Abstract
Purpose
Progression during neoadjuvant chemotherapy (NAT) for early and locally advanced breast cancer is generally uncommon. However, these patients tend to do poorly, and salvage therapy (ST) use is variable and often not well defined. We aimed to establish the characteristics and outcomes of breast cancer (BC) patients progressing on NAT, report the patterns of institutional ST usage, and identify predictors of ST failure.
Methods
A retrospective review was conducted using the “Biomatrix” institutional database. Fisher’s exact test was used to study the association between baseline characteristics and progression after ST. Survival outcomes were estimated using Kaplan–Meier. Disease-Free Survival 1 (DFS1) and DFS2 represent the time between diagnosis and first progression, and the first and second progression, respectively. The log-rank test was used to compare survival outcomes between different ST types.
Results
Thirty patients out of 413 (7.2%) progressed on primary NAT, with a median follow-up of 28.52 months (13.77–46.97) and a mean age of 57 years (standard deviation: 12). The two most frequently used ST modalities were surgery (43%) and radiation with concurrent cisplatin chemotherapy (CT/RT) (40%). Eighty percent of the patients made it to subsequent surgery and among those, 11 (69%) were initially not operable and their tumors were rendered surgically removable after ST. The initial tumor stage and grade, and the presence of lymphovascular invasion predicted progression after ST (p = 0.02, p = 0.03 and p = 0.01, respectively). Median DFS1, DFS2, and overall survival were 4.4 months (95% CI 3.6–5.7), 14.8 months (95% CI 2.37–NR), and 39.5 months (95% CI 22.73–NR), respectively. No difference in survival outcomes based on ST type was seen.
Conclusion
In this evaluated cohort and despite potential poorer outcomes, patients progressing on NAT responded well to ST, became operable, and had promising survival outcomes. Appropriate selection of ST is crucial, and can help improve outcomes in such patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Locally advanced breast cancer (LABC) accounts for 5–15% of new breast cancer (BC) cases in high income nations, and for 40–60% of new cases in lower income nations [1]. Typically, LABC patients are treated with pre-operative, or neoadjuvant chemotherapy (NAT) followed by surgery, with tumor downstaging and breast conservation as major goals. NAT also enables the primary tumor response to serve as an in vivo chemosensitivity test: a reduction in the primary tumor volume can be thought to represent a reduction in micrometastatic disease [2].
The proven benefits of NAT in downstaging tumor burden, increasing breast conservation rates, and improving outcomes justify its routine clinical use in LABC. However, the routine use of NAT in early operable BC is less clear, given the lack of prospective evidence supporting its superiority to adjuvant chemotherapy in terms of overall survival [3]. However, the retrospective data are limited and largely based on older trials with older chemotherapy regimens. In addition, as the burden of residual disease after NAT is an important prognostic factor, and a complete pathologic response (pCR) is known to correlate with excellent patient outcomes [4–7], as such, many clinicians support the use of NAT in early, operable BC, particularly with high-risk phenotypes. A recent consensus recommends that it should be considered for all patients who are candidates to receive adjuvant chemotherapy [8].
Several studies have attempted to determine predictors of response to NAT and pCR rates [9–12]. Among them, ER-negative tumors, HER2-positive tumors, high Ki-67 expression, and the presence of tumor-associated lymphocytes appear to be the most robust markers yet reported [13–19]. However, predictors of progression during NAT are rarely studied mainly because only a minority of patients actually progress during NAT. A large series from MD Anderson reported that approximately 5% of the NAT patients progressed during treatment, and found that predictors of disease progression included race, large initial tumor size, ER and PR negativity, high Ki-67 scores, and high nuclear grade [20]. Interestingly, many of these tumor characteristics that correlated with progression have also been associated with a likelihood of complete response to NAT, as mentioned above.
Patients who progress during NAT tend to have poor outcomes, and no standard salvage treatment (ST) has been established for such patients. Different ST approaches are used, which include switching systemic therapies, radiation therapy (with or without additional chemotherapy), and surgery. Ultimately, it is important to identify these patients in a timely manner in order to optimize their subsequent treatments and outcomes.
