Abstract
Background
Most patients with breast cancer treated with neoadjuvant chemotherapy (NAC) experience clinical benefit, however, a small proportion progress. We aimed to characterize factors predicting in-breast tumor progression and impact on distant recurrence.
Patients and Methods
We reviewed all patients with clinical stage I–III breast cancer treated with NAC in 2006–2021 at our institution. We compared in-breast progressive disease (PD), defined as ≥ 20% increase in tumor size, with stable disease (SD) or response. Distant recurrence-free survival (DRFS) was analyzed using the Kaplan–Meier method and Cox proportional hazards regression.
Results
Of 1403 patients, 70 (5%) experienced in-breast PD, 243 (17%) SD, 560 (40%) partial response (PR), and 530 (38%) breast pathologic complete response (breast pCR, ypT0/Tis). The rate of PD varied by tumor subtype (8% in HR+/HER2−, 5% TNBC, 2% HER2+, p < 0.001). With median 48 months follow-up, the rates of DRFS were significantly different according to clinical breast response as follows: PD 56%, SD 68%, PR 82%, or breast pCR 93%, p < 0.001. In patients with PD on multivariable analysis, post-NAC grade (adjusted HR 2.9, p = 0.002) and ypT3–4 category (adjusted HR 2.4, p = 0.03) were the strongest predictors of DRFS. Combining these factors, 23% had neither, 44% had one, and 33% had both, which stratified outcome in PD with 3-year DRFS of 100%, 77%, and 30%, respectively (p < 0.001).
Conclusions
While in-breast PD during NAC is uncommon (5%), it predicts poor survival. Among patients with in-breast PD, post-NAC tumor grade and T category predict outcomes and may be useful to guide treatment escalation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Neoadjuvant chemotherapy (NAC) has emerged as an important component of breast cancer treatment. It allows for de-escalation of surgery with increased utilization of breast conservation and sentinel lymph node surgery by decreasing the burden of disease in the breast and axilla.1,2,3,4 However, the greatest advantage of NAC is likely the prognostic information gained from assessment of disease response.3,5,6,7,8 These factors have driven the increased use of NAC over time, most notably in patients with human epidermal growth receptor 2 (HER2) positive or triple negative breast cancer (TNBC).9,10
The advantages for NAC over adjuvant chemotherapy are clear; however, not all patients who undergo NAC have tumor response and some experience disease progression. While progressive disease (PD) is uncommon (1.6–7.2%), uncertainty remains about which patients are most likely to develop PD and the best clinical management.11,12,13,14,15,16,17 Caudle et al. evaluated a cohort of 59 patients with PD between 1994 and 2007 and identified African American race, greater tumor (T) category, higher AJCC clinical stage, higher tumor grade, and ER/PR negative receptor status as factors predicting PD.11 Progressive disease impacted operative plan in a small percentage of these patients (0.5%).12 In a more recent cohort of 124 patients with PD identified from 2005 to 2015, triple negative receptor status was independently associated with a decrease in overall survival (OS) in patients with PD. Likewise, inflammatory breast cancer and triple negative receptor status were both independently associated with distant failure in this group.16
While the population of patients with PD is small, it is critical to identify clinical and pathological factors associated with an increased risk for PD so that this population may be more closely monitored during NAC and to identify alternative treatment approaches. The aim of this study was to characterize patients who experience in-breast PD and to evaluate the impact of in-breast PD on survival.
