Abstract
Objectives
To assess the impact of sarcopenia and alterations in body composition parameters (BCPs) on survival after surgery for oesophageal and gastro-oesophageal junction cancer (OC).
Methods
200 consecutive patients who underwent resection for OC between 2006 and 2013 were selected. Preoperative CTs were used to assess markers of sarcopenia and body composition (total muscle area [TMA], fat-free mass index [FFMi], fat mass index [FMi], subcutaneous, visceral and retrorenal fat [RRF], muscle attenuation). Cox regression was used to assess the primary outcome parameter of overall survival (OS) after surgery.
Results
130 patients (65 %) had sarcopenia based on preoperative CT examinations. Sarcopenic patients showed impaired survival compared to non-sarcopenic individuals (hazard ratio [HR] 1.87, 95 % confidence interval [CI] 1.15–3.03, p = 0.011). Furthermore, low skeletal muscle attenuation (HR 1.91, 95 % CI 1.12–3.28, p = 0.019) and increased FMi (HR 3.47, 95 % CI 1.27–9.50, p = 0.016) were associated with impaired outcome. In the multivariate analysis, including a composite score (CSS) of those three parameters and clinical variables, only CSS, T-stage and surgical resection margin remained significant predictors of OS.
Conclusion
Patients who show signs of sarcopenia and alterations in BCPs on preoperative CT images have impaired long-term outcome after surgery for OC.
Key Points
• Sarcopenia is associated with impaired OS after surgery for oesophageal cancer.
• Other body composition parameters are also associated with impaired survival.
• This influence on survival is independent of established clinical parameters.
• Sarcopenia provides a better estimation of cachexia than BMI.
• Sarcopenia assessment could be considered in risk/benefit stratification before oesophagectomy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among cancers of the digestive tract, oesophageal cancer (OC) is one of the leading causes of mortality as well as treatment-associated morbidity worldwide [1, 2]. Since most patients are diagnosed in advanced stages, management of patients with OC remains challenging. Surgery remains the only potentially curative treatment, which still bears high post-procedural morbidity and mortality [3]. Patient selection, therefore, is crucial in this disease, with a complex interplay between high-risk procedures and, often, multiple comorbidities [4].
Dysphagia and weight loss are often encountered in patients with OC, and multiple approaches have been reported to link those disease-specific symptoms to adverse outcomes [5]. More recently, the concept of sarcopenia as a more accurate means to describe muscle loss [6, 7] and cancer-related cachexia [8] has been introduced. Sarcopenia has been defined by international consensus [9] as a skeletal muscle index (SMI) of ≤39 cm2/m2 for women and ≤55 cm2/m2 for men. SMI reflects the total cross-sectional skeletal muscle area at the level of lumbar vertebra L3 and has been shown to correlate well with whole-body muscle mass. Furthermore, the assessment of fat-free mass (FFM) and total fat mass (FM) seems to better correlate with a relevant reduction in muscle mass than changes in body weight or body mass index (BMI) alone [8]. All those parameters can be measured on readily available abdominal CTs.
The presence of sarcopenia has been linked to poor long-term outcome in pancreatic [10], respiratory tract, colorectal [8] and liver cancer [11]. Since patients with OC are prone to malnutrition and often present with marked weight loss, a high prevalence of sarcopenia in this patient population is expected, and has been described to be as high as 57 % [12]. Interestingly, BCP alterations are associated with incomplete resections (R1, microscopic tumour cells at the resection margin) [13] and increased postoperative morbidity [14] in patients with OC. However, a clear association with impaired survival has not been demonstrated yet [15].
The goal of this study was to assess whether the presence of sarcopenia and alterations in other BCPs, which can be measured with CT, were associated with impaired survival in patients with OC.
Methods
The local institutional review board approved the study, and informed consent for the retrospective analysis was waived.
Patients who had CT scans for suspected oesophageal malignancies between January 2006 and December 2013 at our tertiary centre were included. We matched this list of patients with a consecutive list of patients who had surgery for suspected OC or cancer of the gastro-oesophageal junction at the Department of Surgery at our institution. For semantic purposes, all those patients are referred to as OC patients. We excluded 21 patients whose images were either not retrievable or who did not have a complete abdominal examination covering the level of the lumbar vertebra 3 (L3). After further exclusion of patients who had metastatic disease, or who were not resected for any reason or were not shown to have malignancy, 200 patients with OC remained who had undergone potentially curative surgery and were suitable for analysis of sarcopenia and BCPs. The selection process is summarized in Fig. 1.
