NACR was associated with an increased risk of death (HR 1.93 per SD, 95% CI 1.21–3.09, P = 0.006) and cancer progression (HR 2.01 per SD, 95% CI 1.31–3.09, P = 0.001). Of note, the one-sample Kolmogorov–Smirnov (K-S) tests verified that the distribution of ΔSMI% was normal (P = 0.000). The optimal cut-off point of ΔSMI was 0.12 using Maxstat method (Fig. 3B). On the basis of these novel findings, we further classified patients into four groups according to ΔSMI% quartile. The clinicopathological characteristics of the four groups are listed in Table 1. On initial survival analysis, the 5-year cumulative OS and DFS rates were, respectively, 65.0% in quartile I, 66.1% in quartile II, 69.1% in quartile III, and 40.2% in quartile IV (log-rank P = 0.009), and 57.1% in quartile I, 66.0% in quartile II, 67.3% in quartile III, and 38.1% in quartile IV (log-rank P = 0.002) (Fig. 3C, D). Patients in the quartile IV group compared with those in the quartile II or III groups had a higher risk of death (HR 1.96, 95% CI 1.14–3.35 or HR 2.14, 95% CI 1.26–3.59, respectively) and were associated with higher probability of recurrence (HR 2.03, 95% CI 1.24–3.30 or HR 2.18, 95% CI 1.35–3.51, respectively). In addition, on multivariable analysis of OS, quartile IV was the independent prognostic factor (HR 1.76, 95% CI 1.03–2.99, P = 0.039) and significant difference was also found on DFS analysis (HR 1.75, 95% CI 1.07–2.87, P = 0.025) (Table 2). Namely, patients suffering intense loss of SMI greater than 12% showed poor survival and disease progression during NACR.
(A) Distribution of patients with different percent changes in skeletal muscle index; (B) optimal cut-off point for overall survival by using receiver operating characteristic (ROC) curve; (C) overall survival across different groups of percent changes in SMI (ΔSMI %); (D) disease-free survival across different groups of percent changes in SMI (ΔSMI %)
Secondary Outcomes
Effect of Sarcopenia on Postoperative Outcomes
In the groups defined as sarcopenia and non-sarcopenia before NACR, no significant differences were found in terms of postoperative complication (Supplementary Table 2). These negative results notwithstanding, several meaningful results were found in the groups defined by post-NACR SMI. Patients in the post-NACR sarcopenia group had a higher rate of pneumonia, classified as CD grades 3–5 (11.0% versus 5.0%, P = 0.038), and respiratory failure and readmission ICU (8.3% versus 1.9%, P = 0.010). In total, all severe postoperative complication (CD grades 3–5) rates were 11.9% in post-NACR sarcopenia group and 5.0% in post-NACR non-sarcopenia group (P = 0.019). Summarily, patients with sarcopenia were associated with worse short-term surgical outcomes, notwithstanding, this result was only proven by evidence on post-NACR body composition.
Effect of the Changes of SMI during NACR on Postoperative Outcomes
The rates of postoperative complications classified as CD grades 1–2 were 28.0% in quartile I, 30.4% in quartile II, 22.8% in quartile III, and 23.3% in quartile IV (P = 0.588). The more severe postoperative (CD grades 3–5) were 9.7% in quartile I, 2.2% in quartile II, 5.4% in quartile III, and 11.1% in quartile IV (P = 0.075). The anastomotic leakage rate in quartile I or IV groups was nearly twice as high as other groups (P = 0.133). The median postoperative length of stay was 11 days, similar in all four groups (P = 0.246). In terms of 30-day mortality rates, no significant difference was found in the four groups (P = 0.799) (Supplementary Table 3).
Discussion
This study summarized the role of sarcopenia through neoadjuvant chemoradiotherapy of locally advanced esophageal cancer, and in short-term and long-term outcomes, providing novel results suggesting that sarcopenia and changes in SMI should be evaluated as potential risk markers. Sarcopenia diagnosed after NACR was associated with high risk of death and disease progression, high incidence of postoperative complications, and adverse oncological outcomes. Moreover, additional SMI loss per 9.0% after NACR increased the risk of death and disease progression nearly onefold. Patients suffering SMI decline more than 12% after NACR had a disadvantage of disease-free survival and short-term outcomes.
