Abstract
Background Breast cancers with a triple negative tumor (TNT) subtype (as defined by lacking protein expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2)) preclude the use of available targeted therapies and may contribute to poor outcome and to the historically poorest survival observed among African–American (AA) women. This study examines association of the ER/PR/HER2 subtypes with race and breast cancer survival. Methods Breast tumors from a population-based cohort of 116 AA and 360 white Atlanta women aged 20–54, diagnosed from 1990 to 1992 were centrally reviewed and tested by immunohistochemistry. Multivariate survival analyses within subtypes (TNT, ER−PR−HER2+, ER+/PR+HER2+, ER+/PR+HER2−) were conducted using weighted Cox regression and included socio-demographic, prognostic, and treatment factors. Results TNTs were more prevalent among young women and particularly among AA women (Odds Ratio [OR] = 1.9, 95% Confidence Interval [CI] 1.2–2.9), adjusting for age, stage, grade, and poverty index. Overall mortality was higher for AA women (Hazard Ratio [HR] = 1.9, 95% CI, 1.5–2.5) and differed by subtypes (P < 0.001). Within the TNT subtype, racial differences in survival persisted, after additional adjustment for treatment and comorbidities (HR = 2.0, 95% CI 1.0–3.7). TNTs were uniquely associated with high expression of p16, p53, and Cyclin E; and low Bcl-2 and Cyclin D1 expression. Conclusions The high prevalence of TNTs among younger women and particularly younger AA women, along with unique protein expression patterns and poorer survival, suggests varying gene–environment etiologies with respect to age and race/ethnicity and a need for effective therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among women in the U.S., breast cancer continues to be the most frequently diagnosed cancer and the second most common cause of cancer death. African–American (AA) women have increased incidence of early onset disease and die at a higher rate than any other racial/ethnic group [1]. While a socio-demographic component is a major factor in this disparate burden, the role of tumor biology or genetics cannot be ignored as breast cancers, like all cancers, arise as a result of genetic alterations [2–9].
The heterogeneity of breast cancer has recently been demonstrated through genetic profiling which has identified the presence of several major breast tumor subtypes, each with distinct gene-expression patterns [10–16]. These major subtypes have important implications in breast cancer etiology, the systemic therapies prescribed, the effectiveness of such therapies, and in outcome, both recurrence and survival [14, 17–24]. Breast cancer-related proteins correspond with the major genetic subtypes; specifically, protein expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) [10, 11, 13, 18, 25]. The triple negative tumor (TNT) phenotype, denoted by lack of expression of all three protein receptors (ER−PR−HER2−) has recently emerged as a distinct subtype. TNTs may arise at an earlier age, are basal-like and almost exclusively high grade tumors. These characteristics suggest distinct molecular origins [14, 17, 20, 26, 27]. Unfortunately, these tumors also currently have no available effective target therapies [27].
TNTs account for between 10–30% of all invasive breast cancers in the U.S. and AA women appear to be twice as likely as white women to present with these tumors [18, 28–30]. Singly and in combination, the earlier age at onset, lack of viable therapeutic targets and highly aggressive features of the TNTs may contribute to poor outcome and may explain in part the historically poorest survival observed among AA women.
Several U.S. studies have recently reported a higher prevalence of TNTs among younger and African–American women [18, 28–30]. However, only two published studies addressing race, breast cancer subtypes, and survival are population-based and both have several limitations [18, 28]. Testing of the biomarkers and ascertainment of other pathology components was not performed in a centralized/uniform manner, treatment information was not available hence was not accounted for in survival analyses, nearly 45% of the biomarker information to construct subtypes was missing in one study [28], and neither study examined other factors such as stage, grade, poverty index, and treatment factors which could explain racial differences in survival among the breast cancer subtypes. Our population-based study compensates for these limitations and further augments the biologic understanding of breast tumor subtypes in several ways. In our study all pathology review and immunohistochemical (IHC) testing was centrally conducted by one pathologist (P.L.P.). In addition to standard prognostic factors, our survival analyses account for delay in treatment, type of treatment received, and co-morbidities. With a study population of 476 younger women (age 20–54 at diagnosis) and 30% AA representation, we were able to simultaneously consider multiple factors in our assessment of racial differences in subtypes and survival, with the aim of identifying factors which could explain these racial differences. Finally, this is also the first population-based study to investigate the association of the subtypes with expression of breast tumor proteins important to regulation of the cell-cycle or to programmed cell death (apoptosis). The proteins we investigated are those which are fairly common in breast cancers and may have particular importance in early age breast carcinogenesis, aggressiveness, or clinical outcome [31]. We investigate the prevalence of triple subtypes of breast cancer (based on immunohistochemical (IHC) protein expression of ER, PR, and HER2) and their association with race and overall survival; adjusting for socio-demographic, prognostic, and treatment factors.
Methods
Population
This study builds on the metropolitan Atlanta arm of a multicenter population-based, case–control study of breast cancer incidence and risk factors in younger women (aged 20–54) [32]. The original study identified 950 African–American (AA) or white women, aged 20–54, who were residents of the three largest counties in metropolitan Atlanta, Georgia (Cobb, Fulton, and DeKalb) when newly diagnosed with unilateral invasive breast cancer between May 1, 1990 and December 31, 1992. The 3-county area represented a population of over 1.64 million residents (36.4% AA and 60.5% white). Case identification was through rapid ascertainment of hospital admission, surgery, and pathology records. Completeness of ascertainment was assessed by periodic checks against the Metropolitan Atlanta Surveillance Epidemiology and End Results (SEER) Cancer Registry, part of the NCI-funded SEER program. The overall response rate for Atlanta interview of patients in the parent case–control study was 87.9% (835/950): 87.2% (251/288) for AA women and 88.2% (584/662) for white women. For the current study, four of the 835 women originally interviewed were excluded (1 later self-reported ‘other’ race and 3 were interviewed as controls, prior to becoming cases), resulting in 831 eligible women for the current study (251 AA and 580 white). After obtaining Institutional Review Board (IRB) approval at each collaborating institution, a more comprehensive medical record review was conducted and tumor specimens were obtained. Of the 831 women, slides for centralized pathology review and archival tissue specimens appropriate for further laboratory analysis were obtained for 476 (116 AA and 360 white).
