Abstract
Alzheimer’s disease (AD), characterized by cognitive impairment, brain plaques, and tangles, is a global health concern affecting millions. It involves the build-up of amyloid-β (Aβ) and tau proteins, the formation of neuritic plaques and neurofibrillary tangles, cholinergic system dysfunction, genetic variations, and mitochondrial dysfunction. Various signaling pathways and metabolic processes are implicated in AD, along with numerous biomarkers used for diagnosis, risk assessment, and research. Despite these, there is no cure or effective treatment for AD. It is critically important to address this immediately to develop novel drug delivery systems (NDDS) capable of targeting the brain and delivering therapeutic agents to modulate the pathological processes of AD. This review summarizes AD, its pathogenesis, related signaling pathways, biomarkers, conventional treatments, the need for NDDS, and their application in AD treatment. It also covers preclinical, clinical, and ongoing trials, patents, and marketed AD formulations.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a type of dementia characterized by cognitive impairment. This disease impacts brain regions especially, the hippocampus and entorhinal cortex [1]. AD is marked by extracellular plaques containing amyloid-β (Aβ-40,42) and intracellular neurofibrillary tangles(NTs) containing tau protein [2]. Aβ plaques are clumps of misshapen proteins that accumulate in the spaces between neurons. Whereas, NTs are twisted masses of tau protein that form inside nerve cells. Another hallmark of this condition is the deterioration of neural connections within the brain [3]. Furthermore, the pathology of AD is linked to both, abnormal amyloid precursor protein (APP) processing, Tau hyperphosphorylation, generating Aβ peptide and aggregation [4].
In addition to this, AD has several forms, including early-onset, late-onset, and familial AD. Early-onset AD (EoAD) is an uncommon form of illness that affects individuals below the age of 65, generally between 40 to 50 [5]. Individuals with EoAD often exhibit more Alzheimer-related brain changes, including tangles, plaques, and loss of brain volume. EoAD has been associated with a genetic defect on chromosome 14 [6]. Late-onset AD (LoAD) is the most common form, typically affecting individuals aged 65 or older. Researchers have not yet identified a specific gene responsible for LoAD. Family AD (FAD) is a less common type of AD with a known genetic link [7]. It is associated with three genes: APP located on chromosome 21, the gene for presenilin 1 (PSEN1) on chromosome 14, and the gene for presenilin 2 (PSEN2) on chromosome 1 [8].
The exact cause of AD is not fully understood. Still, some researchers suggest that dysfunction in the cholinergic system is a significant risk factor for Alzheimer’s, while others propose that changes in amyloid β-protein production and processing may be the primary trigger [9].
Moreover, genetic variances and a range of health, environmental, and lifestyle factors can play a role in the development of AD. As AD progresses, individuals may encounter memory loss, including difficulties recalling their past, diminished awareness of their surroundings, and challenges recognizing familiar individuals [10].
While there is no cure for AD, specific medications like Donepezil, Galantamine, and Rivastigmine may be prescribed to individuals in the early to mid-stages of the disease. These cholinesterase inhibitors can help mitigate some cognitive and behavioral symptoms by preventing the breakdown of acetylcholine, a crucial brain chemical for memory and cognition. In addition to medication, self-care strategies can assist in managing AD symptoms.
A report from the Alzheimer’s and Related Disorders Society of India (ARDSI) estimates that there are more than 5.3 million individuals in India living with dementia, with AD being the most prevalent form. Projections indicate that this number may increase to 7.6 million by 2030 [11]. Furthermore, according to the WHO, At present, the population exceeds 55 million individuals worldwide living with dementia, and approximately 10 million new cases are diagnosed each year [12]. Globally, AD is the most common cause of dementia, accounting for an estimated 60–70% of cases [13]. In the United States, an estimated 6.2 million people aged 65 and older are living with Alzheimer’s dementia in 2021, and this number is projected to grow to nearly 13 million by 2050 [14]. The economic impact of AD is substantial, with the global cost of dementia estimated at $1.3 trillion in 2019 and expected to rise to $2.8 trillion by 2030 [15]. This review provides a concise introduction to AD, covering its pathogenesis, biomarkers, traditional treatments, the need for novel drug delivery systems (NDDS), ongoing clinical trials, and AD-related patents.