Rationale
Given the variability of salvage therapies for patients progressing on NAT, and the paucity of data on predictors of ST failure, we believe it is important to establish the characteristics of BC patients treated with NAT at our own center who progressed and required ST.
Objectives
The primary objective of this study was to identify and describe the characteristics of patients with disease progression during NAT at our institution, to determine the types of salvage therapies used, and in particular, to establish patterns and predictors of ST failure. The ultimate aim is to identify the best ST strategy to use in that setting and the clinical features of the patients with the highest risk of failure after ST. The secondary objective was to study the association between the different ST types and survival outcomes including overall survival (OS) and disease-free survival (DFS). A particular unique endpoint was to establish DFS both before and after ST (DFS1 and DFS2).
Methods
Study design
This was a retrospective cohort study using data housed within the Sunnybrook Biomatrix. This is a secure, privacy-protected institutional data warehouse, currently housing several breast cancer databases. The web-based platform allows investigators to enter patient-level data throughout the cancer journey and facilitate REB-approved retrospective research. The Biomatrix currently houses a dedicated LABC database, which captures patients treated with neoadjuvant therapy at our institution, with patient consent. REB approval was granted to use these data for the project. Baseline de-identified patient, tumor, and treatment characteristics were evaluated, including age, tumor stage, receptor status (Estrogen, progesterone, HER2), the presence of lymphovascular invasion, histologic type, tumor grade, and type of surgery (breast and axillary). Clinical and pathologic staging was based on the seventh edition of the American Joint Committee on Cancer staging criteria [21] and tumor grading was based on the Nottingham score.
Patient population and treatment
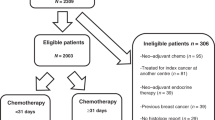
All patients within the Biomatrix LABC database with evaluable data who progressed clinically and/or radiologically during their NAT were included in the study; this included early breast cancer patients treated with NAT. Patients with metastatic disease (stage 4) at diagnosis and those treated with upfront radiation therapy or endocrine therapy were excluded.
Progression was defined as any clinical and/or radiographic increase in tumor size or new development of palpable lymphadenopathy or distant metastasis during NAT, which prompted salvage treatment (switch in NAT, radiation therapy with or without chemotherapy, and/or early surgery), or a complete cessation of curative-intent treatments (including initiation of palliative systemic therapy for established distant metastases). If the clinical exam was equivocal and suspicious for progression, an ultrasound, a mammogram, and/or a breast MRI were performed to confirm the progression and in some cases a computed tomography scan was added to rule out any metastasis. The change in NAT (chemotherapy) was considered as salvage if it was a regimen different than the planned initial treatment (considering most planned treatments including polychemotherapy with both anthracyclines and taxanes). As such, patients were considered as progressing if they showed progression on their anthracycline or taxane therapy. A switch to the taxane portion of the regimen was considered ST if the anthracycline portion was stopped and the switch happened earlier than what the initial regimen dictated. The goal of ST was to achieve surgical operability; in some cases, tumors were thought to be operable at the time of progression and no further systemic therapy or radiation was utilized. In these cases, surgery itself was considered as the primary ST as long as it happened before the end of the pre-planned systemic therapy.
Treatment information included initial NAT (chemotherapy), any interruption or adverse events from NAT, and salvage treatments used (different systemic therapy, radiation, and/or surgery). Type of progression (local, regional, distant) and methods of evaluation (clinical, radiographic) were also collected. pCR rates were established for those patients that were able to proceed with surgery. pCR was defined as the complete absence of invasive carcinoma in breast and lymph nodes; ductal carcinoma in situ was allowable.
Statistical analyses
All continuous variables were reported as means and medians with standard deviation and interquartile ranges as appropriate. All categorical variables were reported as frequency counts and proportions.