Data sources & elements
Data sources
There were several sources of data for this study, including information previously collected in the original study and Atlanta SEER registry data. Follow-Up telephone interviews and extensive medical record abstraction from hospitals, physicians’ offices, diagnostic and radiation facilities, and pathology laboratories were conducted beginning in 1997. Trained medical record abstractors and interviewers conducted the interviews and medical record abstraction. In cases where multiple sources for the same data conflicted, extensive expert review (J.W.E.) was conducted.
Patient-related factors
Data from the parent study were the primary source of the non-tumor factors, including race, age, education, and poverty index. A detailed description of these factors was published previously [32]. Racial identity was self-reported. The youngest age category (20–34) included by necessity a larger range because there were so few cases in this age range as previously described [33]. A poverty index (as an indicator of socio-economic status) was calculated based on the combination of annual household income and the number of people supported by that income, with the annual household income divided by the 1991 national poverty level income for a family of the corresponding size [33, 34]. The lowest ordinal category represents those living at the federally defined poverty level. Co-morbid illness information was obtained from the original patient interview and abstraction of hospital records. The co-morbid variable sums the number of co-morbid medical conditions for each patient and includes only those illnesses thought to contribute to treatment and/or survival: Diabetes, drug abuse, gastrointestinal disease, heart disease, HIV, hypertension, renal disease, liver disease, lung disease, neuropathic disease, psychiatric disorders, and rheumatoid arthritis. These measurements represent the time period up to the date of initial diagnosis.
Treatment-related factors
Treatment data were obtained from abstraction of hospital, medical (oncology) office, and radiation facility records, cancer registry data, and from the original interview. Chemotherapy, hormonal and radiation therapies were categorized as receipt, non-receipt, or unknown. Type of surgery, based on the most extensive surgery performed, was categorized as breast conserving surgery (BCS), mastectomy, or none. Diagnosis delay was defined at the elapsed time between first medical consultation and biopsy-proven diagnosis of primary invasive breast cancer. Treatment delay was defined as the time between biopsy-proven diagnosis and the date of either definitive surgery, initiation of neo-adjuvant chemotherapy, or treatment for metastatic disease.
Tumor-related factors
Stage and its components (tumor size, nodal status, and distant metastasis) were obtained from the cancer registry and/or independent abstraction of medical records. Stage at diagnosis was defined using the American Joint Committee on Cancer Staging criteria that were in use during the case ascertainment period (1990–1992) [35]. Stage represents pathologic stage at the time of the first diagnostic procedure confirming invasive breast cancer (or clinical stage if the patient received neo-adjuvant therapy) and was divided into groups, based on similarity of expected prognosis and to achieve adequate numbers in our analyses: I, IIA, IIB, and III/IV.
Pathology
A detailed description of the pathology review and laboratory methodology was previously reported [31]. All components of the pathology review and immunohistochemical (IHC) assays were conducted without knowledge of patient characteristics or clinical outcome. Tumor tissue specimens were reviewed by a single pathologist (P.L.P) for Nottingham tumor grade [36, 37]. The levels of tumor marker proteins were assayed using standard IHC techniques, including antigen retrieval when appropriate, on tumor tissue sections using the antibodies specified below [38–42].
Representative tumor blocks were selected for testing, and the expression levels of the following proteins were assessed: estrogen receptor (ER; ERID5; Immunotech, Westbrook, ME) [43–45], progesterone receptor (PR; 1A6; Novocastra, Newcastle-Upon-Tyne, United Kingdom) [46], c-ErbB-2 (HER-2/neu) oncogene protein (AO485/DAKO15; Dako, Capinteria, CA) [47], p53 tumor suppression gene protein (pAb 1801; Oncogene Science, Manhassett, NY) [48–50], Ki-67 proliferation-related antigen (MIB-1; Immunotech) [38, 39], and the cell-cycle regulatory proteins cyclin E (cyclin E polyclonal; Dr. James M. Roberts, Fred Hutchinson Cancer Research Center, Seattle, WA) [51, 52], cyclin D1 (5D4; Immunotech) [53, 54], p16 (Ab-2(ZJ11); Neomarkers Freemont, CA) [55], p21 (EA10; Calbiochem, San Diego, CA) [56], p27 (Ab1-DCS-72.F6; Neomarkers) [52, 57], p130 (Mab 10; Transductions Labs) [58], pRb (G3–245 PharMingen) [59], and Bcl-2 (6C8 D; Hockenbery Lab, FHCRC, Seattle, WA) [60]. Apoptotic index (AI) was determined by terminal deoxyunucleotidyl transferease-mediated dUTP-biotin nick end labeling (TUNEL) assay [61].