Many studies and meta-analyses had evaluated the effect of sarcopenia on the short-term and long-term outcomes for patients with esophageal cancer.7,8,9,10,11,12,13,25,26,27,28 This welcome progress notwithstanding, these results were contradictory. Three studies concluded that patients with sarcopenia had no impact on overall survival.9,25,28 There are many methods to assess SMI, including CT images at L3, dual energy X-ray absorptiometry (DXA), and bioelectrical impedance analysis (BIA). It is very simple to diagnosis sarcopenia through routine CT images, however, the cut-off values of SMI at L3 are reported differently. For patients with locally advanced esophageal cancer receiving NACR followed by surgery, it is more important to examine changes in SMI instead of SMI at presentation. As reported in previous studies, SMI loss was associated with poor survival.13,14,15,16,17,18 Kamitani et al. reported that SMI loss greater than 12% was significantly associated with poor survival.16 Similarly with our study, we found a meaningful cut-off point of 12% for the changes in SMI. Additionally, our results also demonstrated that the probability of death and disease progression was increased along with a significant SMI decline. Additionally, Yoon et al. reported that patients with SMI loss greater than 10% had poor overall survival and recurrence-free survival.14
The mechanism of sarcopenia with cancer still remains unclear. Inflammatory, oxidative stress, mitochondrial function disorders, and so on were associated with sarcopenia.29,30 Many inflammation-induced signal pathways can lead to unintentional skeletal muscle loss, which is mediated by serval known factors such as interleukin-6 (IL-6), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and tumor necrosis factor (TNF).31 Mitochondrial function disorders also plays an important role in sarcopenia, which can also be induced by anti-cancer drugs such as doxorubicin and paclitaxel.32 In this context, NACR itself can change skeletal muscle status and the body composition of patients with esophageal cancers. Chemotherapeutic drugs such as cisplatin can cause skeletal muscle decline through Akt pathway, and patients with sarcopenia in esophageal cancer suffered a higher rate of dose-limiting chemotoxicity.33,34,35
Moreover, many researchers not only investigated the body composition at certain times, but also systematically examined the changes in SMI and sarcopenia during neoadjuvant therapy. Generally, numbers of studies demonstrated that the rates of sarcopenia increased and SMI mostly decreased from neoadjuvant therapy to surgery.13,14,15,16,17,18 To date, the influence of neoadjuvant therapy on nutrition status remains unclear, and many potential factors may affect it, such as efficacy of neoadjuvant therapy, use of corticosteroids, adverse treatment-related events, and individual differences during neoadjuvant therapy, and these may contribute to body composition changes either increasing or declining. Thus, the meaning of ΔSMI% may differ between patients with and without pre-NACR sarcopenia. As shown in Supplementary Fig. 4, 71.7% patients in the pre-NACR sarcopenia group show an increased SMI. Hence, it was better to use changes in SMI to predict long-term survival outcomes. Different from previous studies, our results showed patients suffering SMI loss only accounted for half of included patients, and the sarcopenia rate did now show a significant increase. Moreover, patients with a SMI increase of more than 12% were associated with poor disease-free survival in our analysis compared with those with a SMI decrease of less than 12%. These novel results demonstrated that it was better to maintain skeletal muscle during neoadjuvant therapy. According to a report of the International Sarcopenia Initiative, exercise (resistance training, combined exercise/physical activity) and nutrition (protein supplements, essential amino acid supplementation) interventions were the main treatment strategies to enhance muscle mass and function in patients with sarcopenia.36 Nutritional intervention played an important role in the treatment of sarcopenia, and a randomized controlled trial (RCT) proved that home enteral nutrition and preoperative nutritional support was safe and feasible for patients’ recovery after esophagectomy.37 On the basis of our results, when patients had sarcopenia after neoadjuvant therapy or show a SMI loss of more than 12%, it was suggested that specifical interventions should be incorporated into clinical management, for example, providing them with essential amino acid and protein through oral feeding or intravenous infusion peri- and preoperatively might be advantageous to decreasing the rates of postoperative complications and thus benefit survival outcomes.
In the present study, pathological factors across different quartile groups showed significantly different. High SMI loss (ΔSMI% > 12%) was associated with higher risk of a tumor with ypTNM III. In addition, differences in TRS and R0 resection were observed in patients with post-NACR sarcopenia, and they had a lower rate of TRS 0/1 and a higher rate of non-R0 resection compared with those with non-sarcopenia. Consistent with previous studies, patients with major SMI loss or sarcopenia had poor oncological outcomes. In terms of postoperative complications, all severe complications and pneumonia (CD grades 3–5) occurred frequently in patients with post-NACR sarcopenia. These results were consistent with our newly published meta-analysis.38
The main strength of this study was that it included the largest numbers of patients with esophageal cancer who received NACR followed by surgery at present. Further, we could divide the patients into four groups according to cut-off point, and compare survival and postoperative outcomes between different ranges of the changes in SMI.
