Abstract
Background
Neoadjuvant systemic therapy (NST) is standard for locally advanced breast cancer and is now frequently considered for those with early-stage and node-positive disease. We aimed to evaluate the treatment course and outcomes in patients with disease progression during NST.
Methods
Patients diagnosed with unilateral stage I–III breast cancer between 2005 and 2015 with documented local-regional progression while receiving NST, by clinical examination and/or imaging after two or more cycles of chemotherapy, were identified from a prospective database, stratified by receipt of surgery and outcomes analyzed.
Results
Of 6362 patients treated with NST during the study period, 124 (1.9%) developed disease progression. At a median live follow-up of 71 months, 23.4% were alive without disease and 70.2% had died from breast cancer. Median overall survival (OS) time for patients with progression was 26 months and median distant disease-free survival (DFS) was 14 months. Triple-negative breast cancer was associated with a higher likelihood of death (p < 0.001) and development of distant metastasis (p = 0.002). Among patients who had surgery (104, 89.3%), 40 (38.5%) developed local-regional recurrence, 67 (64.4%) developed distant metastasis, and 69 (66.3%) died from breast cancer. Median OS and median distant DFS in this subgroup was 31 and 16 months, respectively.
Conclusions
High rates of local-regional and distant failure were seen following disease progression while receiving NST. This suggests aggressive tumor biology and the need to study novel systemic therapies. Poor survival outcomes despite surgical management highlight the importance of careful patient selection when considering operative intervention after progression while receiving NST.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The role of neoadjuvant systemic therapy (NST) in the treatment of breast cancer continues to evolve with increased understanding of biologic subtypes and advances in drug development. NST is currently the standard of care for locally advanced breast cancer and is a strategy utilized in the treatment algorithm for selected early-stage and node-positive patients. The use of NST has several potential benefits, including downstaging of disease to facilitate less extensive surgical management and assessment of tumor response to therapy, providing a window into the biological behavior of the tumor while it remains in vivo. Existing data have demonstrated that a pathologic complete response (pCR) to NST is associated with better prognosis, with increased overall survival (OS) and longer disease-free survival (DFS) intervals.1,2,3,4,5 While most patients have either a partial or complete response to NST, a small proportion of patients experience disease progression while receiving NST. Given the poor response to systemic therapy, many of these patients proceed to surgical management.6 We previously evaluated the outcomes of patients who experienced disease progression while receiving NST and found that it was a negative predictor of distant DFS and OS.7 The treatment course and clinical outcomes of patients with disease progression while receiving NST have not been evaluated in contemporary datasets. As such, our study aims to evaluate the prognostic significance of progression of disease (PD) while receiving NST, to assess factors impacting clinical outcomes and to determine the role of surgical management in breast cancer patients with local-regional progression during NST.
Methods
Patient Population and Treatment Considerations
A prospectively maintained institutional database was queried to identify patients with stage I–III breast cancer diagnosed between 1 January 2005 and 31 December 2015. Patients undergoing NST with documented disease progression after two or more cycles of treatment were included in the analysis, whereas patients receiving neoadjuvant endocrine therapy alone were excluded. PD during NST was defined as growth of local in-breast or regional nodal disease or the development of new ipsilateral breast or nodal lesions, and was determined by radiologic evaluation and/or physical examination by a treating physician or team of physicians and documented in the medical record. Patients with bilateral breast cancer, recurrent disease, or a synchronous cancer were excluded from this study. Demographic, clinicopathologic, and treatment characteristics were extracted and reviewed.
Initial evaluation and staging consisted of a combination of physical examination and radiologic evaluation utilizing ultrasound and mammography with selective use of magnetic resonance imaging (MRI). Choice of the NST regimen and frequency of interval evaluation during NST with physical examination and imaging was per institutional standard clinical guidelines. The treatment algorithm after documented PD included a change in NST regimen, enrollment in a clinical trial, or surgical management, and the next treatment course was made in a multidisciplinary fashion.
Statistical Analysis
Descriptive statistics were used to summarize demographic, clinicopathologic, and treatment information. Endpoints included the rate of distant metastasis, the rate of local-regional failure after surgery, OS, and distant DFS. The Kaplan–Meier method was used to determine OS and distant DFS. Differences between groups were evaluated using the log-rank test. Events were measured from the date of breast cancer diagnosis. Patients who were without a documented event at the end of the study period were censored at the date of last follow-up or date of death, where appropriate. In order to determine factors independently associated with OS and distant DFS, univariate and multivariate Cox proportional hazard regression was utilized and adjusted odds ratios and 95% confidence intervals (CIs) were computed. Analysis was conducted for the entire cohort and patients were stratified by receipt of surgery, and analyzed independently. A two-tailed p-value <0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics version 26 (IBM Corporation, Armonk, NY, USA). Institutional Review Board approval was obtained prior to initiation of the retrospective analysis.