IHC assays were scored according to staining intensity and/or percentage of tumor cells that were positive. Scores were then collapsed into positive (high) and negative (or low) categories. For ER and PR, any nuclear staining was considered indicative of positivity. Antibody staining for HER2 was performed using the AO485 antibody from Dako and was initiated before the acceptance of the HercepTest kit (Dako) as the Food and Drug Administration-approved technique for the evaluation of HER2expression [62]. Tumor specimens were categorized as positive if there was ≥2+ positive membranous staining observed relative to normal breast epithelium. The clinical use of the marker to indicate therapy was not the objective of this current study and Herceptin for the treatment of HER2+ (c-ErbB-2 +) tumors was not a standard therapeutic option during the relevant study follow-up years (1990–2003). For p53, nuclear staining of ≥10% of tumor cells was categorized as positive. Expression levels of cyclin E and p27 were assigned scores ranging from 1 (negative) to 7 (highest intensity), with subsequent groupings as previously described [31]. Bcl-2 was categorized as positive if intermediate or high intensity cytoplasmic staining was detected and negative if no or low intensity cytoplasmic staining was detected.
The remaining tumor markers (Ki-67, p21, p16, pRb, p130, cyclin D1, AI) were graded according to the percentage of tumor nuclei with positive staining in four high power fields (HPF). An average of >1000 tumor cells were evaluated for each case. The results were categorized into tertiles (excluding zero values, which comprised a fourth group). They were then further dichotomized as “low” if there was no positive staining or if the percentage of positive cells was in the lowest tertile, and “high” if the percentage of positive stainings were in the two highest tertiles.
Triple subtypes
The breast cancer cases were divided into 4 IHC subtypes based on whether ER, PR, and HER2 expression were positive (+) or negative (−): Triple negative (ER−PR−HER2−), ERand/orPR+, HER2−; ERand/orPR+, HER2+; and ER−PR−HER2+. These four subtypes are approximately equivalent to the following respective subtypes developed through genetic hierarchal clustering: basal-like, Luminal A, Luminal B, and HER2+ (but distinct from Luminal B) [27, 63].
Follow-up and survival (Time to death)
Survival information was obtained from the Atlanta SEER registry, which utilizes multiple sources for active and passive follow-up, and routinely matches all cancers with the State of Georgia vital statistics database. Women who no longer resided in Georgia were followed-up on a yearly basis through the National Death Index. Death certificates were requested from the Georgia State Health Department or from the state where the participant resided at the time of death. For living patients, the follow-up interviews and additional medical record abstraction were also used to update last contact information. Cause of death was obtained from death certificates where underlying cause of death codes 174.0–174.9 or C50.0–C50.9 were classified as death due to breast cancer (International Classification of Diseases for Oncology). Survival (time to death from any cause) was calculated from the elapsed time between the date of diagnosis and the date of death or most recent follow-up. For this study, follow-up was truncated at January 1, 2003.
Statistical analysis
As previously reported, we determined if the inability to obtain tumor specimens on all of the eligible women may have caused selection bias [31]. We compared the pathology cohort to the entire eligible population. This comparison indicated that AA women accounted for 30% of the eligible population but only 24% of the pathology cohort. AA women in the pathology cohort were also younger (16% of AA in the eligible population were aged 21–34 years, compared to 20% of the pathology cohort) and more likely to have died of disease (64% vs. 55%) than were AA women in the overall eligible population. These differences between the eligible population and the pathology cohort were not observed among white women. Therefore, to reduce the possibility of overestimating the magnitude of tumor characteristic based on race, this analysis was weighted based on the inverses of the probabilities that women in the eligible population were included in the pathology cohort. Women were categorized into 20 groups, based on race, age at diagnosis (5 groups), and vital status at the time of contact to obtain consent (1998) [31]. For each group, the statistical weighting procedure used the inverse of the proportion of women in the eligible population with the same 3 characteristics, who were included in the pathology cohort. All reported results are based on weighted analyses, as described previously. [31]
In the present analyses, frequency distributions and χ2 tests of independence (or Fisher Exact tests where expected cell counts were less than 5) were used to describe the IHC triple subtypes [ER−PR−HER2−, ER−PR−HER2+, ER/PR+HER−, ER/PR+HER2+] by race and other patient-related (socio-demographic), tumor-related, and treatment-related factors. Odds ratios (unadjusted and adjusted for age and stage at diagnosis) and 95% confidence intervals (CIs) examined the magnitude and precision of the prevalence associations. Racial differences in the prevalence of the IHC triple subtypes were further examined by adjusting for patient/socio-demographic and diagnosis-related factors (age, poverty index, education, stage, and delay in time to diagnosis). The Hosmer–Lemeshow Goodness of Fit (GOF) test was satisfied for the multivariate logistic models we present.
Kaplan Meier curves and log rank tests were used to estimate unadjusted survival functions and to compare them across strata: race, the IHC triple subtypes, and the other patient-related and tumor-related prognostic factors and treatment factors [64]. Cox Proportional Hazards (PH) models were used to estimate the effect of each of the independent variables on the risk of death (unadjusted hazard ratio (HR)). No violations of the PH assumption were observed based on graphical and statistical approaches.
Racial differences in overall survival and within the IHC triple subtypes, were assessed by additional adjustment for factors that were determined a-priori as being clinically relevant (age, stage, grade, education status, poverty index, co-morbid status, and treatment factors [receipt of chemotherapy, radiation therapy, hormonal therapy, delay in receipt of treatment, and type of surgery]). Due to the restrictive sample sizes in the triple subtypes, interaction terms between race and the other covariates were not assessed in survival models.