Follow-up time was estimated using the inversed Kaplan–Meier method [22]. Overall Survival (OS) was defined as the time from diagnosis until death. DFS1 was defined as the time from diagnosis until the date of the first event (local, regional or distant recurrence) or death. DFS2 was defined as the time between the first progression and the second progression (after ST) or the date of last follow-up or death. Patients without an event (death for OS and progression or death for DFS) were censored at the time of their last follow-up. OS and DFS for the whole population were estimated using the Kaplan–Meier method [23]. Fisher’s exact test was used to study the association between baseline characteristics and tumor progression after ST. A log-rank test was used to compare the survival outcomes (OS and DFS2) between groups depending on the ST used: group 1 included patients treated with concomitant chemotherapy/radiation therapy (CT/RT) as ST and group 2 included patients treated with other ST modalities. The choice of groups was based on the number of patients in each ST group and the clinical interest in the CT/RT regimen at our center. A two-tailed p value of <=0.05 was considered statistically significant for our analyses. Statistical analyses were performed with SAS® version 9.4 software (Cary, NC, USA).
Results
Patient characteristics
A total of 413 patients treated with NAT were eligible for evaluation; of these, 30 (7.2%) had progression on primary NAT, and were included in our analyses. The median study follow-up was 28.52 (13.77–46.97) months. Baseline patients and tumor characteristics are presented in Table 1. Of note, 57% of the patients with progression (17/30) had a triple-negative BC phenotype (TN) and 23% (7/30) were HER2-positive.
Treatment characteristics of patients with progression
All 30 patients who progressed received conventional chemotherapy (anthracycline–taxane based) in the neoadjuvant setting, and had a clinical and/or radiographic progression during their therapy. A dose reduction of the pre-planned neoadjuvant chemotherapy regimen was noted in six patients (20%) due to toxicity. Subsequently, they received ST with an interruption in their planned NAT course. A multidisciplinary team, including the treating medical oncologist, radiation oncologist and surgeon, generally decided the choice of ST. The different primary NAT regimens and types of ST used are presented in Table 2. Figure 1 represents the type of ST used by BC phenotype; patients with HER2-positive phenotype had upfront surgery as ST in 72% of the cases, while patients with TN phenotype had CT/RT as ST in 53% of the cases.
Response to salvage therapy
Eighty percent of the patients with progression (24/30) made it to surgery. This included patients treated with surgery as primary ST and patients treated with non-surgical ST who became operable thereafter. Forty-three percent (13/30) of the patients had upfront surgery as ST and 69% of the patients initially deemed to be non-operable at the time of progression (11/16 evaluable) were treated with non-surgical ST modalities and rendered operable and had surgery subsequently. Response by type of ST is presented in Table 3. One patient (1/24, 4%) was found to have a pCR at the time of surgery and one patient progressed during ST (1/30, 6%).
Predictors of progression after salvage therapy
After the end of ST, 57% of the patients (17/30) developed a progression subsequently. Among those, 53% were TN (9/17) and 29% were HER2+ (5/17). It was evaluated if baseline tumor characteristics were associated with progression after ST (ST failure). It was found that higher tumor stage (p = 0.02), higher tumor grade (p = 0.01), and the presence of LVI (p = 0.03) were significantly associated with progression after ST. A multivariable logistic regression analysis was not performed because of the small number of events observed (N = 17), which did not allow for any statistical model with more than one variable to be fit and to control for potential confounders. The univariate analysis for all baseline characteristics is presented in Table 4.
Survival analysis
As a secondary analysis, we reported the survival outcomes of our study cohort. Median DFS1 was 4.4 months (95% CI 3.6–5.7), median DFS2 was 14.8 months (95% CI 2.37–NR), and median OS was 39.5 months (95% CI 22.73–NR) (Figs. 2, 3 4, respectively). At 2 years after the first progression, 43.3% of patients remained recurrence-free and 66% were still alive.
We also evaluated if any particular ST was associated with better patient outcomes. We used the CT/RT treatment regimen as a standard comparator to all the other salvage modalities because of our specific interest in this regimen and preliminary data showing its efficacy especially in TNBC. There did not appear to be any difference in survival outcomes based on ST; the OS and DFS2 were not statistically different between the CT/RT regimen and the rest of the regimens used. However numerically, the survival outcomes were in favor of the CT/RT regimen. At 3 years after the first progression, 53.3% versus 30.5% and 65.5% versus 45.9% of the patients who received CT/RT versus other treatment modalities as ST were recurrence-free and alive, respectively (log-rank test, p = 0.38 and p = 0.35) (Fig. 5 and 6).
