Abstract
Purpose
To investigate the change in skeletal muscle mass and evaluate the prognostic impact of sarcopenia on esophageal cancer (EC) patients
Methods
The subjects of this retrospective study were 90 EC patients who were treated with neoadjuvant chemotherapy (NAC) and subsequent esophagectomy. The skeletal muscle index (SMI) was defined according to computed tomography (CT) imaging of the total cross-sectional muscle tissue, measured at the third lumbar level using a volume analyzer before NAC and surgery. The SMI was calculated by normalization according to height, and skeletal muscle loss (SML) was defined as (pre-NAC SMI value − preoperative SMI value) × 100/pre-NAC SMI.
Results
Sarcopenia was evident in 72 (80.0%) patients before NAC and 77 (85.6%) patients before NAC and surgery. The SMI value was decreased in 28 (68.9%) patients and the median SML was 3.3%. The 3-year overall survival rate was 68.9% in the low SML group and 0% in the high SML group (P < 0.001). Sarcopenia before NAC or surgery was not significantly associated with overall survival. Multivariable analysis identified high SML as an independent prognostic factor.
Conclusions
These results suggest that skeletal muscle loss is associated with a worse long-term outcome for EC patients treated with NAC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcopenia is defined as the progressive loss of skeletal muscle mass (SMM) related to aging or disease and is common in patients with cancer [1]. Sarcopenia is associated with various outcomes, such as postoperative complications as well as response and tolerance to anticancer therapy [2,3,4,5]. Moreover, several studies have provided evidence that the presence of sarcopenia has a negative prognostic impact on various types of cancer [2, 3, 6, 7].
Although esophagectomy is currently the mainstay of treatment for esophageal cancer (EC), the prognosis of patients with EC remains poor [8]. Therefore, a multimodal treatment approach, combining surgery with neoadjuvant chemotherapy (NAC) or neoadjuvant chemoradiotherapy (NACRT), has been attempted [9, 10]. Recently, a more intensive chemotherapeutic regimen, with the combination of docetaxel, cisplatin, and 5-fluorouracil [5-FU], also known as “DCF”), was tested in the neoadjuvant setting [11]. Chemotherapy is frequently associated with a variety of adverse effects that can lead to SMM reduction and nutritional status deterioration [12,13,14]. However, chemotherapy also has the potential to decrease the tumor bulk and reduce the risk of micrometastasis. Therefore, an increase in SMM may be achieved in responders to chemotherapy, but thus far, there is limited information regarding the influence of NAC on SMM and the prognostic importance of sarcopenia in patients with EC treated with NAC. We conducted the present study to investigate the changes in SMM and the prognostic impact of sarcopenia on EC treated with NAC and subsequent esophagectomy.
Methods
Patients and data
A total of 119 patients received NAC followed by esophagectomy for clinical Stage IB–III EC between February, 2007 and August, 2018 at the Nara Medical University Hospital, Nara, Japan. Patients without available computed tomography (CT) data prior to and after NAC (n = 25) and those who underwent R2 resection (n = 4) were excluded from the study. The final analysis population comprised 90 patients. This study was approved by the Local Ethics Committee on Clinical Investigation of Nara Medical University (No. 2164) and written informed consent was obtained from all patients.
The following clinicopathological characteristics of the patients were collected retrospectively from medical records: age, gender, clinical tumor depth before NAC, clinical lymph node metastasis before NAC, pathological tumor depth, pathological lymph node metastasis, pathological response to NAC, adverse events caused by NAC, the serum carcinoembryonic antigen (CEA) value and the presence of squamous cell carcinoma immediately before surgery, and postoperative complications.
The adverse events caused by NAC were evaluated according to the National Cancer Institute Common Toxicity Criteria version 4.0. The pathological response to NAC was evaluated according to the Japanese Classification of Esophageal Cancer as follows: Grade 0, no recognizable cytological or histological therapeutic effect; Grade 1a, viable cancer cells account for two-thirds of the tumor tissue; Grade 1b, viable cancer cells account for one–two-thirds of the tumor tissue; Grade 2, viable cancer cells account for less than one-third of the tumor tissue; and Grade 3, no viable cancer cells are present [15].