Results
IHC triple subtype associations with race and other factors
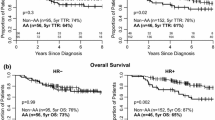
Characteristics of the study population by breast cancer triple subtypes and race are presented in Table 1. The most common subtype was ER+/PR+/HER2−, representing 56.3% of all breast tumors, followed by triple negative tumors (29.5%), with HER2+ expressing subtypes being the rarest (14.1%); ER−PR−Her2+ (6.7%) and ER/PR+HER2+ (7.3%). Triple negative tumors (TNTs) were the most prevalent subtype among African–American (AA) women, accounting for 46.6% of their tumors. In contrast, among white women, TNTs comprised only 21.8% of their tumors, while nearly two-thirds (64.3%) were the ER+/PR+/HER2− subtype. The racial differences in TNTs strongly persisted within age strata (Fig. 1) and stage strata (Fig. 2).
The four triple subtypes significantly differed by several other factors, including age at diagnosis, poverty index, and multiple tumor characteristics (Table 2). Patients with TNTs were younger and impoverished (Table 2). TNTs also demonstrated the largest proportions of high grade and Ki-67; high expression of p16, p53, and Cyclin E; and low Bcl-2, Cyclin D1, and p130 expression. TNTs, along with the ER−PR−HER2+ subtype, were least likely to be found in Stage I disease. TNTs were the rarest subtype among tumors ≤1.0 cm. There were no differences among the four subtypes by nodal status or distant metastasis.
Odds ratios for the triple subtypes, adjusted for age and stage at diagnosis, are presented in Table 3. The most common subtype (ER/PR+HER2−) serves as the referent group. The TNT subtype was strongly associated with AA women (OR = 3.0, 95% CI 2.1–4.2); as well as younger age (<40 years) and low poverty level. The TNTs were also significantly and strongly associated with high grade (OR = 9.4), as well as high expression of p53 (OR = 9.1), p16 (OR = 3.2), cyclin E (OR = 8.8), and low expression of cyclin D1 (OR = 8.3) and Bcl-2 (OR = 6.3).
The high TNT prevalence observed among AA women was attenuated, but persisted, after further adjustment for grade, poverty index, and diagnosis delay (OR = 1.9, 95% CI 1.2–2.9), (Table 4). After this same further adjustment, AA and white women did not differ with respect to the other breast cancer subtypes: ER−PR−HER2+ (OR = 1.3, 95% CI 0.6–2.7) and ER/PR+HER2+ (OR = 1.1, 95% CI 0.5–2.2).
Survival, race, and the triple subtypes
Median follow-up was 11.4 years and did not differ by race (AA = 11.5 years and white = 11.3 years). Of the 476 women in the cohort, 131 were deceased at the end of the study period, 57 AA and 74 white women, representing 39.5% and 23.6% of their respective populations (P < 0.001). In this young population of women ages 20–54, breast cancer was cited as an underlying cause of death in over 88% and did not differ by race.
Survival significantly differed by the triple subtypes and by race. Because survival curves could not be adjusted for weighting, Fig. 3 presents unadjusted/unweighted survival curves, stratified on race. Table 5 presents hazard ratios (HR) for all-cause mortality, comparing AA women to white women overall and within each IHC triple subtype. Overall, AA women were twice as likely to die (HR = 1.9, 95% CI 1.5–2.5). After adjustment for age, stage, grade, and poverty index this survival disparity was fully attenuated (OR = 0.9, 95% CI 0.7–1.3). Additional adjustment for treatment, including receipt of surgery, radiation, chemotherapy, and hormonal therapy drove the racial difference toward better survival for AA women, though non-significant (OR = 0.8, 95% CI 0.6–1.2).
Within every triple subtype, AA women also experienced poorer survival, when no other factors were considered (Table 5). The worst survival experiences for AA women were among the TNT group (OR = 2.1, 95% CI 1.3–3.3) and the ER−PR−HER2+ group (OR = 3.5, 1.8–7.1). Adjustment for age, stage, grade, and poverty index, had no affect on the disparate survival observed in the TNT subtype; AA women continued to experience statistically significant poorer survival (HR = 2.1, 95% CI 1.1–4.0). In the ER−PR−HER2+ group, composed of only 33 women, the survival differences were decreased by 50% (HR = 2.3, 95% CI 0.8–6.4) after this adjustment. Additional adjustment for treatment did not relevantly impact the observed survival differences in the TNT group (HR = 2.0, 95% CI 1.0, 3.7).
Discussion
Our study of this cohort of women under age 55 diagnosed with breast cancer in the early 1990s revealed that triple negative tumors (TNT) were the most common breast cancer subtype diagnosed among African–American (AA) women; TNT accounted for nearly 47% of their breast tumors, compared to 22% among white women. Moreover, every age subgroup and every stage subgroup presented this marked racial difference in TNT prevalence, suggesting that in this already young population it is not merely age or stage at diagnosis that is driving these differences. Indeed, the significant double prevalence for AA women persisted, when concurrently considering differences in age, stage at diagnosis, tumor grade, diagnosis delay, and socio-demographic factors (OR = 1.9). Our findings are consistent with the Carolina Breast Cancer Study (CBCS), where Carey et al. [18] reported that pre-menopausal AA women were most likely to present with TNTs (39%) vs. pre-menopausal non-AA women (14%); but racial differences were not observed in post-menopausal women. After adjustment for age and stage, TNTs were similarly twice as likely among AA women vs. white women in that population-based study (OR = 2.1). A California registry study, although missing nearly 45% of the data to construct triple subtypes, also found the highest prevalence for TNT among AA women (25%), followed by Hispanics (17%), and white women (11%) [28]. Among non-population based studies in Michigan and Philadelphia, higher TNT prevalences among AA women compared to white women have also been noted [29, 30]. Similar to previous studies [18, 30], we observed no racial differences in the other subtypes, including the other ER−PR− subtype (ER−PR−HER2+).