The following demographic and tumour-related parameters were extracted from the hospital information system: age, gender, weight, height, tumour location, histology, type of neoadjuvant treatment, type of surgery, margin status, tumour grading, T-stage and N-stage.
Clinical and radiological follow-up was routinely performed on a 3-month basis during the first 2 years after surgery and every 6 months thereafter, either by the treating surgeons or by the medical oncologists at our or outside institutions. Overall survival (OS) was defined as time from surgery to death due to any reason.
CT protocol
At our institution, patients underwent contrast-enhanced CT using a 64-row multi-detector CT (Siemens Somatom 64, or Biograph TruePoint64, both Siemens, Erlangen, Germany). After fasting for 4 hours, patients were instructed to drink 1,000–1,500 mL of water immediately before the scan, since it has been shown that this helps to distend the oesophageal and gastric lumen in order to better aid with the detection and characterization of oesophageal tumours [16]. A contrast-enhanced scan of the neck, chest and abdomen was performed after the injection of 2 mL/kg (at least 100 mL) Iomeron 300® (Bracco, Milan, Italy), using a flow rate of 4 mL/s, followed by a saline flush (50 mL). Scan delay for the arterial phase was 30 s (covering the oesophagus and upper abdomen), followed by a portal venous phase of the abdomen with a delay of 60 s. Section collimation was 64 × 0.6 mm, slice thickness was 3 mm with 2-mm increments, and a 512 × 512 matrix was used. Tube voltage was 120 kVp. Coronal and sagittal reconstructions were performed with a 3- to 5-mm slice thickness, depending on patient size. Various scanners and protocols were used in patients who had had a preoperative staging examination at outside institutions, but at least a portal venous phase and coverage of L3 were required for those examinations to be included.
Image analysis
For the analysis of CT images, venous-phase axial images of the abdomen were exported to a workstation using OSIRIX© V5.0 (Pixmeo, Sarl, Switzerland). A single slice on the level of L3, with both transverse processes visible, was selected. Semi-automated, specific tissue demarcation was performed using the following Hounsfield unit (HU) thresholds: −29 to +150 (skeletal muscle excluding visceral organs); −190 to −30 (subcutaneous and intramuscular adipose tissue); and −150 to −50 (visceral adipose tissue), as described previously [13] (Fig. 2a–d). Manual corrections were performed in case other structures outside the respective compartment were detected. At the level of L3, the cross-sectional area of total skeletal muscle (TMA), subcutaneous and visceral adipose tissue was assessed [13]. Furthermore, the mean muscle attenuation in was measured in HU. Retrorenal fat diameter (RRF) was measured at the midlevel of each kidney, since this has also been reported as a prognostic parameter for various adverse outcomes in other cancers [17]. Measurements were performed by a single reader (DT), were saved as JPEG files and were cross-read by a second operator (MP) before entering into the database. Total FFM and FM were calculated as follows [13]:
Measurement of body composition parameters using computed tomography at the level of L3. (a) Delineation of total muscle area (TMA), using a threshold of −29 to +150 HU. (b) Delineation of visceral fat area, using a threshold of −150 to −50 HU. (c) Delineation of subcutaneous fat area, using a threshold of −190 to −30 HU. (d) Measurement of retrorenal fat (RRF). A representative section on the mid-level of each kidney is selected and the minimal fat diameter is measured
Both parameters were corrected for body habitus, similarly to that performed for BMI:
For the assessment of sarcopenia, the skeletal muscle index (SMI, cm2/m2) must be calculated, which is TMA at L3 / (height [m] × height [m]). Sarcopenia was defined by international consensus [9] as an SMI of ≤39 cm2/m2 for women and ≤55 cm2/m2 for men.
Statistics
SPSS (Version 21.0, SPSS Inc., Chicago, IL, USA) was used for the statistical analysis.
All variables are depicted as median and interquartile range (IQR) or 95 % confidence intervals (CIs). Variables were compared between the respective subgroups using the Mann-Whitney U test for two subgroups. Correlations were calculated using the Pearson rank correlation test. P-values <0.05 were considered significant.