Results
Patient Demographics and Surgical Treatment
Of 6362 patients treated with NST during the study period, 124 (1.9%) developed local-regional progression while receiving treatment. A total of 104 (83.9%) patients proceeded to surgery after PD. Demographic and clinicopathologic features of the entire cohort and stratified by receipt of surgery are summarized in Table 1. In the cohort of patients with PD while receiving NST, 45.2% of patients had triple-negative breast cancer (TNBC) and 25.8% had inflammatory breast cancer (IBC). There was a higher proportion of premenopausal, cT4, cN3, and IBC patients among those patients who did not have surgery.
Among the 20 patients who did not receive surgery after progression while receiving NST, 17 (85%) developed distant metastasis, two (10%) developed unresectable regional disease, and 1 (5%) had concomitant comorbidities precluding operative management. Patients who did not receive surgery had a median distant DFS of 6 months. At the end of the study period, 18 (90%) patients died from metastatic breast cancer and 2 (10%) were alive with disease. The median OS for patients who were not treated with surgery was 14 months.
Patients proceeding to surgery received an average of six total cycles of NST (range 2–24). Of 104 patients receiving surgical management, 95 (91.3%) underwent mastectomy and 9 (8.7%) were treated with breast-conserving therapy. Management of the axilla was axillary lymph node dissection in 82 (78.7%) patients and sentinel lymph node dissection in 15 (14.4%) patients. Negative margins were achieved at index operation in 97 (93.3%) patients, none of whom had a pCR on final pathology. Of 15 (14.4%) patients undergoing postmastectomy reconstruction, 11 (73.3%) received immediate reconstruction and 4 (26.7%) underwent delayed autologous reconstruction. In the group of patients receiving immediate reconstruction, 3 (27.3%) received autologous tissue flaps for chest wall coverage, 2 (18.2%) received autologous reconstruction for reconstruction of the breast mound, and 6 (54.5%) received implant-based reconstruction. Adjuvant radiation was delivered in 65 (62.5%) patients. Treatment characteristics and results of surgical pathology for patients receiving surgery are summarized in Table 2.
Survival Analysis
Median live follow-up for all patients was 71 months (range 4–166). A total of 86 (69.4%) patients developed distant metastasis during the period of study. At a median overall follow-up of 25 months, only 29 (23.4%) patients were alive without disease and 87 (70.2%) died from metastatic breast cancer. Median distant DFS was 14 months (95% CI 11.6–16.4) and median OS was 26 months (95% CI 20.9–31.1). Multivariate analysis of factors independently associated with OS demonstrated that patients with TNBC were significantly more likely to die than those with hormone receptor (HR)-positive or HER2-positive breast cancer (hazard ratio [HR] 2.44, 95% CI 1.55–3.84, p < 0.001). Patients with TNBC and progressive disease while receiving NST also had a higher risk of distant failure (HR 2.06, 95% CI 1.29–3.30). IBC was independently associated with distant failure on multivariate analysis (HR 3.23, 95% CI 1.29–8.06).
Outcomes After Surgical Management
In patients undergoing surgical management after progression while receiving NST, at a median overall follow-up of 26 months, 29 (27.9%) patients were alive without evidence of disease and 69 (66.3%) patients died from metastatic breast cancer. Analysis demonstrated a median distant DFS of 16 months (95% CI 10.1–21.9) and a median OS of 31 months (95% CI 24.1–37.9) in patients undergoing surgery. Local-regional failure after surgery occurred in 40 (38.5%) patients at an average of 12.6 months. Local-regional failure occurred in 16 (24.6%) patients who received adjuvant radiation, compared with 24 (61.5%) patients who did not receive radiation (p < 0.001). Median OS was 35 months in patients who received adjuvant radiation therapy, compared with 25 months in those who did not receive adjuvant radiation (p = 0.006). Follow-up, vital status, and survival in patients managed with surgery after progression while receiving NST are summarized in Table 3.