Discussion
To our knowledge, our study is only the second institutional series of patients progressing on NAT in the literature, the first being a patient cohort from MD Anderson [20]. Predictors of tumor response to NAT are well described [9–19]; however, predictors of progression, and the outcomes of these patients are less well studied; one likely reason for this is the low proportion of patients (5-10%) that actually do progress on NAT.
While in the MD Anderson series, predictors of tumor progression were identified; for the first time, we evaluated features that correlate with ST failure, and the outcomes of patients who receive these secondary treatments. Many of the baseline tumor characteristics identified (such as receptor status), which correlate with primary progression on NAT and progression on ST, are also known to correlate with response [14–17]; hence, these features themselves would not be useful markers to help predict patient response or progression upfront.
Interestingly, a significant number of non-operable patients who initially progressed on NAT (69%) were able to undergo surgery after receiving different types of non-surgical ST. This study not only described the different ST modalities used but also showed that non-surgical ST modalities were efficient enough to change the course and the surgical outcome for the majority of these patients especially if we consider that the ultimate goal was to make it to surgery.
One of the interesting regimens is the CT/RT regimen, which has been developed and used at our institution among a few others centers in our region; this regimen uses weekly cisplatin (25 mg/m2) along with daily radiation therapy for 5 weeks. Previous studies have assessed similar protocols using other chemotherapy drugs (taxanes or capecitabine) in different settings such de novo NAT for LABC or treatment for local recurrence, and the results were also promising [24, 25]. Work is ongoing using this ST for TN patients who progress on NAT.
Although no difference in survival was detected between all the ST regimens used from a statistical perspective, the clinical differences appeared to be meaningful comparing the CT/RT salvage regimen compared to all others. Numerically, the survival outcomes were in favor of the CT/RT regimen especially for the TN phenotype. However, due to the low number of events, conclusions from this analysis are limited. It is quite notable, however, that OS and DFS after ST are quite promising despite these patients historically having a poor prognosis. The follow-up interval was also rather short, and so longer follow-up would be ideal to establish if these relatively good outcomes persist.
Limitations to this work include the retrospective and exploratory nature of the analyses, and the lack of multivariable regression analyses to account for potential confounders. Furthermore, the low number of patients and events may have underpowered our analysis to detect any statistically significant survival difference between the ST regimens. In addition, we recognize that it would have also been interesting to assess the effect of dose reduction during NAT on progression rates. However, the number of patients we encounter in the clinical setting, who progress during NAT, is very low (5–10%), and renders such analyses challenging.
Conclusion and future work
This study identified for the first time predictors of ST failure in patients who progress initially during NAT. Despite their known poor outcome, a significant number of patients were rendered operable after receiving different non-surgical ST and their survival outcomes were quite promising. The CT/RT regimen seemed as an interesting ST option mainly for TNBC patients and this is currently being evaluated at our institution.
On another hand, In order to identify these patients at an early stage and ultimately change our treatment strategies and their outcomes, new biomarkers such tumor infiltrating lymphocytes need to be studied and validated in well-designed prospective studies.