Neoadjuvant chemotherapy
The NAC regimens included DCF (n = 30; 33.3%), divided DCF (n = 58; 62.5%) and a combination of 5-FU and cisplatin (FP) (n = 2; 2.2%). DCF therapy consisted of docetaxel (70 mg/m2) on day 1, cisplatin (70 mg/m2) on day 1, and 5-FU (700 mg/m2) on days 1–5 of a 21-day cycle. The divided DCF therapy consisted of docetaxel (35 mg/m2) on day 1, cisplatin (6 mg/m2) on days 1–5, and 5-FU (350 mg/m2) on days 1–5 of a 14-day cycle. The FP regimen consisted of cisplatin (5 mg/m2) and 5-FU (250 mg/m2) on days 1–5 of a 14-day cycle. In principle, two courses of each regimen were planned. A total of 79 (87.8%) patients completed these two courses of therapy. Six (6.7%) patients underwent only one course of the therapy because of adverse events related to chemotherapy and three (5.5%) patients underwent three courses.
Preoperative assessment
The patients principally underwent upper gastrointestinal barium meal examination, upper gastrointestinal endoscopy, and enhanced CT from the neck to the abdomen before the initiation of NAC and surgery, for the evaluation of tumor stage and response to NAC. The tumor stage was classified according to the seventh edition of the American Joint Committee on Cancer TNM classification system [16].
Surgical treatment
All patients underwent esophagectomy within 4 weeks after the completion of NAC. Subtotal esophagectomy was performed in 87 (96.7%) patients and middle–lower esophagectomy was performed in 3 (3.3%) patients. Three- and two-field lymph node dissections were performed in 79 (87.8%) and 11 (12.2%) patients, respectively.
Measurement of SMM and analysis
Computed tomography (CT) scans were used to assess the total cross-sectional transverse areas of skeletal muscles at the third lumbar vertebra. The psoas, quadratus lumborum, paraspinal, transverse abdominal, external oblique, internal oblique, and rectus abdominal muscles were included. The images were analyzed using the volume analyzer SYNAPSE VINCENT software (Fujifilm Company, Tokyo, Japan). A threshold range of − 29 to 150 Hounsfield units was used to define the muscles. The measured total muscle area (cm2) was normalized according to height in square meters and reported as the skeletal muscle index (SMI). Sarcopenia was defined based on sex-specific cutoff values. The threshold of the SMI for sarcopenia was 52.4 cm2/m2 in men and 38.5 cm2/m2 in women [17]. Skeletal muscle loss (SML) was defined as follows: %SML = (pre-NAC SMI value − preoperative SMI value) × 100/pre-NAC SMI.
Statistical analysis
Continuous variables are expressed as the median with ranges and the medians were compared using the Mann–Whitney U test. The mean pre-NAC and preoperative SMI values were compared using the paired t test. Categorical variables are presented as numbers and percentages and the groups were compared using Fisher exact test.
At the time of final follow-up in January, 2019, the median follow-up period was 20.8 months. Overall survival (OS) was defined as the period from the day of operation to the day of death. Disease-specific survival (DSS) was defined as the period from the day of operation to the day of death caused by EC. Survival curves were produced using the Kaplan–Meier method and differences between the curves were analyzed using a log-rank test. The hazard ratio (HR) was calculated using the Cox proportional hazards model. A cutoff value for the SML was selected to provide the optimal separation between low and high risk in terms of the OS. A P value of < 0.05 denoted significance and the 95% confidence interval (CI) was calculated. All statistical analyses were performed using the SPSS software (version 22.0, SPSS, Chicago, IL, USA).
Results
OS and DSS
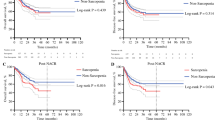
Sarcopenia was diagnosed in 72 (80.0%) and 77 (85.6%) patients before NAC and surgery, respectively (P = 0.43). The mean pre-NAC and preoperative SMI were 45.0 and 43.2, respectively (P = 0.023). The SMI was decreased in 28 patients (68.9%) and the median SML was 3.3% (− 104.5% to 62.2%). Regarding OS, the HR for high SML was highest when the cutoff SML was 12.5%; therefore, the cutoff value of the SML was set at 12.5%. Consequently, 10 patients (11.1%) with SML ≥ 12.5% and 80 patients (88.9%) with SML < 12.5% were classified into high-SML and low-SML groups, respectively. Table 1 outlines the associations between the clinicopathological characteristics of patients and survival rates. The 3-year OS rate was 68.9% in the low-SML group and 0% in the high-SML group (P < 0.001; Fig. 1a). The 3-year DSS rate was 71.6% in the low-SML group and 0% in the high-SML group (P < 0.001; Fig. 1b). There were no significant differences in the OS and DSS rates in terms of sarcopenia before NAC and surgery.