Several inherent caveats of our study should be acknowledged. First, since this is a retrospective analysis based on our non-randomized controlled cohort, we cannot exclude potential selection bias. Second, owing to our retrospective study design, several inflammation indicators such as CRP, IL-1, IL-6, PCT, and TNF-a could not be investigated. Third, patients’ individual characteristics, such as the use of glucocorticoids, capacity for eating, and external nutrition support during neoadjuvant chemoradiotherapy, could not be collected and analyzed. Finally, since there is no gold standard for the cut-off value of L3-SMI to diagnosis sarcopenia, this study used the value proposed by a relatively large unhealthy population-based study, which was used widely in many studies. This optimal cut-off value based on high-quality research notwithstanding, future studies are warranted to find the optimal cut-off value of L3-SMI for defining sarcopenia in accordance with Asian individuals.
In conclusion, post-neoadjuvant chemoradiotherapy sarcopenia is a significant factor for overall survival, disease-free survival, oncological outcomes, and postoperative complications in this study. Patients with sarcopenia have worse survival and adverse short-term outcomes. Moreover, greater loss in skeletal muscle index is associated with increased risks of death and disease progression during neoadjuvant chemoradiotherapy, with maximum impact noted with SMI loss greater than 12%.
References
Li X, Chen L, Luan S, et al. The development and progress of nanomedicine for esophageal cancer diagnosis and treatment. Semin Cancer Biol. 2022;86:873–85.
Ajani JA, D’Amico TA, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers, Version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(7):855–83.
Eyck BM, van Lanschot JJB, Hulshof M, et al. Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS Trial. J Clin Oncol: Offic J Am Soc Clin Oncol. 2021;39(18):1995–2004.
Kano K, Yamada T, Yamamoto K, et al. The impact of pretherapeutic naples prognostic score on survival in patients with locally advanced esophageal cancer. Ann Surg Oncol. 2021;28(8):4530–9.
Yodying H, Matsuda A, Miyashita M, et al. Prognostic significance of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in oncologic outcomes of esophageal cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2016;23(2):646–54.
McGovern J, Dolan RD, Horgan PG, Laird BJ, McMillan DC. Computed tomography-defined low skeletal muscle index and density in cancer patients: observations from a systematic review. J Cachexia Sarcopenia Muscle. 2021;12(6):1408–17.
Jogiat UM, Sasewich H, Turner SR, et al. Sarcopenia determined by skeletal muscle index predicts overall survival, disease-free survival, and postoperative complications in resectable esophageal cancer: a systematic review and meta-analysis. Ann Surg. 2022;276(5):e311–8.
Ishida T, Makino T, Yamasaki M, et al. Quantity and quality of skeletal muscle as an important predictor of clinical outcomes in patients with esophageal cancer undergoing esophagectomy after neoadjuvant chemotherapy. Ann Surg Oncol. 2021;28(12):7185–95.
Elliott JA, Doyle SL, Murphy CF, et al. Sarcopenia: prevalence, and impact on operative and oncologic outcomes in the multimodal management of locally advanced esophageal cancer. Ann Surg. 2017;266(5):822–30.
Nakashima Y, Saeki H, Nakanishi R, et al. Assessment of sarcopenia as a predictor of poor outcomes after esophagectomy in elderly patients with esophageal cancer. Ann Surg. 2018;267(6):1100–4.
Saeki H, Nakashima Y, Kudou K, et al. Neoadjuvant chemoradiotherapy for patients with cT3/nearly T4 esophageal cancer: is sarcopenia correlated with postoperative complications and prognosis? World J Surg. 2018;42(9):2894–901.
Paireder M, Asari R, Kristo I, et al. Impact of sarcopenia on outcome in patients with esophageal resection following neoadjuvant chemotherapy for esophageal cancer. Eur J Surg Oncol. 2017;43(2):478–84.
Huang CH, Lue KH, Hsieh TC, Liu SH, Wang TF, Peng TC. Association between sarcopenia and clinical outcomes in patients with esophageal cancer under neoadjuvant therapy. Anticancer Res. 2020;40(2):1175–81.
Yoon HG, Oh D, Ahn YC, et al. Prognostic impact of sarcopenia and skeletal muscle loss during neoadjuvant chemoradiotherapy in esophageal cancer. Cancers (Basel). 2020;12(4).
Reisinger KW, Bosmans JW, Uittenbogaart M, et al. Loss of skeletal muscle mass during neoadjuvant chemoradiotherapy predicts postoperative mortality in esophageal cancer surgery. Ann Surg Oncol. 2015;22(13):4445–52.
Kamitani N, Migita K, Matsumoto S, et al. Association of skeletal muscle loss with the long-term outcomes of esophageal cancer patients treated with neoadjuvant chemotherapy. Surg Today. 2019;49(12):1022–8.