The population proportion of TNT we report is substantially greater than prior estimates [23]. This could partly be attributable to differences in assay kits and antibodies, processing and storage, cutoff points for assays, and disease stage. Additionally, most prior estimates were based on studies consisting primarily of homogenous Caucasian populations, both pre-and post-menopausal. Thus, the differences may be attributable to our younger cohort with large representation of AA women. Similar to AA women in the United States, early onset and more aggressive breast cancers are very common in sub-Saharan African women, but peak incidence occurs at an earlier age in the sub-Saharan region, between 35 to 45 years of age [65]. In a study of breast cancer among Nigerian women, 59% of cases were ER−negative and HER2−negative [66]. That this distinctly high prevalence of TNT among AA women in our study remained after accounting for age, stage, and grade at diagnosis, as well as poverty index, suggests it is not merely differences in access to care or social deprivation that drives this propensity for triple negative tumors in AA women. The consistency across the studies strongly suggests a role for African ancestry, associated genetic variations, unmeasured risk and biological factors, and gene–environment interactions in the etiology of TN breast cancers.
There is a clear need to refine etiologic heterogeneity through evaluating interactions among genes and environmental exposures i.e. behavioral risk factors or system-related factors (gene–environment interactions ) or through surrogates such as our study which bases subtypes on tumor protein expression [phenotype–environment interactions]. Very few studies have examined risk factors or racial differences in risk factors that could contribute to heterogeneity in breast cancer subtypes. A population-based study of Polish women identified increased BMI as a factor which significantly reduced risk of the Luminal A (ER+PR+HER2−) subtype (OR = 0.71), but not the basal-like (ER−PR−HER2−, CK5+ and/or HER1+) subtype (OR = 1.18, 95% CI 0.86–1.64). Furthermore, the association was exclusively in pre-menopausal women. This risk heterogeneity is consistent with our findings that overweight/obese women were at 2–3 fold increased odds for the TNT subtype (data not shown). However, the Polish study also suggests that our findings are driven more by the decreased propensity for Luminal A cancers among overweight/obese women, rather than overweight/obesity increasing risk for TNTs. In the very recent study of the CBCS population, Millikan et al. [67] suggest that racial differences in parity and breast feeding history account for much of the increased prevalence of TNTs among AA women.
There is compelling evidence that TNT, or at least those that are the basal-like phenotype, may arise through distinct stem cells or through early genetic alterations e.g. inherited susceptibility as in BRCA1 carriers, thus predisposing to earlier-onset disease [26, 63, 68]. Similar to other studies, TNT in our study exhibited a ‘BRCA1-ness’ in that they were uniquely associated with high grade and mitotic activity, medullary and atypical medullary histologies, high expression of p53 and cyclin E, and low expression of cyclin D1 [24, 68, 69]. The proclivity for hereditary BRCA-related breast cancers in Ashkenazi Jews is well established. Among AA women, a similar defect in the BRCA pathway that is yet to be identified, hereditary or sporadic (genetic or epigenetic), may underlie the high prevalence of TNT that we and others have reported.
To improve patient outcome, it will be necessary to identify tumor targets/proteins for directed therapies. Potential targets have been proposed in the last year, but the literature suggests that no one target is 100% up-regulated among triple negative tumors and those most effective treatments will be those that target a combination of proteins [23, 70]. In our study, although several proteins were uniquely up-regulated in the TNT subtype (i.e. Cyclin E, p16, and p53), the highest proportion was seen for p53; over-expression was noted in 51%. We previously reported racial differences in this population for many of these same tumor proteins, suggesting that AA descent may be a determinant, or surrogate, for defects in specific pathways [31]. Our current study suggests this may be particularly so for those associated with the aggressive TNT subtype. Thus, different and more aggressive therapeutic strategies will be required in treating these tumors. Phase I and II trials targeting other specific proteins and pathways are underway [23]. Considering the high prevalence of TNTs among AA women, current and future trials must take care that there be commensurate AA, and other minority, representation.
Through earlier detection and improved treatments, mortality rates among women with breast cancer have declined over the last decades and 5-year relative survival has risen from 75% to 90% [1]. Yet mortality differences between AA and white women have grown ever wider and survival for AA women today is equivalent to that of white women from 25 years ago. Our study suggests that, beyond factors related to socioeconomic status, a large proportion of AA women have not been able to reap the benefits of improved treatments because of the intrinsic biology of their tumors. Consistent with other studies [13, 18], the poorest survival in our study was among women with ER−PR−HER2+ expressing tumors and the TNT, the worst for the former subtype. This subtype now benefits from trastuzumab therapy (Herceptin) which specifically targets HER2+ tumors, and has lead to marked improvements in outcome [71, 72]. Thus, TNT may now carry with them the worst clinical prognosis. Furthermore, within the TN subtype, we found that the risk of death was twice as high for AA women as for white women, after adjusting for other tumor, treatment, and socio-demographic factors. Additional adjustment for co-morbid illnesses had no affect on the mortality differences (data not shown); although in this young cohort of women, very few had co-morbid illnesses that would impact treatment options or survival. This suggests that beyond socio-demographic and system-related factors that may lead to poorer outcome among AA women, the currently available chemotherapeutic regimens may be less effective in AA women. The poorer survival associated with TNT, combined with the high prevalence of TNT among young and AA women, bode a poor clinical course for young women, AA women, and particularly young AA women with breast cancer. Considering the high prevalence of TNTs among AA women and the accompanying worse prognosis, strategies must encompass socio-demographic factors and treatment that is both targeted and adherent in order to overcome the foreboding poor outcome.