References
Hunter CP (2000) Epidemiology, stage at diagnosis, and tumor biology of breast carcinoma in multiracial and multiethnic populations. Cancer 88(supp 5):1193–1202
Forrest AP, Levack PA, Chetty U et al (1986) A human tumor model. Lancet 2:840–842
Mauri D, Pavlidis N, Ioannidis JP (2005) Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst 97:188–194
Kong X, Moran MS, Zhang N et al (2011) Meta-analysis confirms achieving pathological complete response after neoadjuvant chemotherapy predicts favourable prognosis for breast cancer patients. Eur J Cancer 47:2084–2090
Broglio KR, Quintana M, Foster M et al (2016) Association of pathologic complete response to neoadjuvant therapy in HER2-positive breast cancer with long-term outcomes: a meta-analysis. JAMA Oncol. 2(6):751–760
Cortazar P, Zhang L, Untch M et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384(9938):164–172
De Mattos-Arruda L, Shen R, Reis-Filho JS, Cortés J (2016) Translating neoadjuvant therapy into survival benefits: one size does not fit all. Nat Rev Clin Oncol. 13(9):566–579
Symmans WF, Peitinger F, Hatzis Ch et al (2007) Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 25:4414–4422
Zambetti M, Mansutti M, Gomez P et al (2011) Pathological complete response rates following different neoadjuvant chemotherapy regimens for operable breast cancer according to ER status, in two parallel randomized phase II trials with and adaptive study design (ECTO II). Breast Cancer Res Treat. doi:10.1007/s10549-011-1660-6
Straver ME, Rutger EJ, Rodenhuis S et al (2010) The relevance of breast cancer subtypes in the outcome of neoadjuvant chemotherapy. Ann Surg Oncol 17:2411–2418. doi:10.1245/s10434-010-1008-1
Buzdar AU, Ibrahim NK, Francis D et al (2005) Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol 23:3676–3685
Gianni L, Eiermann W, Semiglazov V et al (2010) Neoadjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer: primary efficacy analysis of the NOAH trial. Cancer Res. 69:640–647
Untch M, Rezai M, Loibl S et al (2010) Neoadjuvant treatment with trastuzumab in HER’s-2 positive breast cancer: results from the GeparQuattro study. J Clin Oncol 28:2024–2031
Smith IC, Heys SD, Hutcheon AW et al (2002) Neoadjuvant chemotherapy in breast cancer; significantly enhanced response with docetaxel. J Clin Oncol 20:1456–1466
Heys SD, Hutcheon AW, Sarkar TK et al (2002) Neoadjuvant docetaxel in breast cancer: 3-year survival results from the Aberdeen trial. Clin Breast Cancer 3(Suppl 2):S69–S74
Von Minckwitz G, Blohmer JU, Raab G et al (2005) In vivo chemo sensitivity-adapted preoperative chemotherapy in patients with early-stage breast cancer: the GEPARTRIO pilot study. Ann Oncol 16:56–63
Von Minckwitz G, Blohmer JU, Costa S et al (2011) Neoadjuvant chemotherapy adapted by interim response improves overall survival of primary breast cancer patients—results of the GeparTrio trial. Cancer Res 71:S3
Kim Kwan Il, Lee Kyung Hee, Kim Tae Ryung et al (2014) Ki-67 as a predictor of response to neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer 17(1):40–46
Denkert C, Loibl S, Noske A et al (2010) Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 28(1):105–113
Caudle AS, Gonzalez-Angulo AM, Hunt KK et al (2010) Predictors of tumor progression during neoadjuvant chemotherapy in breast cancer. J Clin Oncol 28(11):1821–1828
Edge SB, Byrd DR, Compton CC et al (eds) (2010) AJCC Cancer Staging Manual, 7th edn. Springer, New York, pp 347–376
Schemper M, Smith TL (1996) A note on quantifying follow-up in studies of failure time. Control Clin Trials 17:343–346
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Amer Statist Assn 53(282):457–481
Mandilaras V, Bouganim N, Spayne J et al (2015) Concurrent chemoradiotherapy for locally advanced breast cancer-time for a new paradigm? Curr Oncol 22(1):25–32
Shaughnessy JN, Meena RA, Dunlap NE et al (2015) Efficacy of concurrent chemoradiotherapy for patients with locally recurrent or advanced inoperable breast cancer. Clin Breast Cancer 15(2):135–142
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Raphael, J., Paramsothy, T., Li, N. et al. A single-institution experience of salvage therapy for patients with early and locally advanced breast cancer who progress during neoadjuvant chemotherapy. Breast Cancer Res Treat 163, 11–19 (2017). https://doi.org/10.1007/s10549-017-4167-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4167-y