Patients and Methods
With institutional review board approval, clinical and pathologic data from patients with stage I–III breast cancer who underwent NAC were identified from a prospectively maintained institutional database. Patients diagnosed from 2006 to 2021 were included. Patients treated with neoadjuvant endocrine therapy were excluded, as were those who did not undergo surgery at our institution. Demographic, clinicopathologic, and treatment data were extracted from patient charts and included for analysis. Tumor size on pretreatment ultrasound, and/or magnetic resonance imaging (MRI) where available, were recorded for each patient. The largest estimated tumor size across all imaging modalities was used as the pre-NAC clinical tumor size for the purposes of defining in-breast response. Post-treatment pathologic tumor size was extracted from the final surgical pathology report. The percent change in tumor size between pre-NAC imaging size and the post-treatment pathologic tumor size was calculated for each patient. PD was identified in accordance with revised Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (version 1.1), where PD was defined as ≥ 20% increase in the sum of the diameter of the target lesion and an absolute increase of ≥ 5 mm and was limited to disease progression in the breast.18 We did not include patients with disease progression only in the regional nodes or outside of the breast and nodal basins if there was no progression in the breast. In this study, HR+ was defined as either estrogen receptor (ER) or progesterone receptor (PR) ≥ 1%. HER2 status was defined as 3+ on immunohistochemistry (IHC) or a positive result on fluorescence in situ hybridization (FISH).
All patients undergoing NAC were characterized by their in-breast tumor response to treatment. Response to treatment was defined using modified RECIST criteria focused only on response in the breast as complete pathologic response (pCR, ypT0/ypTis), partial response (PR, ≥ 30% decrease), stable disease (SD, response < 30% or increase < 20%), or PD. Response to treatment was evaluated in relation to tumor receptor status [hormone receptor positive (HR+)/(HER2−), HER2+, or TNBC], clinical T category at time of initial diagnosis, tumor histology (invasive ductal, invasive lobular, mixed, or other), tumor grade, type of breast surgery, and type of axillary surgery.
Statistical Analysis
Data were summarized overall and by modified RECIST category using the median (range) for continuous variables and frequency (percentage) for categorical variables. The associations of baseline demographic and clinical factors with modified RECIST category were assessed using Wilcoxon rank-sum or chi-squared tests. A binary variable characterizing the patient as having PD versus not was modeled using univariate and multivariable logistic regression to identify baseline demographic and clinical factors associated with PD. To assess the impact of modified RECIST category on long-term oncologic outcome, the endpoint of distant recurrence-free survival (DRFS) was assessed. DRFS was defined as the months from surgery to first distant recurrence, death from any cause, or last follow-up in those without an event. DRFS was estimated using the Kaplan–Meier method and compared across modified RECIST categories using a log-rank test.
Univariate and multivariable Cox proportional hazards regression were used to assess factors associated with DRFS within the subset of patients with PD. Given the small sample size in the PD group, the Firth penalized likelihood bias-reduction method was applied to improve performance with sparse data. Variables included in multivariable models were chosen as the combination of variables yielding the best score statistic after consideration of all possible models of appropriate size given the number of events available. Analysis was performed using SAS (version 9.4, SAS Institute Inc., Cary, NC) and R software (version 4.2.2, www.R-project.org). Estimates were reported with 95% confidence intervals (CI) and p values < 0.05 were considered statistically significant.
Results
Of 1403 patients who underwent NAC and definitive surgical management, 530 (38%, 95% CI 35–40%) had breast pCR, 560 (40%, 95% CI 37–43%) PR, 243 (17%, 95% CI 15–19%) SD, and 70 (5%, 95% CI 4–6%) had in-breast PD. Median age of the patients with PD was 51.5 years, with median clinical tumor size at diagnosis of 3.2 cm. A total of 63% (44/70) had cT3–4 disease, 70% (49/70) were cN+, and 51% (36/70) had grade 3 disease at time of diagnosis, prior to initiation of NAC. The demographic, clinical, and pathologic data from the cohort overall and by modified RECIST category are summarized in Table 1.
Variables Associated with Response to NAC
Patients with breast pCR or PR were slightly younger than patients with SD or PD (median 50.5 versus 52.6 years, p = 0.005). Regarding tumor biology, breast pCR rates were highest in HER2+ disease and TNBC at 56% and 44% compared with those with HR+/HER2− disease (breast pCR rate of 19%, p < 0.001). PD was more common in HR+/HER2− disease (8% with PD) compared with HER2+ (2%) and TNBC (5%), p < 0.001; see Fig. 1A. Furthermore, pretreatment grade 1/2, invasive lobular or other histology, and higher cT category had higher rates of in-breast PD (see Fig. 1B–D). The most common other histology was metaplastic carcinoma, comprising 4/12 (33%), with 1/4 (25%) developing PD.