This is the first population-based study to characterize major breast cancer subtypes based on centralized pathology review and testing, permitting standardized criteria for assessment thereby reducing the potential for measurement error. It is also the first to investigate the association of TNTs with breast tumor proteins important to regulation of the cell-cycle or to programmed cell death (apoptosis); proteins which may have particular importance in early age breast carcinogenesis, aggressiveness, or clinical outcome [31]. In addition to standard prognostic factors, our survival analyses are the first to account for delay in treatment, type of treatment received, and co-morbidities. The comprehensive array of standard prognostic factors, treatment factors, and socio-demographic indicators is, we believe, a major strength. Finally, the nearly 500 cases and 30% African–American representation, allowed for multivariate adjustment of these factors by major triple subtypes of breast cancer, so as to examine factors which could explain these racial differences. However, there is the possibility that we may have not completely accounted for SES and lifestyle differences across the lifecourse that may have contributed to the higher TNT prevalence among AA women. We were also limited in our ability to include interaction terms in our multivariate analyses. Also, though not completely concordant, TNT are typically associated with the basal-like phenotype, the latter characterized by over-expression of cytokeratins 5, 6, and 17 or by up-regulation of EGFR (HER1), as well as TN status. While we analyzed a number of proteins potentially associated with breast cancer early carcinogenesis and outcome, we did not analyze markers considered specific to the basal-like phenotype. Additionally, we considered HER2 IHC values of 2+ as positive (and these were not confirmed by FISH analyses), hence a large proportion of our HER2+ cancers could in fact be triple negative. This would likely increase the incidence of TNTs in both AA and white women, but should not have major impact on the racial differences we report. Also, our findings may not be generalizable to women of all ages or from other national or international regions as in this population-based setting, only younger women from three counties of Atlanta were included. A more serious limitation is that tumor characteristics could not be assessed for all women in the cohort. However, as in prior analyses, we weighted the sample to address these issues and we believe our estimates are not largely influenced by this bias, suggesting that residual selection bias does not significantly alter our findings.
In conclusion, the high prevalence of aggressive triple negative tumors among younger women and particularly younger AA women, along with the unique protein expression patterns and poorer survival noted with these breast cancers, suggests varying gene–environment etiologies with respect to age and race/ethnicity and ultimately a direct connection to prognosis [73]. The necessity to identify gene–environment interactions that program biologically distinct breast cancer subtypes such as triple negative tumors (TNTs) among young women, African–American women, and in nearly half of young AA women, is apparent. Regarding survival; poverty, tumor biology, and treatment impact overall survival, but fail to explain the poorer survival experienced by AA women with the TN subtype. The increased risk of death for women with TNTs, and particularly AA women, underscores the need for better characterization of TNT signatures and to develop effective targeted therapies and expeditiously translate them into clinical practice. It is clearly time for breast cancer to be interrogated as a heterogeneous group of diseases with acknowledgment of the observed differences in its molecular biology; racial, age, and otherwise. In doing so, we may be able to offer new hope to those in greatest jeopardy.
References
Ries LAG, Melbert D, Krapcho M et al (2006) SEER cancer statistics review, 1975–2004. National Cancer Institute, Bethesda, MD
Amend K, Hicks D, Ambrosone C (2006) Breast cancer in African–American women: differences in tumor biology from European–American women. Cancer Res 66(17):8327–8330
Chlebowski RT, Chen Z, Anderson GL et al (2006) Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst 97(6):439–448
Eley JW, Hill HA, Chen VW et al (1994) Racial differences in survival from breast cancer. Results of the National Cancer Institute Black/White Cancer Survival Study. JAMA 272(12):947–954
Jatoi I, Chen BE, Anderson WF, Rosenberg PS (2007) Breast cancer mortality trends in the United States according to estrogen receptor status and age at diagnosis. J Clin Oncol 25(13):1683–1690
Newman LA, Griffith KA, Jatoi I et al (2006) Meta-analysis of survival in African American and white American patients with breast cancer: ethnicity compared with socioeconomic status. J Clin Oncol 24(9):1342–1349
Shavers VL, Harlan LC, Stevens JL (2003) Racial/ethnic variation in clinical presentation, treatment, and survival among breast cancer patients under age 35. Cancer 97(1):134–147
Wojcik BE, Spinks MK, Optenberg SA (1998) Breast carcinoma survival analysis for African American and white women in an equal-access health care system. Cancer 82(7):1310–1318
Dayal HH, Power RN, Chiu C (1982) Race and socio-economic status in survival from Breast cancer. J Chronic Dis 35(8):675–683
Abd El-Rehim DM, Ball G, Pinder SE et al (2005) High-throughput protein expression analysis using tissue microarray technology of a large well-characterised series identifies biologically distinct classes of breast cancer confirming recent cDNA expression analyses. Int J Cancer 116(3):340–350