Pathophysiology
AD is marked as the gradual accumulation of neuritic plaques (NP) & NTs [16] which are present around the brain’s meningeal, cerebral, and grey matter regions. These plaques and tangles interfere with neurotransmission by affecting neuronal cells [17]. NP is defined as round, small lesions comprised of an Aβ-peptide core. This peptide originates from a transmembrane protein called APP [18]. This is cut from APP by enzyme proteases: α, β, γ secretase [19]. This cleavage further results in the formation of Aβ 42. Furthermore, they can clump together & harm the neuronal cells [20]. In addition to this, Aβ 42 also leads to the accumulation of fibrillary amyloid protein clusters instead of normal APP degradation [21]. As, a result of this there is hyperphosphorylation of the tau protein. This further leads to tau protein aggregation & forms NTs [22]. These are twisted pairs of helical filaments that primally affect the hippocampus & cerebral cortex. As a result of this, there is an impairment in cognition functions [23].
In addition to this, in AD Acetoacetyl CoA level increases which converts into HMG-CoA with the help of HMG-CoA reductase [24]. Further, it activates the mevalonate pathway which results in the formation of Isopentenyl Pyrophosphate (IPP), Geranyl Pyrophosphate (GPP), and Farnesyl Pyrophosphate (FPP) [25]. Afterward, FPP leads to the formation of geranylgeranyl pyrophosphate (GGPP). This further promotes the formation of Ras-related C3 botulinum toxin substrate (Rac) and Ras Homologous (Rho). Finally, it leads to the oxidation of NADH [26]. This ultimately causes mitochondrial dysfunction. As a result of this, there is the formation of Reactive oxygen species (ROS) which activates microglial & causes neuroinflammation [27].
In addition to this, genetic variations are also one of the implicating reasons for the pathogenesis of AD. The genes that are mainly affected in AD include AAP on chromosome 21, Presenilin2 (PSEN2) on chromosome 1, and Presenilin1 (PSEN1) on chromosome 14 [28]. These genetic alterations result in the production and accumulation of Aβ peptide by disrupting the functioning of gamma-secretase. These mutations are responsible for approximately 5–10% of AD cases, predominantly in EoAD [29]. Besides this, the other genes that are altered in AD include Apolipoprotein E (APOE), CLU, CR1 (Complement Receptor 1), Bridging Integrator1, Sortilin-related Receptor 1, and TREM2 [30]. The genetic variation in APOE and CLU genes results in the aggregation of Aβ protein. Hence, results in impairment in the functioning of the brain [31]. Whereas, alteration in the Bridging Integrator 1 gene results inhibition of cellular processes such as endocytosis. This leads to a buildup of Aβ protein, thereby increasing the susceptibility to AD [32]. Dysregulated endocytosis contributes to the pathogenesis of AD by enhancing the production and accumulation of Aβ, disrupting cellular homeostasis, and impairing neuronal function [33]. Similarly, alterations in the SORL1 gene result from an impairment in APP processing. Hence, promotes the accumulation of tau proteins and NTs in the brain [34]. Finally, leads to an impairment in cognition functions. Whereas, alterations in the TREM 2 gene result from an impairment in the microglial function and immune response in the brain. Overall, these events result in AD (Fig. 1) [35].
Signaling Pathways
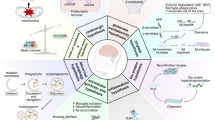
AD involves several cells’ signaling systems and metabolic pathways (Fig. 2) [36].
The Aβ aggregation pathway is a central process in AD development [37]. Decreasing Aβ production, preventing its aggregation, or promoting its clearance to change the disease’s progression [38]. It starts with APP cleavage into Aβ peptides that can aggregate into Aβ fibrils [39]. These fibrils contribute to oxidative stress, inflammation, and the formation of NTs) leading to neuronal damage [40]. Aβ plaques a characteristic of AD accumulates but smaller Aβ aggregates also play a role in disease. Aβ oligomers may interact with cell membranes or accumulate at synapses affecting synaptic proteins and glutamate receptors [41]. Microglia the brain’s primary immune cells surround these plaques forming a protective barrier and contributing to Aβ fibril clearance [42]. Additionally, degradation of acetylcholine (Ach) is accelerated leading to neurotransmitter deficiency and cognitive impairment [43]. Tau hyperphosphorylation: The tau protein when excessively phosphorylated leads to the destabilization of microtubules, a process linked to AD [44]. Tau hyperphosphorylation plays a critical role in AD by causing tau proteins to misfold and aggregate into NTs [45]. These tangles disrupt neuronal function, impairing synaptic communication and leading to cell death, which contributes to cognitive decline and memory loss characteristic of AD [46]. Alzheimer’s is marked by the buildup of amyloid plaques and tau protein clusters in various brain regions. The formation of NTs and neuropil threads results in tau phosphorylation [47]. The tau phosphorylation at Ser202/Thr205 labeling is used to determine the Braak stage based on the presence of NTs [48]. The phosphorylation of tau at Tyr18 and Thr231 in the transentorhinal region at Braak stage III/IV indicates a progressive increase with advancing Braak stages [49]. These insights imply that tau hyperphosphorylation could be a key factor in the development of AD from its early stages making it a potential target for therapeutic strategies [50].