Looking at type of breast surgery performed, mastectomy rates were slightly higher in patients with in-breast PD (80%) compared with those with SD (76%), PR (73%), or breast pCR (70%), but this was not significant (p = 0.18). Axillary surgery, however, was more extensive in patients with in-breast PD. Among clinically node negative (cN0) patients with in-breast PD, 33% underwent axillary lymph node dissection (ALND), while the rates of ALND in cN0 patients were 31% for SD, 17% for PR, and 7% for breast pCR, p < 0.001. A majority of patients who were clinically node positive (cN+) at presentation underwent ALND in patients with in-breast PD, although a similar trend was observed across modified RECIST categories (88% for PD, 84% for SD, 78% for PR, 55% for breast pCR, p < 0.001); see Fig. 2.
Factors Associated with In-Breast PD
On univariate analysis, factors associated with PD included clinical T3 or T4 category at diagnosis, histology, and tumor subtype of HR+/HER2− or TNBC (Table 2). Patient age, body mass index (BMI), and biopsy tumor grade were not significantly associated with PD.
On multivariable analysis, factors associated with increased odds of PD were cT4 disease (adjusted odd ratio [aOR] 3.65, 95% CI 1.63–8.17, p = 0.002 for cT4a–c and aOR 6.08, 95% CI 2.84–13.03, p < 0.001 for cT4d, each versus cT0–2) and biologic subtype other than HER2+ disease (aOR 3.15, 95% CI 1.37–7.26, p = 0.007 for TNBC and aOR 4.92, 95% CI 2.31–10.46, p < 0.001 for HR+/HER2−, each versus HER2+), Table 2. Invasive ductal or mixed invasive ductal lobular carcinoma was associated with decreased odds of progression (aOR 0.44, 95% CI 0.22–0.91, p = 0.03). This model showed good discrimination in identifying patients at higher risk for PD with area under the curve (AUC) of 0.72 (95% CI 66–78%) and adequate calibration (Hosmer–Lemeshow goodness-of-fit test p = 0.42).
Distant Recurrence-Free Survival
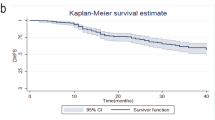
With a median follow-up of 48 months, 36 patients with PD experienced a DRFS event, resulting in a DRFS estimate of 79% (95% CI 70–89%) at 1 year, 67% (95% CI 57–79%) at 3 years, and 56% (95% CI 45–70%) at 5 years. These 36 DRFS events included 32 patients with distant recurrence and 4 patients who died from other or unknown causes without breast cancer recurrence. The sites of first distant recurrence were as follows: bone (14), lung (9), liver (5), brain (3), distant lymph nodes (6), and other sites in 3, with 9 patients having multiple sites of first distant recurrence.
DRFS varied significantly (p < 0.001) according to in-breast response to treatment (Fig. 3A). DRFS at 5 years was 93% (95% CI 90–95%) for patients achieving a breast pCR, 82% (95% CI 79–86%) for those with PR, and 68% (95% CI 62–76%) for SD, compared with the 56% (95% CI 45–70%) DRFS in those with PD at 5 years. When stratified by tumor biologic subtype, a stepwise decrease in DRFS was also observed to a degree within each subtype but with the clearest differentiation in patients with TNBC. Among patients with PD, DRFS was worst in patients with TNBC followed by those with HER2+ and HR+/HER2− breast cancer (Fig. 3B–D).
Kaplan–Meier curve of distant recurrence-free survival (DRFS) through 5 years post-surgery in patients undergoing NAC based on response to treatment stratified by tumor biologic subtype A overall, B in patients with TNBC, C in patients with HER2+ breast cancer, and D in patients with HR+/HER2− breast cancer
Factors Associated with DRFS in Patients with PD
DRFS in patients with PD did not differ by age (p = 0.48), BMI (p = 0.87), or ypN status (p = 0.84). On univariate analysis, factors associated with DRFS in patients with PD included histology, biopsy tumor grade 3, post-NAC tumor grade 3, ypT3–4 category, and tumor; see Table 3.