Abd El-Rehim DM, Pinder SE, Paish CE et al (2004) Expression of luminal and basal cytokeratins in human breast carcinoma. J Pathol 203(2):661–671
Sorlie T, Perou CM, Tibshirani R et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98(19):10869–10874
Sorlie T, Tibshirani R, Parker J et al (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 100(14):8418–8423
Sorlie T, Wang Y, Xiao C et al (2006) Distinct molecular mechanisms underlying clinically relevant subtypes of breast cancer: gene expression analyses across three different platforms. BMC Genomics 7:127
van‘t Veer LJ, Dai H, van de Vijver MJ et al (2002) Gene expression profiling predicts clinical outcome of breast cancer[comment]. Nature 415(6871):530–536
Sotiriou C, Neo SY, McShane LM et al (2003) Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci USA 100(18):10393–10398
Birnbaum D, Bertucci F, Ginestier C et al (2004) Basal and luminal breast cancers: basic or luminous? (review). Int J Oncol 25(2):249–258
Carey LA, Perou CM, Livasy CA et al (2006) Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 295(21):2492–2502
Haffty BG, Yang Q, Reiss M et al (2006) Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol 24(36):5652–5657
Perou CM, Sorlie T, Eisen MB et al (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752
Sotiriou C, Wirapati P, Loi S et al (2006) Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst 98(4):262–272
Yang XR, Sherman ME, Rimm DL et al (2007) Differences in risk factors for breast cancer molecular subtypes in a population-based study. CEBP 16(3):439–443
Cleator S, Heller W, Coombes RC (2007) Triple-negative breast cancer: therapeutic options. Lancet Oncol 8(3):235–244
Rakha EA, El-Rehim DA, Paish C et al (2006) Basal phenotype identifies a poor prognostic subgroup of breast cancer of clinical importance. Eur J Cancer 42(18):3149–3156
Nielsen TO, Hsu FD, Jensen K et al (2004) Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 10(16):5367–5374
Anderson WF, Matsuno R (2006) Breast cancer heterogeneity: a mixture of at least two main types?[comment]. J Natl Cancer Inst 98(14):948–951
Rakha EA, El-Sayed ME, Green AR et al (2007) Prognostic markers in triple-negative breast cancer. Cancer 109(1):25–32
Bauer KR, Brown M, Cress RD et al (2007) Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2–negative invasive breast cancer, the so-called triple-negative phenotype: A population-based study from the California cancer Registry. Cancer 109(9):1721–1728
Morris GJ, Naidu S, Topham AK et al (2007) Differences in breast carcinoma characteristics in newly diagnosed African–American and Caucasian patients: a single-institution compilation compared with the National Cancer Institute’s Surveillance, Epidemiology, and End Results database. Cancer 110(4):876–884
Stark A, Kapke A, Schultz D (2007) Advanced stages and poorly differentiated grade are associated with an increased risk of HER2/neu positive breast carcinoma only in White women: findings from a prospective cohort study of African–American and White-American women. Breast Cancer Res Treat [epub ahead of print]
Porter PL, Lund MJ, Lin MG et al (2004) Racial differences in expression of cell cycle regulatory proteins in breast cancer: Study of young African American and white women in Atlanta. Cancer 100(12):2533–2542
Brinton LA, Daling JR, Liff JM et al (1995) Oral contraceptives and breast cancer risk among younger women. J Natl Cancer Inst 87(11):827–835
Gwyn K, Bondy ML, Cohen DS et al (2004) Racial differences in diagnosis, treatment, and clinical delays in a population-based study of patients with newly diagnosed breast carcinoma. Cancer 100(8):1595–1604
Department of Health, Human Services. (1991) HHS poverty guidelines. Fed Regist 47:15417–15418
SEER Summary Staging Manual (2000) Codes and coding instructions. National Cancer Institute, Bethesda, MD
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19(5):403–410
Tavassoli FA, Deville P, Aas T (2003) Pathology and genetics of tumours of the breast and female genital organs. Oxford University Press, Oxford
Cattoretti G, Becker M, Key G et al (1992) Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol 168(4):357–363
Gerdes J, Becker MH, Key G et al (1992) Immunohistological detection of tumour growth fraction (Ki-67 antigen) in formalin-fixed and routinely processed tissues.[see comment]. J Pathol 168(1):85–86
Hsu SM, Raine L, Fanger H (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures[see comment]. J Histochem Cytochem 29(4):577–580
Hsu SM, Soban E (1982) Color modification of diaminobenzidine (DAB) precipitation by metallic ions and its application for double immunohistochemistry. J Histochem Cytochem 30(10):1079–1082
Taylor CR, Shi SR, Chaiwun B et al (1994) Strategies for improving the immunohistochemical staining of various intranuclear prognostic markers in formalin- paraffin sections: androgen receptor, estrogen receptor, progesterone receptor, p53 protein, proliferating cell nuclear antigen, and Ki-67 antigen revealed by antigen retrieval techniques[see comment]. Human Pathol 25(3):263–270
Andersen J, Poulsen HS (1989) Immunohistochemical estrogen receptor determination in paraffin-embedded tissue. Prediction of response to hormonal treatment in advanced breast cancer. Cancer 64(9):1901–1908
Parl FF, Posey YF (1988) Discrepancies of the biochemical and immunohistochemical estrogen receptor assays in breast cancer. Human Pathol 19(8):960–966
Shousha S, Stamp T, James K et al (1989) Immunohistochemical study of oestrogen receptors in breast carcinomas that are biochemically receptor negative. J Clin Pathol 43:239–242
Giri D, Goepel J, Rogers K (1988) Immunohistological demonstration of progesterone receptor in breast carninomas: correlation with radioligand binding assays and oestrogen receptor negative. J Clin Pathol 41:444–447