Prognostic value of the SML
In the univariate analysis for OS, the HR for high SML was 5.601 (95% CI 2.287–13.720, P < 0.001). Other factors that correlated significantly with the OS were clinical tumor depth before NAC (P = 0.023) and pathological tumor depth (P < 0.001). In the multivariate analysis, SML was identified as an independent prognostic factor (Table 2). In the univariate analysis for DSS, the HR for high SML was 5.990 (95% CI 2.427–14.790, P < 0.001). The multivariable analysis identified SML as an independent predictor for DSS.
Cause of death
At the time of final follow-up, 33 patients (28.9%) had died, including 26/80 (32.5%) patients in the low-SML group and 7/10 (70%) patients in the high-SML group (P = 0.034). The causes of death were relapse of EC in 20 (25%) and 6 (60%, P = 0.031) patients, other cancers in 3 (3.8%) and 0 (P > 0.999) patients, and reasons other than cancer in 3 (3.8%) and 1 (10%, P = 0.381) patients, respectively.
SML and clinicopathological characteristics
We subsequently evaluated the relationship between the SML and the clinicopathological characteristics of the patients (Table 3). Pathological tumor depth was significantly greater in the high-SML group than in the low-SML group (P = 0.001).
Discussion
The present study evaluated the prognostic impact of sarcopenia in patients with EC treated with NAC and subsequent esophagectomy. Some investigators have linked sarcopenia to a negative prognostic impact [6], whereas others have not detected a significant association between sarcopenia and prognosis in EC patients treated with NAC [18, 19]. Recently, Liu et al. [12] evaluated the relationship between change in the psoas muscle index (PMI) and the clinical outcomes of 84 patients with EC who received NACRT. They demonstrated that a decreased PMI during neoadjuvant therapy (NAT) was independently associated with postoperative survival. Moreover, Järvinen et al. [13] evaluated the prognostic impact of sarcopenia and loss of SMM during NAT, including NACRT and NAC, in 115 patients with EC or junctional cancer, and identified a greater decrease in SMI as an independent predictor of OS. To date, there are no data on the prognostic impact of SML in EC patients treated with NAC. In the present study, the rate of OS was significantly lower in the high-SML group than in the low-SML group. Moreover, the presence of sarcopenia before NAC and surgery was not significantly correlated with OS. Notably, SML was identified as a predictor of OS, independent of various factors. These results suggest that high-SML may be a reliable predictor of the long-term outcome of EC patients treated with NAC.
A previous study found that high loss of muscle mass during the course of preoperative chemotherapy was an independent predictor of poor relapse-free survival for patients with colorectal liver metastases [14]. To date, there are limited data on the prognostic impact of a change in the SMM in patients with EC. Liu et al. [12] reported that patients with a decreased PMI during NAT died of EC more frequently than those with an increased or stable PMI. In the present study, SML was identified as an independent predictor of DSS and death caused by EC relapse was significantly more common for patients with high SML than for those with low SML. These results suggest that the ongoing reduction of the SMM after NAC may promote the proliferation of residual EC cells, leading to disease recurrence in EC patients treated with NAC.
SMM appears to be affected by various factors. In the present study, we evaluated the association between SML and various clinicopathological factors. High SML was linked to a greater risk of a tumor with pathological T3 and T4 depth than those with low SML. These results indicate that it may be difficult to maintain the SMM of patients with bulky tumors, possibly because of the reduced intake and greater degree of systemic inflammatory responses [1, 13, 20]. In contrast, previous studies suggest that the SMM may differ between responders and non-responders to chemotherapy. Ohta et al. [5] reported that a low SMI was an independent predictor of poor pathological response to NAC for EC. Sato et al. [21] reported that among the patients with unresectable EC who underwent chemoradiotherapy, the response rates were significantly lower in those with a low SMI than in those with a high SMI. A study of patients with advanced urothelial carcinoma receiving chemotherapy by Fukushima et al. [7] also found that a change in the SMM was significantly associated with response to chemotherapy. In contrast to these studies, the present study did not reveal a significant association between SML and the pathological response to NAC. Further investigations are warranted to clarify the mechanism underlying SML after NAC in patients with EC.