On multivariable analysis, post-NAC grade 3 versus 1–2 [adjusted HR (aHR) 2.91, 95% CI 1.46–5.79, p = 0.002] and ypT3–4 versus ypT1–2 category (aHR 2.44, 95% CI 1.10–5.40, p = 0.03) were the strongest predictors of DRFS in patients with PD (Table 3). Combining these two factors, 23% (16/70) had neither, 44% (31/70) had one, and 33% (23/70) had both factors, which stratified outcome in the setting of PD with 3-year DRFS of 100%, 77% (95% CI 63–94%), and 30% (95% CI 16–56%), respectively (p < 0.001); see Fig. 4. Although biologic subtype was not included directly in this model, the risk categories defined by post-NAC grade and ypT category were strongly related to tumor biologic subtype, with the two lowest risk groups predominantly HR+/HER2− disease [81% (13/16) and 87% (27/31), respectively] and with TNBC comprising > 50% of the highest risk group (12/23); see Supplementary Fig. 1. Further, a large majority of HR+/HER2− patients with PD (40/45 or 89%) fell into the two lowest risk groups, while the majority of patients with HER2+ [75% (6/8)] and TNBC [71% (12/17)] with PD fell into the highest DRFS risk group.
Discussion
In-breast disease progression during NAC is uncommon, and in this study occurred in 5% of patients. Patients with in-breast PD had significantly worse outcomes than those with breast pCR, PR, or SD. Our data demonstrate a significantly lower DRFS in patients with in-breast disease progression on NAC. Furthermore, among patients who do have PD, factors associated with poorer survival were post-NAC tumor grade 3 and residual breast tumor category of ypT3–4. Although DRFS in patients with PD was only 56% overall at 5 years, our analysis identified a subgroup of patients with PD, those with post-NAC grade 1–2 and ypT1–2, who had good DRFS during early follow-up despite their in-breast tumor progression during NAC.
Poorer DRFS in patients with PD is consistent with findings from prior studies including reports of DDFS of 23.2 months and 16 months.16,17 In comparison with those with PD, patients with breast pCR, PR, or SD in our cohort were found to have significantly better DRFS, further highlighting the critical relationship between response to NAC and overall outcomes. A systematic review and meta-analysis of 27,895 cases from 1999 to 2016 supports this finding and demonstrated that patients who achieved a pCR after NAC, compared with those who did not, experienced a significantly better event-free survival (EFS) and OS.19 This echoes findings published in an earlier international meta-analysis.20 Furthermore, in a pooled analysis of 5161 patients from the USA and Europe from 2018 to 2019, residual cancer burden (RCB) score and class were independently prognostic in all subtypes of breast cancer.8 A total of 38% of patients in our cohort achieved pCR, which is consistent with what has been published previously.21
The literature has demonstrated that response to NAC provides unique information about survival when stratified by tumor biologic subtypes. In particular, early data from the I-SPY1 clinical trial demonstrated that the extent of advantage conferred by pCR in terms of survival is specific to tumor biologic subtype.5 When looking more closely at pCR, multiple studies have demonstrated a survival benefit in patients experiencing pCR with HER2+ disease and TNBC.19,22,23
Interestingly, in our cohort of patients with in-breast PD, we found that post-NAC tumor grade and T category were the strongest predictors of DRFS in patients with progressive disease, suggesting that survival in PD may be more heavily influenced by the way these features impact tumor response to NAC than tumor biologic subtype. However, the univariate significance of subtype and the strong relationship between subtype and post-NAC grade and ypT category, as well as the limitation of our small sample size with PD, suggest that subtype may be important, though we were unable to establish an independent association with DRFS in the setting of PD with this study.