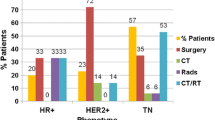
Subgroup analysis was conducted to evaluate patterns of local-regional failure and to identify factors associated with distant DFS and OS in patients receiving surgical management in the setting of disease progression while receiving NST. Twenty (62.5%) IBC patients were treated with surgery after progression while receiving NST. Local-regional failure occurred in 12 (60%) IBC patients. Patients with IBC had a median distant DFS of only 7 months (95% CI 6.2–7.8), compared with 19 months (95% CI 11.8–26.2) in non-IBC patients treated with surgery (p < 0.001). OS in patients with IBC was 18 months (95% CI 11.6–24.4), compared with 32 months (95% CI 25.0–39.0) in non-IBC patients (p < 0.001) [Fig. 1]. Forty-seven (83.9%) patients with TNBC were treated with surgery after progression while receiving NST. No difference in local-regional failure was noted in patients with TNBC compared with those with HR-positive or HER2-positive disease. Patients with TNBC were noted to have worse survival outcomes compared with patients with HR-positive disease. Median distant DFS was 12 months (95% CI 10.2–13.7) and median OS was 18 months (95% CI 14.5–21.5) in patients with TNBC, compared with 24 months (95% CI 8.6–39.4) and 38 months (95% CI 27.0–49.0), respectively, in patients with HR-positive disease (Fig. 2). Both IBC and TNBC were independently associated with distant failure on multivariate analysis (HR 3.71, 95% CI 1.20–11.49; and HR 2.19, 95% CI 1.29–3.71, respectively).
A total of 29 (23.4%) patients were alive without evidence of disease at the end of the study period. Among the group who were alive without disease, patients were younger, with a mean age of 43 years, and 72.4% were premenopausal. Fifteen (51.7%) patients had no nodal disease at presentation despite PD. Compared with the patients who died, those alive at the end of the study were significantly more likely to have T1 disease, non-IBC, and non-TNBC (p < 0.05 for all) breast cancers.
Discussion
PD while receiving NST in women with breast cancer often represents a clinical challenge that requires a multidisciplinary approach to care. Although most patients experience partial or complete response to NST, our data indicate that 1.9% of patients at our comprehensive cancer center experienced PD while receiving NST. This rate of PD is lower than historical data from our institution. Caudle et al. published the first evaluation of PD while receiving NST in a cohort of women treated between 1994 and 2007 at our institution and found that 3% of patients experienced PD.7 The difference is likely due to advances in systemic therapies, including adoption of targeted therapies that have led to appreciable improvements in tumor response. Other contemporary series have reported rates of PD while receiving NST ranging from 3.8 to 7.2%.8,9,10 To our knowledge, our study represents the largest contemporary dataset to evaluate PD while receiving NST, and the role of surgical management.
Breast cancer represents a heterogenous spectrum of diseases with variable response to therapy based on tumor characteristics. We noted overrepresentation of certain populations of patients within the cohort of patients with PD while receiving NST compared with the global breast cancer population. TNBC represents only 12% of all new breast cancer diagnoses, however in11 our cohort of patients who had PD while receiving NST, 45.2% carried a diagnosis of TNBC. While the biologic behavior of TNBC confers a more aggressive phenotype compared with HR-positive disease, the TNBC subtype has been shown to have higher rates of pCR to NST.12,13 Given the reported chemosensitivity in many series, the high rates of TNBC within the PD population suggests more aggressive tumor biology within this group that warrants further genomic profiling of nonresponders and the development of targeted therapies. Our data also demonstrate a high prevalence of patients with IBC, representing 25.8% of all patients with PD while receiving NST. IBC represents only 3.6% of all breast cancer patients evaluated at our institution during the period of study. NST remains the standard of care in the treatment algorithm for IBC. Despite improvements in survival over time, IBC patients remain a management challenge, with lower rates of response to NST therapy.14,15
In our population of patients with PD while receiving NST, we also noted that 19.2% of the women were African American (AA). When evaluating all patients diagnosed with breast cancer between 2005 and 2015 seen at our institution, 11.8% of patients were AA. Given the prevalence of TNBC in our study population and the high rates of TNBC in AA women, this may account for the overrepresentation of AA patients in this population.16,17 In fact, 52% of patients with TNBC who experienced PD while receiving NST were AA. Despite investigation into the biological basis and social determinants of health, worse survival outcomes continue to be reported in AA women.18,19 Although patients with PD while receiving NST represent a small proportion of breast cancer patients, determining optimal therapy for this population of patients may contribute towards mitigating the survival disparities seen in AA patients.