Anticancer treatment, including chemotherapy and chemoradiotherapy, reduces tumor bulk. However, these modalities have the potential to worsen a patient’s condition and nutritional status because of treatment-related toxicities. To date, the influence of NAC on the SMM remains uncertain. Yip et al. [22] demonstrated that the rate of sarcopenia after NAC increased from 26 to 43% in EC patients. Awad et al. [23] also found that the rate of sarcopenia increased from 57 to 79% and that the SMI decreased significantly during NAC in patients with EC. Järvinen et al. [13] also reported that the median SML after NAT was 2.98%. In the present study, the SMI decreased after NAC in 62 patients (68.9%) and the median SML after NAC was 3.3%. These results suggest that chemotherapy has a negative impact on the SMM of patients with EC.
Considering the poor prognosis of patients with high SML, the maintenance of the SMM during NAC may be of great importance to prevent worse long-term outcomes for patients with EC. Improving nutritional status may help maintain SMM during NAC. A randomized controlled trial demonstrated that lean body mass after esophagectomy was preserved significantly better in a group given eicosapentaenoic acid than in a control group [24]. Another trial found that the perioperative use of an oral nutritional supplement significantly reduced the number of patients with a low SMI among those undergoing radical cystectomy [25].
Physical exercise has also been suggested to be effective for improving muscle mass and strength. A case–control study of patients with hepatocellular carcinoma demonstrated significantly less loss of the SMM in those who underwent in-hospital exercise versus the control group [26]. However, the effects of SMM maintenance during NAC on the long-term outcomes of patients with cancer remain unclear. Further trials are required to clarify whether nutritional intervention and physical exercise maintain the SMM and thereby contribute to prolonging the survival of EC patients treated with NAC.
Previous studies have found that the pathological response to NAC is an important predictor of patient survival [27]. Inconsistent with these results, the present study did not identify pathological response to NAC as a prognostic factor for EC patients treated with NAC. While the relatively small number of patients included in this study would have influenced this result, the precise reason remains unclear.
The present study had several limitations. First, it was a retrospective analysis of a small study population. Second, the patients received various chemotherapeutic regimens. Third, the timing of CT evaluation and the operation were decided independently by each surgeon without any clear criteria and based on the general condition of the patient, the degree of adverse events related to chemotherapy, and other factors. These limitations restrict our ability to draw definite conclusions; therefore, further studies are warranted to validate the present results.
In conclusion, the present study demonstrated that SML was associated with a worse long-term outcome for EC patients treated with NAC. Intervention involving nutritional support and/or physical exercise during NAC is necessary for patients with EC undergoing this type of treatment.
References
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on sarcopenia in older people. Age Ageing. 2010;39:412–23.
Harimoto N, Shirabe K, Yamashita YI, Ikegami T, Yoshizumi T, Soejima Y, et al. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg. 2013;100:1523–30.
Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M, Tokunaga R, Kurashige J, et al. Sarcopenia is a negative prognostic factor after curative resection of colorectal cancer. Ann Surg Oncol. 2015;22:2663–8.
Murimwa GZ, Venkat PS, Jin W, Leuthold S, Latifi K, Almhanna K, et al. Impact of sarcopenia on outcomes of locally advanced esophageal cancer patients treated with neoadjuvant chemoradiation followed by surgery. J Gastrointest Oncol. 2017;8:808–15.
Ota T, Ishikawa T, Endo Y, Matsumura S, Yoshida J, Yasuda T, et al. Skeletal muscle mass as a predictor of the response to neo-adjuvant chemotherapy in locally advanced esophageal cancer. Med Oncol. 2019;36:15.
Paireder M, Asari R, Kristo I, Rieder E, Tamandl D, Ba-Ssalamah A, et al. Impact of sarcopenia on outcome in patients with esophageal resection following neoadjuvant chemotherapy for esophageal cancer. Eur J Surg Oncol. 2017;43:478–84.
Fukushima H, Kataoka M, Nakanishi Y, Sakamoto K, Takemura K, Suzuki H, et al. Posttherapeutic skeletal muscle mass recovery predicts favorable prognosis in patients with advanced urothelial carcinoma receiving first-line platinum-based chemotherapy. Urol Oncol. 2018;36(156):e9–16.
Klein CA, Stoecklein NH. Lessons from an aggressive cancer: evolutionary dynamics in esophageal carcinoma. Cancer Res. 2009;69:5285–8.
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJH, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20.