Jarvinen T, Ilonen I, Kauppi J, Salo J, Rasanen J. Loss of skeletal muscle mass during neoadjuvant treatments correlates with worse prognosis in esophageal cancer: a retrospective cohort study. World J Surg Oncol. 2018;16(1):27.
Mayanagi S, Tsubosa Y, Omae K, et al. Negative impact of skeletal muscle wasting after neoadjuvant chemotherapy followed by surgery on survival for patients with thoracic esophageal cancer. Ann Surg Oncol. 2017;24(12):3741–7.
Liu J, Motoyama S, Sato Y, et al. Decreased skeletal muscle mass after neoadjuvant therapy correlates with poor prognosis in patients with esophageal cancer. Anticancer Res. 2016;36(12):6677–85.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7.
Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–35.
Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33(5):997–1006.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13.
Darragh TM, Colgan TJ, Cox JT, et al. The Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med. 2012;136(10):1266–97.
Grotenhuis BA, Shapiro J, van Adrichem S, et al. Sarcopenia/muscle mass is not a prognostic factor for short- and long-term outcome after esophagectomy for cancer. World J Surg. 2016;40(11):2698–704.
Yip C, Goh V, Davies A, et al. Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer. Eur Radiol. 2014;24(5):998–1005.
Tamandl D, Paireder M, Asari R, Baltzer PA, Schoppmann SF, Ba-Ssalamah A. Markers of sarcopenia quantified by computed tomography predict adverse long-term outcome in patients with resected oesophageal or gastro-oesophageal junction cancer. Eur Radiol. 2016;26(5):1359–67.
Murimwa GZ, Venkat PS, Jin W, et al. Impact of sarcopenia on outcomes of locally advanced esophageal cancer patients treated with neoadjuvant chemoradiation followed by surgery. J Gastrointest Oncol. 2017;8(5):808–15.
Rolland Y, Czerwinski S, Abellan Van Kan G, et al. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging. 2008;12(7):433–50.
Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int. 2010;21(4):543–59.
Cesari M, Kritchevsky SB, Baumgartner RN, et al. Sarcopenia, obesity, and inflammation–results from the trial of angiotensin converting enzyme inhibition and novel cardiovascular risk factors study. Am J Clin Nutr. 2005;82(2):428–34.
Calvani R, Joseph AM, Adhihetty PJ, et al. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol Chem. 2013;394(3):393–414.
Awad S, Tan BH, Cui H, et al. Marked changes in body composition following neoadjuvant chemotherapy for oesophagogastric cancer. Clin Nutr. 2012;31(1):74–7.
Anandavadivelan P, Brismar TB, Nilsson M, Johar AM, Martin L. Sarcopenic obesity: a probable risk factor for dose limiting toxicity during neo-adjuvant chemotherapy in oesophageal cancer patients. Clin Nutr. 2016;35(3):724–30.
Tan BH, Brammer K, Randhawa N, et al. Sarcopenia is associated with toxicity in patients undergoing neo-adjuvant chemotherapy for oesophago-gastric cancer. Eur J Surg Oncol. 2015;41(3):333–8.
Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. 2014;43(6):748–59.
Liu K, Ji S, Xu Y, et al. Safety, feasibility, and effect of an enhanced nutritional support pathway including extended preoperative and home enteral nutrition in patients undergoing enhanced recovery after esophagectomy: a pilot randomized clinical trial. Dis Esophagus. 2020;33(2):doz030.
Fang P, Zhou J, Xiao X, et al. The prognostic value of sarcopenia in oesophageal cancer: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2022;14(1):3–16.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 81970481), 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (grant nos. 2020HXFH047 and 20HXJS005), and Sichuan Science and Technology Program (grant no. 2022YFS0048).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
The Institutional Review Board (IRB) of West China hospital approved our study (IRB no. 2022-853), and the writing of informed consent was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Fig. S1
Flow chart of the study. Fig. S2 Correlation between change in skeletal muscle index (SMI) and weight. Fig. S3 Mean values of Δweight and ΔSMI across four groups. Fig. S4 Distribution of patients with increased or declined SMI in the sarcopenia and non-sarcopenia groups before neoadjuvant therapy (DOCX 47 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiao, X., Fang, PH., Zhou, JF. et al. Impact of Skeletal Muscle Loss and Sarcopenia on Outcomes of Locally Advanced Esophageal Cancer during Neoadjuvant Chemoradiation. Ann Surg Oncol 31, 3819–3829 (2024). https://doi.org/10.1245/s10434-024-14936-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-024-14936-3