Survival analysis was performed using a Cox proportional hazards regression model, and hazard ratios (HRs), with 95 % CIs, were used for the data presentation. Optimal cut-off determination was performed as described by Budczies et al. [18], and variables were dichotomized based on their association with OS, if applicable. For the purpose of multivariable analysis, a Cox proportional hazards model with backward elimination was used. All variables with p < 0.05 on the univariate analysis were entered into the model. Furthermore, age, gender, BMI and histology were used to correct for potential factors associated with sarcopenia. Collinearity was excluded using pairwise Pearson regression analysis (see Table S1, Supplemental Digital Content, which shows Pearson regression coefficients of all investigated variables).
Results
Patients
Of the 200 patients who had surgery for OC in the designated time period, 81 died during the follow-up period. The median OS was 39.4 (95 % CI, 28.7–50.1) months. The estimated 1-, 3- and 5-year survival of the entire cohort was 78.3 %, 52.1 % and 40.3 %, respectively. The median follow-up was 35.1 (95 % CI, 28.6–41.5) months.
Demographics and tumour details
The demographic distribution and summary of tumour-related details are depicted in Table 1, both for the entire cohort and with regard to the presence of sarcopenia. Based on CT parameters, 130 patients (65 %) in our cohort had sarcopenia.
Distribution of body composition parameters (BCPs) in relation to sarcopenia
Patients with sarcopenia had lower total TMA, SMI and FFMi, as expected by the definition of this condition. There was no difference in the attenuation of skeletal muscles (median HU 36 vs. 37, p = n.s.) with regard to sarcopenia.
The subcutaneous fat area, as well as FMi, was also reduced in sarcopenic patients, while visceral fat, retrorenal fat and the total fat-to-muscle ratio was evenly distributed among patients. These findings are summarized in Table 2.
Distribution of BCPs in relation to histology
Since all tumour-related variables except tumour type (adenocarcinoma vs. squamous cell carcinoma) were well balanced with regard to sarcopenia, we performed a separate analysis of body composition parameters (Table 3). Patients with squamous cell carcinoma had reduced values in both muscle parameters (SMI, TMA, FFMi) and fat parameters (FMI, FMR, visceral and subcutaneous fat area, RRF) compared to patients with adenocarcinoma. There was no difference between those two groups with regard to muscle attenuation (median HU 36 and 37, respectively).
Univariate analysis of BCP and clinical variables in association with overall survival (OS)
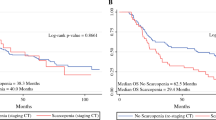
Patients with sarcopenia had worse survival compared to non-sarcopenic patients (HR 1.87 [1.15–3.03], p = 0.011; median OS 31.5 [22.0–40.9] vs. 76.5 [46.5–106.6] months, Fig. 3a). For the remaining parameters of body composition, optimal cutoff parameters were determined as described in the Methods section. For muscle attenuation, the optimal cutoff value was 39.5 HU, which was rounded to the next even number (40 HU). Patients with muscle attenuation values ≤40 HU had impaired OS compared with patients with a >40 HU (HR 1.91 [1.12–3.28], p = 0.019; median OS 34.6 [22.2–47.0] months vs. not reached, Fig. 3b). For FMi, a cutoff of 5.85 kg/m2 was calculated. Patients with a higher FMi of >5.85 kg/m2 had worse OS than patients with a low FMi (HR 3.47 [1.27–9.50], p = 0.016; median OS 34.6 [24.3–44.9] months vs. not reached, Fig. 3c), similar to patients with a total FMR of >1.38 (HR 2.16 [1.11–4.20], p = 0.023; median OS 35.4 [25.8–45.1] months vs. not reached). Clinical variables associated with tumour stage (T-stage, nodal stage, grading), as well as surgical resection margin, were predictive of worse OS, while age, gender, BMI and tumour histology were not associated with worse outcomes. These findings are summarized in Table 4.
Kaplan-Meier analysis of overall survival in relation to various body composition parameters. (a) Presence of sarcopenia; (b) muscle attenuation; (c) fat mass index (kg/m2); (d) Composite sarcopenia score, for each fulfilled criterion depicted in Fig. 3a-c, one point is added to achieve a possible score of 0–3. HR hazard ratio
Composite sarcopenia score
In order to allow easier interpretation and comprehensive evaluation of the effect of BCP on survival, we chose three dichotomized variables, which were associated with OS in the univariate analysis, and did not show a high correlation with other variables of BCP (Table S1). Those three parameters were the presence of sarcopenia (yes/no), muscle attenuation (≤40 HU or >40 HU) and FMi (>5.85 kg/m2 or ≤5.85 kg/m2). For each fulfilled criterion, one point was added, leading to a possible score of 0 to 3. In the univariate analysis, the score was associated with worse survival, with an HR of 1.93 (95 % CI 1.39–2.67, p < 0.001, Fig. 3d).