The advent of targeted therapies for HER2+ disease as well as the use of immunotherapy for TNBC has changed the landscape of NAC. As trastuzumab and pertuzumab have been widely adopted in the neoadjuvant setting for HER2 positive disease, rates of pCR in this population have increased. An analysis of 6994 HER2+ patients included in the NCDB from 2013 to 2016 treated with multiagent HER2 directed chemotherapy in the neoadjuvant setting has demonstrated pCR rates as high as 46.6% in patients with cT1–T2/N0 disease.24 When pCR was stratified by receptor subtype, this number rose to 63% in patients with HR−, HER2+ disease. Prospective studies have suggested that the number of patients achieving a pCR with multiagent HER2 directed chemotherapy in the neoadjuvant setting may be even higher.25 In our cohort, the proportion of patients experiencing PD was lowest in those with HER2+ disease, which is suggestive of the efficacy of targeted anti-HER2 NAC in patients with HER2+ disease.
While our cohort primarily captured patients prior to the wide adoption of targeted immunotherapy for TNBC, the last 2–3 years of our cohort included patients who received pembrolizumab as part of their neoadjuvant regimen as established by the KEYNOTE-522 trial. KEYNOTE-522 demonstrated a pCR rate of 60% in patients who received pembrolizumab in combination with chemotherapy in the neoadjuvant setting, compared with a pCR rate of 35–45% observed with conventional chemotherapy prior to adoption of this NAC regimen in patients with TNBC.22,26 A smaller proportion of patients with TNBC in our study cohort experienced PD, though it is difficult to attribute this smaller proportion of patients exclusively to the inclusion of immunotherapy in NAC regimens. Future evaluation of cohorts who develop PD will better elucidate the impact that these new therapies have had on the proportion of patients with TNBC developing PD.
Clinical T4 category and HR+/HER2− or TNBC tumor biology were associated with increased odds of disease progression on multivariable analysis in our cohort. We found that patients with T4a–c or T4d (inflammatory breast cancer) disease were more likely to experience disease progression. While this factor has been suggested in correlation with PD in previous studies, its predictive relationship has not been well characterized prior to this cohort. In particular, T4d category has been demonstrated to have significant impact on DFS and OS in previous studies, though it has not previously been identified as an independent predictor of PD prior to our cohort.16
With regard to biologic subtype, HR+/HER2− and TNBC were found to be independent predictors of PD in our cohort. Interestingly, this is the first cohort to identify HR+/HER2− biologic subtype as an independent predictor of PD. A previously published cohort examining patients from 1994 to 2007 demonstrated TNBC as an independent predictor of PD, which was also reflected in our cohort.11 We anticipate that in future cohorts the proportion of patients with TNBC who experience progression may diminish due to recent advances in targeted NAC regimens in this group. While we have presented a more modern cohort in this study, the cohort was not recent enough to adequately capture these shifts in treatment.
We exclusively examined patients with PD who underwent surgery in this study and thus excluded patients who progressed and did not undergo surgical resection either due to disease extent, comorbidities, or development of metastatic disease. While this facet of our study provided an interesting window into how breast-specific progression impacts DRFS, a limitation of this approach is that we did not examine progression in the regional nodes or distant progression during NAC. Future studies examining in-breast progression with regional nodal and distant progression may provide more insight into the facets of disease biology and histology that influence progressive disease outside the breast.
There are several other limitations to our study. Firstly, we utilized preoperative imaging size to estimate pretreatment tumor size. The type of imaging used to estimate pretreatment tumor size varied between patients, as we were not able to standardize imaging modalities due to the retrospective nature of our study design. Secondly, imaging after neoadjuvant treatment was not factored into our assessment of progression and was not routinely performed in our patient cohort. Thirdly, there was a very small number of patients with PD in our cohort, which could have affected the precision of our estimates of DRFS and limited our statistical power to detect factors associated with DRFS. As a result, our model could have excluded clinically important factors that were less common or had a smaller effect size. Fourthly, the small number of patients with PD means that our model for predicting DRFS may be overfit to this particular dataset. While we have tried to avoid this by including a number of variables considered reasonable for model stability given the number of events and by using penalized regression methods appropriate for sparse data, this model should be validated in other datasets.