Kleinberg L, Forastiere AA. Chemoradiation in the management of esophageal cancer. J Clin Oncol. 2007;25:4110–7.
Yamasaki M, Yasuda T, Yano M, Hirao M, Kobayashi K, Fujitani K, et al. Multicenter randomized phase II study of cisplatin and fluorouracil plus docetaxel (DCF) compared with cisplatin and fluorouracil plus Adriamycin (ACF) as preoperative chemotherapy for resectable esophageal squamous cell carcinoma (OGSG1003). Ann Oncol. 2017;28:116–20.
Liu J, Motoyama S, Sato Y, Wakita A, Kawakita Y, Saito H, et al. Decreased skeletal muscle mass after neoadjuvant therapy correlates with poor prognosis in patients with esophageal cancer. Anticancer Res. 2016;36:6677–86.
Jarvinen T, Ilonen I, Kauppi J, Salo J, Rasanen J. Loss of skeletal muscle mass during neoadjuvant treatments correlates with worse prognosis in esophageal cancer: a retrospective cohort study. World J Surg Oncol. 2018;16:27.
Okuno M, Goumard C, Kopetz S, Vega EA, Joechle K, Mizuno T, et al. Loss of muscle mass during preoperative chemotherapy as a prognosticator for poor survival in patients with colorectal liver metastases. Surgery. 2019;165:329–36.
Japan Esophageal Society. Japanese Classification of Esophageal Cancer. 10th ed. Tokyo: Kanehara; 2008.
Sobin L, Gospodarowicz M, Wittekind C. TNM classification of malignant tumors. 7th ed. Oxford: Wiley; 2010.
Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–35.
Grotenhuis BA, Shapiro J, van Adrichem S, de Vries M, Koek M, Wijnhoven Bas PL, et al. Sarcopenia/muscle mass is not a prognostic factor for short- and long-term outcome after esophagectomy for cancer. World J Surg. 2016;40:2698–704.
Mayanagi S, Tsubosa Y, Omae K, Niihara M, Uchida T, Tsushima T, et al. Negative impact of skeletal muscle wasting after neoadjuvant chemotherapy followed by surgery on survival for patients with thoracic esophageal cancer. Ann Surg Oncol. 2017;24:3741–7.
Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–9.
Sato S, Kunisaki C, Suematsu H, Tanaka Y, Miyamoto H, Kosaka T, et al. Impact of sarcopenia in patients with unresectable locally advanced esophageal cancer receiving chemoradiotherapy. Vivo. 2018;32:603–10.
Yip C, Goh V, Davies A, Gossage J, Mitchell-Hay R, Hynes O, et al. Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer. Eur Radiol. 2014;24:998–1005.
Awad S, Tan BH, Cui H, Bhalla A, Fearon KC, Parsons SL, et al. Marked changes in body composition following neoadjuvant chemotherapy for oesophagogastric cancer. Clin Nutr. 2012;31:74–7.
Ryan AM, Reynolds JV, Healy L, Byrne M, Moore J, Brannelly N, et al. Enteral nutrition enriched with eicosapentaenoic acid (EPA) preserves lean body mass following esophageal cancer surgery: results of a double-blinded randomized controlled trial. Ann Surg. 2009;249:355–63.
Ritch CR, Cookson MS, Clark PE, Chang SS, Fakhoury K, Ralls V, et al. Perioperative oral nutrition supplementation reduces prevalence of sarcopenia following radical cystectomy: results of a prospective randomized controlled trial. J Urol. 2019;201:470–7.
Koya S, Kawaguchi T, Hashida R, Hirota K, Bekki M, Goto E, et al. Effects of in-hospital exercise on sarcopenia in hepatoma patients who underwent transcatheter arterial chemoembolization. J Gastroenterol Hepatol. 2019;34:580–8.
Suzuki G, Yamazaki H, Ogo E, Abe T, Eto H, Murai K, et al. Multimodal approach for cervical esophageal carcinoma: role of neoadjuvant chemotherapy. Anticancer Res. 2014;34:1989–92.
Acknowledgements
This study did not receive funding from any organization.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kamitani, N., Migita, K., Matsumoto, S. et al. Association of skeletal muscle loss with the long-term outcomes of esophageal cancer patients treated with neoadjuvant chemotherapy. Surg Today 49, 1022–1028 (2019). https://doi.org/10.1007/s00595-019-01846-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-019-01846-1