Patients with disease refractory to systemic therapy are generally treated with surgical management as the next step in the absence of distant metastatic disease or unresectable local-regional disease. At our institution, 83.9% of patients with PD while receiving NST received surgical management. In a series by Raphael and colleagues, outcomes after salvage therapy were evaluated in a group of 30 patients (or 7.2% of the NST population) who experienced PD. They noted that salvage therapy consisted of either immediate surgery or change in chemotherapy or chemoradiation with the goal of rendering operability. Eighty percent of patients in their study were treated with surgery, similar to our study population.8 They conducted an analysis of outcomes after salvage therapy but this was limited by a small absolute number of patients treated with surgery and did not evaluate survival outcomes specifically after surgical management. To our knowledge, our present review represents the only study to conduct a focused analysis into the surgical management of patients with PD while receiving NST.
In our population of patients with PD while receiving NST treated with surgery, median distant DFS was 14 months and OS was 26 months. The 5-year survival rate for patients with PD while receiving NST was 32%, not markedly different from the rate of 28% seen in women with metastatic breast cancer.20 Our data indicate an interesting pattern of disease recurrence in patients treated with surgery. Local-regional failure occurred in 38.5% of patients, with distant metastasis occurring in 64.6% of patients. In 39.4% of patients treated with surgery, distant metastasis occurred before evidence of local-regional recurrence. An additional 40.4% of patients developed concurrent local-regional recurrence with distant metastasis. The high rates of distant failure preceding or concurrent with local-regional failure calls into question the role for local-regional treatment with respect to patient outcomes. Surgery is not without risk of morbidity, and, even in the absence of complications, creates an interval where additional systemic and radiation therapy cannot be administered. Further understanding of patients who do not receive a benefit from surgical therapy is critical.
Patients with TNBC and IBC with PD while receiving NST emerged as populations with poor distant DFS and OS despite surgical management. Patients with TNBC had a median OS of 18 months, with a median distant DFS of 12 months and a 5-year survival rate of 11%. Notably, IBC patients with PD while receiving NST experienced comparable outcomes to patients who did not receive surgery. In IBC patients, median distant DFS was 7 months, with a median OS of 18 months, compared with a median distant DFS of 6 months and a median OS of 14 in patients who did not receive surgery. Furthermore, 50% of IBC patients who received surgery and adjuvant radiation developed local-regional recurrence. The short interval to development of distant metastasis and poor OS in TNBC and IBC patients, and the poor local-regional control despite surgery and adjuvant radiation in IBC patients, highlights the need for careful consideration when offering surgery to these patients in the setting of PD while receiving NST.
Immunotherapy has emerged as a promising adjunct to standard chemotherapeutic regimens. Several trials have investigated the role of immune checkpoint inhibitors that target the programmed cell death ligand 1 (PD-L1) or programmed cell death protein 1 (PD-1). In the randomized phase II I-SPY2 trial, the role of pembrolizumab, a monoclonal antibody for PD-1, was investigated. Two-hundred and fifty patients with stage II or III breast cancer were randomized to receive neoadjuvant chemotherapy with or without pembrolizumab. Higher rates of pCR and lower residual cancer burden was noted compared with patients who did not receive pembrolizumab with both HR-positive, HER2-negative and triple-negative breast cancers.21 In the randomized trials KEYNOTE-522 and KEYNOTE-355, the role of pembrolizumab in addition to neoadjuvant chemotherapy was assessed in patients with early-stage TNBC and locally recurrent inoperable or metastatic TNBC, respectively. Pembrolizumab with chemotherapy resulted in higher rates of pCR in both populations compared with chemotherapy alone.22,23 The IMpassion031 randomized, phase III trial evaluated the PD-L1 inhibitor atezolizumab in patients with early-stage TNBC and found significantly higher rates of pCR in patients receiving atezolizumab with chemotherapy compared with patients receiving chemotherapy alone, regardless of PD-L1 status.24 Taken together, the results of these landmark trials demonstrate that immune checkpoint inhibitors will play an important role in the contemporary treatment of breast cancer patients and may decrease the number of patients with PD while receiving NST.
Radiation therapy is a critical component in the management algorithm of patients with PD while receiving NST. In our series, we noted a dramatic difference in local-regional recurrence in patients who received adjuvant radiation compared with those who did not. Although there was a trend towards lower rates of distant metastasis in patients receiving adjuvant radiation, this did not reach statistical significance. Median OS was superior in patients who received adjuvant radiation therapy. This likely represents selection bias, as patients with worse functional status and comorbid conditions with more advanced distant disease may be more likely to have had omission of radiation therapy. A subset of patients may have developed metastatic disease prior to starting radiation therapy. These possibilities were unable to be assessed in the present study design. Our data suggest that adjuvant radiation should be strongly considered in patients with PD while receiving NST who are treated with surgery.