Multivariate analysis
In the multivariate Cox regression model, all clinical variables with p < 0.05 were included, and a correction for age, gender, BMI and tumour histology, which were shown to be associated with sarcopenia but not survival, was performed. Rather than adding all individual variables of BCP into the model, only the CSS was used as a comprehensive measure of cachexia. After elimination in the backward stepwise regression model, only CSS, T-stage and resection margin remained as independent predictors in our cohort (Table 5).
Discussion
In this analysis including 200 patients with OC, we could show that sarcopenia and associated changes in body composition such as increased fat mass and decreased muscle attenuation, are independently associated with poor long-term outcome after potentially curative surgery.
The indicators of sarcopenia can be easily identified on any preoperative CT and might aid in the difficult triage and selection process before those extensive surgeries.
In a recent cohort of 230 patients who underwent transhiatal oesophagectomy for cancer, Sheetz et al. [15] demonstrated an association between improved survival and an increasing psoas muscle area. This study is somewhat comparable to ours, yet those authors could only show a difference in survival in patients without neoadjuvant chemoradiotherapy (n = 64). The study was further limited since only the psoas area was assessed and no comparable measurements of total muscle area at L3, which was shown to correlate best with whole body muscle mass [8, 19], were performed.
The concept of sarcopenic obesity [20], which is widely discussed currently [21], leads to a new understanding that even overweight individuals can be subject to muscle wasting and that BMI is actually not the method of choice to assess nutritional status in those patients. In several studies, patients with sarcopenia and obesity had the worst outcome compared to other analysed subgroups [22, 23].
However, in our study, only a small subgroup of patients was obese, without association to impaired survival (data not shown). We observed, though, that patients with an increased fat mass index >5.85 kg/m2 had a worse outcome than patients with less total fat tissue. Although patients with OC are less likely to be obese due to the inherent characteristics of the disease, this observation shows that increased fat mass is associated with a poor oncological outcome, as is the case in patients with sarcopenic obesity.
One interesting finding, which has already been reported by several groups, was that a low skeletal muscle attenuation ≤40 HU was associated with worse outcomes [8, 24, 25]. This reflects the immanent changes in body musculature induced by cancer cachexia, which might go beyond simple muscle mass loss, and rather relates to a real qualitative alteration in the muscle tissues [26]. At this point, we can only speculate about a potential mechanism for these findings, agreeing with Chang et al. [27] that, due to the deposition of intramuscular lipids, a qualitative as well as a quantitative change in muscle composition might be of importance in cancer patients.
Our study had several limitations: One point of criticism is that not all patients were investigated with the same CT protocol. To truthfully prove our observations, an analysis of patients examined only on one scanner would have to be performed. However, low intra- and inter-reader variability of HU measurements have been reported [28], confirming that a semiautomatic quantification method with manual correction like this can be applied across various devices. Since we performed a single-centre analysis, an independent confirmatory study would also be required in order to transfer these findings to the general population and to confirm the cutoff values we calculated for patients with OC. A larger patient cohort would also allow us to perform more sophisticated subgroup analyses. However, the results of our study show that a simple CT performed in any centre can be used to evaluate sarcopenia, so there might be a broad applicability of those findings, regardless of technical circumstances.
The results from our analysis regarding distribution of fat parameters with regard to histology confirm the clinically well known difference between patients with adenocarcinoma compared to those with squamous cell carcinoma: Patients with squamous cell carcinoma tend to be have less fat and muscle mass compared to patients with adenocarcinoma, probably due to the difference in carcinogenesis and nutritional factors. Since we did not observe a difference in OS with regard to histology, we decided to perform the analysis of the entire cohort with histology as a correction factor in the multivariate analysis.
A further limiting factor is the fact that follow-up could have been longer (at present almost 3 years), in order to better estimate 5-year survival, a well established oncological outcome parameter. Since all calculations were performed with OS as a continuous, time-dependent variable, the effect of dropouts and patients with relatively short follow-up is compensated.