Press MF, Hung G, Godolphin W et al (1994) Sensitivity of HER-2/neu antibodies in archival tissue samples: potential source of error in immunohistochemical studies of oncogene expression. Cancer Res 54(10):2771–2777
Bartek J, Bartkova J, Vojtesek B et al (1990) Patterns of expression of the p53 tumour suppressor in human breast tissues and tumours in situ and in vitro. Int J Cancer 46(5):839–844
Davidoff AM, Herndon JE 2nd, Glover NS et al (1991) Relation between p53 overexpression and established prognostic factors in breast cancer. Surgery 110(2):259–264
Purdie CA, O’Grady J, Piris J et al (1991) p53 expression in colorectal tumors. Am J Pathol 138(4):807–813
Ohtsubo M, Theodoras AM, Schumacher J et al (1995) Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol 15(5):2612–2624
Porter PL, Malone KE, Heagerty PJ et al (1997) Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nat Med 3(2):222–225
Motokura T, Bloom T, Kim HG et al (1991) A novel cyclin encoded by a bcl1-linked candidate oncogene [see comment]. Nature 350(6318):512–515
Simpson JF, Quan DE, O’Malley F et al (1997) Amplification of CCND1 and expression of its protein product, cyclin D1, in ductal carcinoma in situ of the breast. Am J Pathol 151(1):161–168
Geradts J, Hruban RH, Schutte M et al (2000) Immunohistochemical p16INK4a analysis of archival tumors with deletion, hypermethylation, or mutation of the CDKN2/MTS1 gene. A comparison of four commercial antibodies. Appl Immunohistochem Mol Morphol 8(1):71–79
Barbareschi M, Caffo O, Doglioni C et al (1996) p21WAF1 immunohistochemical expression in breast carcinoma: correlations with clinicopathological data, oestrogen receptor status, MIB1 expression, p53 gene and protein alterations and relapse-free survival. Br J Cancer 74(2):208–215
Cote RJ, Shi Y, Groshen S et al (1998) Association of p27Kip1 levels with recurrence and survival in patients with stage C prostate carcinoma. J Natl Cancer Inst 90(12):916–920
Fusaro G, Wang S, Chellappan S (2002) Differential regulation of Rb family proteins and prohibitin during camptothecin-induced apoptosis. Oncogene 21(29):4539–4548
Saegusa M, Hashimura M, Kuwata T et al (2006) Induction of p16INK4A mediated by beta-catenin in a TCF4-independent manner: implications for alterations in p16INK4A and pRb expression during trans-differentiation of endometrial carcinoma cells. Int J Cancer 119(10):2294–2303
Zutter M, Hockenbery D, Silverman GA et al (1991) Immunolocalization of the Bcl-2 protein within hematopoietic neoplasms. Blood 78(4):1062–1068
Negoescu A, Lorimier P, Labat-Moleur F et al (1996) In situ apoptotic cell labeling by the TUNEL method: improvement and evaluation on cell preparations. J Histochem Cytochem 44(9):959–968
Birner P, Oberhuber G, Stani J et al (2001) Evaluation of the United States Food and Drug Administration-approved scoring and test system of HER-2 protein expression in breast cancer. Clin Cancer Res 7(6):1669–1975
Sorlie T. (2004) Molecular portraits of breast cancer: tumour subtypes as distinct disease entities. Euro J Cancer 40(18):2667–2675
Kleinbaum DG (1996) Survival analysis – a self-learning text. Springer-Verlag, New York
Fregene A, Newman LA (2005) Breast cancer in sub-Saharan Africa: how does it relate to breast cancer in African–American women? Cancer 103(8):1540–1550
Olopade OI, Ikpatt FO, Dignam JJ et al (2004) “Intrinsic Gene Expression” subtypes correlated with grade and morphometric parameters reveal a high proportion of aggressive basal-like tumors among black women of African ancestry. J Clin Oncol (Meeting Abstracts) 22(14 suppl):9509
Millikan RC, Newman B, Tse CK et al (2007) Epidemiology of basal-like breast cancer. Breast Cancer Res Treat [epub ahead of print]
Yehiely F, Moyano JV, Evans JR et al (2006) Deconstructing the molecular portrait of basal-like breast cancer. Trend Mol Med 12(11):537–5544
Foulkes WD, Brunet JS, Stefansson IM et al (2004) The prognostic implication of the basal-like (cyclin E high/p27 low/p53+/glomeruloid-microvascular-proliferation+) phenotype of BRCA1-related breast cancer. Cancer Res 64(3):830–835
Tan DS, Marchio C, Jones RL et al (2006) Triple negative breast cancer: molecular profiling and prognostic impact in adjuvant anthracycline-treated patients. Breast Cancer Res Treat [epub ahead of print]
Romond EH, Perez EA, Bryant J et al (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer.[see comment]. N Engl J Med 353(16):1673–1684
Slamon DJ, Romond EH, Perez EA et al (2006) Advances in adjuvant therapy for breast cancer. Clin Adv Hematol Oncol 4(suppl 1):4–9 [discussion suppl 10]
Spitz MR, Wu X, Mills G (2005) Integrative epidemiology: from risk assessment to outcome prediction. J Clin Oncol 23(2):267–275
Acknowledgements
Supported in part by awards RO1CA64292 (R.J.C., E.W.F., J.W.E., M.J.L.), RO1CA71735 (P.L.P.), the Avon Foundation (M.J.L., P.L.P.), the Glenn Foundation (M.J.L.), the Sindab Endowment (M.J.L., R.M.O.) and the Oak Ridge Institute for Science & Education Research Participation Program/CDC (M.J.L., K.F.T). The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Author information
Authors and Affiliations
Corresponding author
Additional information
This manuscript represents original work and has not been previously published in this form. This work was presented in part at the American Association for Cancer Research Annual Meetings, April 2006, Washington DC, April 2007, Los Angeles, CA.
Rights and permissions
About this article
Cite this article
Lund, M.J., Trivers, K.F., Porter, P.L. et al. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Res Treat 113, 357–370 (2009). https://doi.org/10.1007/s10549-008-9926-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-008-9926-3