Concurrent chemoradiation is another area of investigation that may hold promise for patients with poor response to NST. In a prospective, phase II trial of women with locally advanced breast cancer, neoadjuvant regional radiation was delivered concurrent with docetaxel. Rates of pCR were improved and the regimen was well-tolerated, although no differences in DFS or OS were noted.25 The role of radiation as a supplement to systemic therapy in this population warrants further investigation.
Our study is limited by its retrospective design and change in chemotherapy combinations over a 10-year period. During this time, there have been changes in systemic therapy protocols due to biologic subtyping and the emergence of newer systemic agents and biologic therapies. Due to the overall low prevalence of patients with PD while receiving NST, evaluating a shorter time interval would result in a smaller population of patients, which would further limit the statistical power of subgroup analyses. Although our data provide insight into the outcomes of patients with PD while receiving NST, there is not enough evidence to define the optimal management for these patients.
The poor clinical outcomes in patients with PD while receiving NST raise the ethical dilemma of what constitutes a treatment benefit and when an intervention should be considered futile. Futility can be defined by lack of evidence that an intervention will produce a tangible improvement in outcomes, known as quantitative futility, or that the quality of the benefit produced is poor, known as qualitative futility.26 When defining futility in cancer care, there is no definition of the minimal acceptable benefit of treatment, known as the minimal effectiveness threshold.27 It is also noted that treatment benefit is not limited exclusively to survival outcomes, and, even in the setting of poor outcomes, consideration to preventing local-regional progression resulting in intractable pain or a fungating wound that compromises quality of life is an important consideration. In addition, approximately 23% of patients in the study population remain alive and free of disease, and identification of favorable features to identify patients in this subgroup warrants further attention. Furthermore, advances in systemic therapy, as well as recently reported clinical trials showing improved outcomes with additional systemic treatment in the setting of residual disease after neoadjuvant chemotherapy, may facilitate improved response to neoadjuvant therapy and improved patient outcomes in the small percentage of patients with local-regional progression.28,29 As a provider developing a treatment plan, one must work in a multidisciplinary team to evaluate the role, relative benefit, and possible futility of surgical intervention, and have a candid discussion with patients about goals of care.
Conclusion
Patients with PD while receiving NST represent a small proportion of breast cancer patients who have poor survival outcomes even after surgical resection. As expanded systemic therapy options emerge and the population of patients with PD while receiving NST declines, this group will remain a management challenge. The pattern of distant failure, even in the setting of appropriate local-regional control, highlights the need for expanded systemic therapy options, careful consideration of the role of surgical management, and a multidisciplinary approach to care.
References
Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–85.
Bear HD, Anderson S, Smith RE, Geyer CE Jr, Mamounas EP, Fisher B, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006;24(13):2019–27.
Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16(8):2672–85.
Esserman LJ, Berry DA, DeMichele A, Carey L, Davis SE, Buxton M, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL—CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30(26):3242–9.
Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17(2):460–9.
Caudle AS, Gonzalez-Angulo AM, Hunt KK, Pusztai L, Kuerer HM, Mittendorf EA, et al. Impact of progression during neoadjuvant chemotherapy on surgical management of breast cancer. Ann Surg Oncol. 2011;18(4):932–8.
Caudle AS, Gonzalez-Angulo AM, Hunt KK, Liu P, Pusztai L, Symmans WF, et al. Predictors of tumor progression during neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(11):1821–8.
Raphael J, Paramsothy T, Li N, Lee J, Gandhi S. A single-institution experience of salvage therapy for patients with early and locally advanced breast cancer who progress during neoadjuvant chemotherapy. Breast Cancer Res Treat. 2017;163(1):11–9.
Myller S, Ipatti P, Jääskeläinen A, Haapasaari KM, Jukkola A, Karihtala P. Early progression of breast cancer during neoadjuvant chemotherapy may predict poorer prognoses. Acta Oncol. 2020;59(9):1036–42.
Zheng Y, Ding X, Zou D, Zhang F, Qin C, Yang H, et al. The treatment option of progressive disease in breast cancer during neoadjuvant chemotherapy: a single-center experience. Cancer Biol Ther. 2020;21(8):675–87.