At present, we are only beginning to understand the complexity of cancer-related sarcopenia and cachexia. One potential application could be that patients who show signs of sarcopenia on their preoperative CT scan might be candidates for intense nutritional support both before and after surgery [29], respectively. One reason why many trials of intensive perioperative nutrition have failed [30] could be that perhaps the wrong patients were chosen for those interventions, which were mostly selected based on BMI. It would need prospective studies using sarcopenia and BCP as inclusion criteria to test this hypothesis.
Assessment of sarcopenia could help in the patient selection process, which is key in a disease like OC, where the decision to perform surgery is strongly influenced by high treatment-specific morbidity and mortality of the procedure s. It is crucial to know which patients would benefit from this radical yet potentially curative treatment, and which patients would ultimately live no longer than if he or she received only palliative treatment. Sarcopenia assessment could also be helpful in the follow-up after oesophagectomy to identify patients who develop muscle wasting and therefore decay in their nutritional status. However, the significance of this finding has still to be tested. With regard to potential technical improvements, it would be of interest to evaluate the value of dual-energy CT in the assessment of sarcopenia and BCP. Since dual-energy absorptiometry (DEXA) is a well established method for measurement of whole-body composition, this method could provide more exact estimation of muscle composition together with the ability to perform those cross-sectional measurements derived from CT images. However, we are not aware of any studies looking at this modality with regard to OC.
In conclusion, sarcopenia and other markers of body composition such as fat mass and muscle attenuation were predictive of impaired survival after surgery for OC in our study. They are easily identified on any preoperative abdominal CT scan and might help in patient risk stratification and optimization before oesophagectomy.
Abbreviations
- BCPs:
-
Body composition parameters
- BMI:
-
Body mass index
- CSS:
-
Composite Sarcopenia Score
- FFM(i):
-
Fat-free mass (index)
- FM(i):
-
Fat mass (index)
- FMR:
-
Fat-to-muscle ratio
- HU:
-
Hounsfield unit
- OC:
-
Oesophageal cancer
- OS:
-
Overall survival
- PET:
-
Positron-emission tomography
- RRF:
-
Retrorenal fat
- SMI:
-
Skeletal muscle index
- TMA:
-
Total muscle area
References
Castro C, Bosetti C, Malvezzi M et al (2014) Patterns and trends in esophageal cancer mortality and incidence in Europe (1980-2011) and predictions to 2015. Ann Oncol 25:283–290
Gonzalez L, Magno P, Ortiz AP et al (2013) Esophageal cancer incidence rates by histological type and overall: Puerto Rico versus the United States Surveillance, Epidemiology, and End Results population, 1992–2005. Cancer Epidemiol 37:5–10
Gronnier C, Trechot B, Duhamel A et al (2014) Impact of neoadjuvant chemoradiotherapy on postoperative outcomes after esophageal cancer resection: results of a European multicenter study. Ann Surg 260:764–771
Hoppo T, Jobe BA (2013) Personalizing therapy for esophageal cancer patients. Thorac Surg Clin 23:471–478
Wikman A, Johar A, Lagergren P (2014) Presence of symptom clusters in surgically treated patients with esophageal cancer: implications for survival. Cancer 120:286–293
Baumgartner RN, Koehler KM, Gallagher D et al (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147:755–763
Cosqueric G, Sebag A, Ducolombier C, Thomas C, Piette F, Weill-Engerer S (2006) Sarcopenia is predictive of nosocomial infection in care of the elderly. Br J Nutr 96:895–901
Prado CM, Lieffers JR, McCargar LJ et al (2008) Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9:629–635
Fearon K, Strasser F, Anker SD et al (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12:489–495
Balentine CJ, Enriquez J, Fisher W et al (2010) Intra-abdominal fat predicts survival in pancreatic cancer. J Gastrointest Surg 14:1832–1837
Voron T, Tselikas L, Pietrasz D et al (2014) Sarcopenia impacts on short- and long-term results of hepatectomy for hepatocellular carcinoma. Ann Surg. doi:10.1097/SLA.0000000000000743
Awad S, Tan BH, Cui H et al (2012) Marked changes in body composition following neoadjuvant chemotherapy for oesophagogastric cancer. Clin Nutr 31:74–77
Yip C, Goh V, Davies A et al (2014) Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer. Eur Radiol 24:998–1005