Our study identified that 5% of patients develop progression, which is in congruence with prior recent studies identifying 1.6–7.2% of patients with progression.11,12,13,14,15,16,17 We anticipate that our rate of patients with progression was slightly higher than other studies as we strictly adhered to modified RECIST criteria utilizing final pathologic measurement of the tumor in defining the post-NAC tumor size. This comparison may have identified patients with subclinical progression who did not have PD noted on post-NAC, preoperative imaging. We anticipate that including patients with subclinical progression in our PD cohort may provide a clearer picture of those at risk of developing progression and may capture additional patients at risk for poorer survival outcomes as a result.
While PD during NAC remains uncommon, its significant negative impact on DRFS demands this population be better understood. This study highlights that post-NAC tumor grade and T category are independent predictors of DRFS among patients with PD and may be utilized in the clinical setting as factors that heighten a provider’s suspicion that a patient with PD and these features may experience a poorer overall outcome. As newer targeted systemic therapies become available, the landscape of disease progression during NAC will continue to change, making it imperative that we continue to follow this population over time.
References
Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16(8):2672–85.
Untch M, Konecny GE, Paepke S, von Minckwitz G. Current and future role of neoadjuvant therapy for breast cancer. Breast. 2014;23(5):526–37.
Boughey JC, Ballman KV, Le-Petross HT, et al. Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0–T4, N1–N2) who receive neoadjuvant chemotherapy: results from ACOSOG Z1071 (Alliance). Ann Surg. 2016;263(4):802–7.
Petruolo O, Sevilimedu V, Montagna G, Le T, Morrow M, Barrio AV. How often does modern neoadjuvant chemotherapy downstage patients to breast-conserving surgery? Ann Surg Oncol. 2021;28(1):287–94.
Esserman LJ, Berry DA, DeMichele A, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL–CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30(26):3242–9.
Haddad TC, Goetz MP. Landscape of neoadjuvant therapy for breast cancer. Ann Surg Oncol. 2015;22(5):1408–15.
Shien T, Iwata H. Adjuvant and neoadjuvant therapy for breast cancer. Jpn J Clin Oncol. 2020;50(3):225–9.
Yau C, Osdoit M, van der Noordaa M, et al. Residual cancer burden after neoadjuvant chemotherapy and long-term survival outcomes in breast cancer: a multicentre pooled analysis of 5161 patients. Lancet Oncol. 2022;23(1):149–60.
Puig CA, Hoskin TL, Day CN, Habermann EB, Boughey JC. National trends in the use of neoadjuvant chemotherapy for hormone receptor-negative breast cancer: a national cancer data base study. Ann Surg Oncol. 2017;24(5):1242–50.
Murphy BL, Day CN, Hoskin TL, Habermann EB, Boughey JC. Neoadjuvant chemotherapy use in breast cancer is greatest in excellent responders: triple-negative and HER2+ subtypes. Ann Surg Oncol. 2018;25(8):2241–8.
Caudle AS, Gonzalez-Angulo AM, Hunt KK, et al. Predictors of tumor progression during neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(11):1821–8.
Caudle AS, Gonzalez-Angulo AM, Hunt KK, et al. Impact of progression during neoadjuvant chemotherapy on surgical management of breast cancer. Ann Surg Oncol. 2011;18(4):932–8.
Raphael J, Paramsothy T, Li N, Lee J, Gandhi S. A single-institution experience of salvage therapy for patients with early and locally advanced breast cancer who progress during neoadjuvant chemotherapy. Breast Cancer Res Treat. 2017;163(1):11–9.
Zheng Y, Ding X, Zou D, et al. The treatment option of progressive disease in breast cancer during neoadjuvant chemotherapy: a single-center experience. Cancer Biol Ther. 2020;21(8):675–87.
Myller S, Ipatti P, Jääskeläinen A, Haapasaari K-M, Jukkola A, Karihtala P. Early progression of breast cancer during neoadjuvant chemotherapy may predict poorer prognoses. Acta Oncol. 2020;59(9):1036–42.