American Cancer Society. Breast cancer: facts & figures 2019–2020. 2019 [cited 2020 Dec 16]. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2019-2020.pdf.
Boughey JC, McCall LM, Ballman KV, Mittendorf EA, Ahrendt GM, Wilke LG, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg. 2014;260(4):608–14 (discussion 614–6).
Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer. 2012;48(18):3342–54.
Abraham HG, Xia Y, Mukherjee B, Merajver SD. Incidence and survival of inflammatory breast cancer between 1973 and 2015 in the SEER database. Breast Cancer Res Treat. 2021;185:229–38.
Liu J, Chen K, Jiang W, Mao K, Li S, Kim MJ, et al. Chemotherapy response and survival of inflammatory breast cancer by hormone receptor- and HER2-defined molecular subtypes approximation: an analysis from the National Cancer Database. J Cancer Res Clin Oncol. 2017;143(1):161–8.
Lund MJ, Trivers KF, Porter PL, Coates RJ, Leyland-Jones B, Brawley OW, et al. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Res Treat. 2009;113(2):357–70.
Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–502.
Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J Clin Oncol. 2018;36(1):25–33.
Warner ET, Tamimi RM, Hughes ME, Ottesen RA, Wong YN, Edge SB, et al. Racial and ethnic differences in breast cancer survival: mediating effect of tumor characteristics and sociodemographic and treatment factors. J Clin Oncol. 2015;33(20):2254–61.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
Nanda R, Liu MC, Yau C, Shatsky R, Pusztai L, Wallace A, et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol. 2020;6(5):676–84.
Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–21.
Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396(10265):1817–28.
Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. The Lancet. 2020;396(10257):1090–100.
Brackstone M, Palma D, Tuck AB, Scott L, Potvin K, Vandenberg T, et al. Concurrent neoadjuvant chemotherapy and radiation therapy in locally advanced breast cancer. Int J Radiat Oncol Biol Phys. 2017;99(4):769–76.
von Gruenigen VE, Daly BJ. Futility: clinical decisions at the end-of-life in women with ovarian cancer. Gynecol Oncol. 2005;97(2):638–44.
Buyx AM, Friedrich DR, Schöne-Seifert B. Ethics and effectiveness: rationing healthcare by thresholds of minimum effectiveness. BMJ. 2011;342:d54.
Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147–59.
Von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–28.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Benjamin Smith has received prior research funding from Varian Medical Systems, and has current royalty and equity interest in Oncora Medical. Funda Meric-Bernstam is a consultant for AbbVie, Aduro BioTech Inc., Alkermes, AstraZeneca, DebioPharm, eFFECTOR Therapeutics, F. Hoffman-La Roche Ltd, Genentech Inc., IBM Watson, Infinity Pharmaceuticals, Jackson Laboratory, Kolon Life Science, OrigiMed, PACT Pharma, Parexel International, Pfizer Inc., Samsung Bioepis, Seattle Genetics Inc., Tyra Biosciences, Xencor, and Zymeworks, and is a member of the Advisory Committee for Immunomedics, Inflection Biosciences, Mersana Therapeutics, Puma Biotechnology Inc., Seattle Genetics, Silverback Therapeutics, Spectrum Pharmaceuticals, and Zentalis. She has also undertaken sponsored research for Aileron Therapeutics, Inc. AstraZeneca, Bayer Healthcare Pharmaceutical, Calithera Biosciences Inc., Curis Inc., CytomX Therapeutics Inc., Daiichi Sankyo Co. Ltd, Debiopharm International, eFFECTOR Therapeutics, Genentech Inc., Guardant Health Inc., Klus Pharma, Takeda Pharmaceutical (formerly Millennium Pharmaceutical), Novartis, Puma Biotechnology Inc., and Taiho Pharmaceutical Co., and has received honoraria from Chugai Biopharmaceuticals. Kelly Hunt is a member of the Medical Advisory Board of Armada Health and Merck & Co., and has received research funding to her institution from Cairn Surgical, Eli Lilly and Company, Lumicell, and OncoNano Medicine. Leisha C. Elmore, Henry M. Kuerer, Carlos H. Barcenas, Makesha V. Miggins, Anthony Lucci, Abigail S. Caudle, and Mediget Teshome have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Elmore, L.C., Kuerer, H.M., Barcenas, C.H. et al. Clinical Course of Breast Cancer Patients with Local-Regional Progression During Neoadjuvant Systemic Therapy. Ann Surg Oncol 28, 5477–5485 (2021). https://doi.org/10.1245/s10434-021-10444-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-10444-w