Ida S, Watanabe M, Karashima R et al (2014) Changes in body composition secondary to neoadjuvant chemotherapy for advanced esophageal cancer are related to the occurrence of postoperative complications after esophagectomy. Ann Surg Oncol 21:3675–3679
Sheetz KH, Zhao L, Holcombe SA et al (2013) Decreased core muscle size is associated with worse patient survival following esophagectomy for cancer. Dis Esophagus 26:716–722
Ba-Ssalamah A, Matzek W, Baroud S et al (2011) Accuracy of hydro-multidetector row CT in the local T staging of oesophageal cancer compared to postoperative histopathological results. Eur Radiol 21:2326–2335
House MG, Fong Y, Arnaoutakis DJ et al (2008) Preoperative predictors for complications after pancreaticoduodenectomy: impact of BMI and body fat distribution. J Gastrointest Surg 12:270–278
Budczies J, Klauschen F, Sinn BV et al (2012) Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One 7, e51862
Shen W, Punyanitya M, Wang Z et al (1985) (2004) Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol 97:2333–2338
Koo HK, Park JH, Park HK, Jung H, Lee SS (2014) Conflicting role of sarcopenia and obesity in male patients with chronic obstructive pulmonary disease: Korean National Health and Nutrition Examination Survey. PLoS One 9, e110448
Poggiogalle E, Migliaccio S, Lenzi A, Donini LM (2014) Treatment of body composition changes in obese and overweight older adults: insight into the phenotype of sarcopenic obesity. Endocrine 47:699–716
Anandavadivelan P, Brismar TB, Nilsson M, Johar AM, Martin L (2015) Sarcopenic obesity: A probable risk factor for dose limiting toxicity during neo-adjuvant chemotherapy in oesophageal cancer patients. Clin Nutr. doi:10.1016/j.clnu.2015.05.011
Yip C, Dinkel C, Mahajan A, Siddique M, Cook GJ, Goh V (2015) Imaging body composition in cancer patients: visceral obesity, sarcopenia and sarcopenic obesity may impact on clinical outcome. Insights Imaging. doi:10.1007/s13244-015-0414-0
Martin L, Birdsell L, Macdonald N et al (2013) Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 31:1539–1547
Idoate F, Cadore EL, Casas-Herrero A et al (2015) Adipose tissue compartments, muscle mass, muscle fat infiltration, and coronary calcium in institutionalized frail nonagenarians. Eur Radiol 25:2163–2175
Di Sebastiano KM, Yang L, Zbuk K et al (2013) Accelerated muscle and adipose tissue loss may predict survival in pancreatic cancer patients: the relationship with diabetes and anaemia. Br J Nutr 109:302–312
Chang D, Joseph DJ, Ebert MA et al (2014) Effect of androgen deprivation therapy on muscle attenuation in men with prostate cancer. J Med Imaging Radiat Oncol 58:223–228
Hammarstedt L, Thilander-Klang A, Muth A, Wangberg B, Oden A, Hellstrom M (2013) Adrenal lesions: variability in attenuation over time, between scanners, and between observers. Acta Radiol 54:817–826
Llop-Talaveron JM, Farran-Teixidor L, Badia-Tahull MB et al (2014) Artificial nutritional support in cancer patients after esophagectomy: 11 years of experience. Nutr Cancer 66:1038–1046
Manba N, Koyama Y, Kosugi S et al (2014) Is early enteral nutrition initiated within 24 hours better for the postoperative course in esophageal cancer surgery? J Clin Med Res 6:53–58
Acknowledgments
This paper was presented as an oral presentation at RSNA 2014. The scientific guarantor of this publication is Ahmed Ba-Ssalamah. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
The authors state that this work has not received any funding. One of the authors has significant statistical expertise (Pascal A. Baltzer). Institutional Review Board approval was obtained.
Written informed consent was waived by the Institutional Review Board. No study subjects or cohorts have been previously reported. Methodology: retrospective, observational, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Upper numbers denote the R-value (Pearson regression coefficient), lower numbers are p-values SMI skeletal muscle index, FFMi free fat mass index, HU hounsfield units, FMi fat mass index, FMR fat to muscle ratio, RRF retrorental fat (XLSX 12 kb)
Rights and permissions
About this article
Cite this article
Tamandl, D., Paireder, M., Asari, R. et al. Markers of sarcopenia quantified by computed tomography predict adverse long-term outcome in patients with resected oesophageal or gastro-oesophageal junction cancer. Eur Radiol 26, 1359–1367 (2016). https://doi.org/10.1007/s00330-015-3963-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3963-1