Elmore LC, Kuerer HM, Barcenas CH, et al. Clinical course of breast cancer patients with local-regional progression during neoadjuvant systemic therapy. Ann Surg Oncol. 2021;28(10):5477–85.
Ling Y-X, Xie Y-F, Wu H-L, et al. Prognostic factors and clinical outcomes of breast cancer patients with disease progression during neoadjuvant systemic therapy. Breast. 2023;70:63–9.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumors: revised RECIST guideline (Version 1.1). Eur J Cancer. 2009;45(2):228–47.
Spring LM, Fell G, Arfe A, et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: a comprehensive meta-analysis. Clin Cancer Res. 2020;26(12):2838–48.
Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72.
I-SPY2 Trial Consortium, Yee D, DeMichele AM, Yau C, et al. Association of event-free and distant recurrence-free survival with individual-level pathologic complete response in neoadjuvant treatment of stages 2 and 3 breast cancer: three-year follow-up analysis for the I-SPY2 adaptively randomized clinical trial. JAMA Oncol. 2020;6(9):1355–62.
Nanda R, Liu MC, Yau C, et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol. 2020;6(5):676–84.
van Mackelenbergh MT, Loibl S, Untch M, et al. Pathologic complete response and individual patient prognosis after neoadjuvant chemotherapy plus anti-human epidermal growth factor receptor 2 therapy of human epidermal growth factor receptor 2-positive early breast cancer. J Clin Oncol. 2023;41(16):2998–3008.
An SJ, Duchesneau ED, Strassle PD, et al. Pathologic complete response and survival after neoadjuvant chemotherapy in cT1-T2/N0 HER2+ breast cancer. NPJ Breast Cancer. 2022;8(1):65.
Clark AS, Yau C, Wolf DM, et al. Neoadjuvant T-DM1/pertuzumab and paclitaxel/trastuzumab/pertuzumab for HER2+ breast cancer in the adaptively randomized I-SPY2 trial. Nat Commun. 2021;12(1):6428.
Lee JS, Yost SE, Yuan Y. Neoadjuvant treatment for triple negative breast cancer: recent progresses and challenges. Cancers (Basel). 2020;12(6):1404.
Funding
Funding of this research was provided in part by the Mayo Clinic Breast Cancer Specialized Program of Research Excellence Grant (P50CA 116201).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Judy Boughey receives funding paid to her institution from Eli Lilly and SymBioSis and sits on the Data Safety Monitoring Committee of CairnsSurgical. She has received honoraria from PER, PeerView, OncLive, EndoMag, and Up-To-Date. Dr. Matthew Goetz is the Erivan K. Haub Family Professor of Cancer Research Honoring Richard F. Emslander, M.D. and reports personal fees for CME activities from IDEOlogy Health, Research to Practice, Medscape, and MJH Life Sciences; personal fees for serving as a panelist for a panel discussion from Total Health Conferencing and personal fees for serving as a moderator for Curio Science; consulting fees to Mayo Clinic from ARC Therapeutics, AstraZeneca, Biotheranostics, Blueprint Medicines, eChinaHealth, EcoR1, Lilly, Novartis, RNA Diagnostics, Sanofi Genzyme, Seattle Genetics, Sermonix, Engage Health Media, Laekna, and TerSera Therapeutics/Ampity Health; grant funding to Mayo Clinic from Lilly, Pfizer, Sermonix, Loxo, AstraZeneca, and ATOSSA Therapeutics; and travel support from Lilly.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10434_2024_16178_MOESM1_ESM.tiff
Supplementary Fig. 1. Distribution of tumor biologic subtype within each DRFS risk category for the 70 patients with in-breast progressive disease (PD) on NAC (TIFF 448 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eckert, K.M., Hoskin, T.L., Olson, C.A. et al. In-Breast Tumor Progression During Neoadjuvant Chemotherapy: Impact on and Factors Influencing Distant Recurrence-Free Survival. Ann Surg Oncol (2024). https://doi.org/10.1245/s10434-024-16178-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1245/s10434-024-16178-9