Neurotrophic factor signaling pathway: Brain-derived neurotrophic Factor (BDNF) a type of neurotrophic factor is pivotal for maintaining synaptic plasticity a process vital for memory and learning [51]. Dysregulation of this pathway contributes to neurodegeneration and cognitive decline, highlighting its importance in the development and progression of AD [52]. This makes it a potential therapeutic molecule and diagnostic biomarker for AD [53]. BDNF-TrkB pathway, a significant signal pathway for BDNF contributes to neurodegeneration in AD, especially in brain regions like the hippocampus where BDNF expression is reduced [54]. Furthermore, the ERK/CREB signaling pathway can increase BDNF levels mitigating Aβ-induced neuronal loss and dendritic atrophy [55]. Silencing BDNF antisense RNA can also enhance BDNF, reduce Aβ-induced neurotoxicity, and improve cell viability [52]. In AD apoptosis, or programmed cell death is a key process [56]. The build-up of Aβ and hyperphosphorylated tau proteins in AD activates apoptotic pathways causing neuronal death [57]. This process is controlled by both extrinsic and intrinsic pathways involving a variety of proteins such as Bcl-2 family proteins and caspases [58]. These apoptotic components interact with growth factors and signaling molecules which include Ras-ERK, JNK, GSK-3β, BDNF/TrkB/CREB, and PI3K/AKT/mTOR [59]. Ras-ERK signaling pathway plays a role in cell cycle progression and apoptosis, while upregulation of JNK pathway in AD leads to a decrease in anti-apoptotic proteins [60]. Additionally, the PI3K/Akt/mTOR pathway regulates the balance between autophagy and apoptosis, and GSK-3β stimulates pro-apoptotic factors, leading to a dysregulation of apoptosis [61]. Drugs that target these pathways are being developed to modulate the disease condition [62].
ER stress: ER has a significant impact on AD [63]. It performs vital cellular functions such as protein folding, calcium balance maintenance, and cholesterol synthesis [64]. In AD, the build-up of Aβ peptides triggers chronic ER stress, leading to oxidative stress, calcium ion imbalance, and mitochondrial dysfunction [65]. This cycle further induces ER stress. ER stress response includes unfolded protein response (UPR), activated by accumulation of misfolded proteins like Aβ [66]. The UPR involves three stress sensors: IRE1, PERK, and ATF6 [67]. Prolonged or severe UPR activation can lead to pathological apoptotic cell death [68]. Furthermore, ER stress can induce neuronal apoptosis, with excessive oxidative stress being an ER stress inducer [69]. Insulin signaling is a key player in cognitive functions such as memory and disrupted in AD [70]. Insulin signaling plays a critical role in AD by influencing brain glucose metabolism, amyloid-beta accumulation, and tau phosphorylation [71]. Impaired insulin signaling in the brain, often termed brain insulin resistance, is associated with cognitive decline and the pathogenesis of AD. This disruption often referred to as brain insulin resistance explains the increased AD risk in diabetic patients [72]. This insulin resistance can lead to an increase in Aβ accumulation, tau hyperphosphorylation, and inflammation [71]. In AD, there are reduction in PI3K subunits and Akt kinase phosphorylation [73]. Enhancing PI3K-Akt signaling in the central nervous system through intranasal insulin treatment can improve memory [74]. The microbiota-gut-brain axis, which is believed to play a significant role in neurodegenerative conditions has been observed to be dysregulated in AD [75]. This dysregulation can lead to changes in intestinal permeability, resulting in neuroinflammation and immune dysregulation [76]. This further contributes to protein aggregation and neuronal death in the brain [77]. Further, gut dysbiosis contributes to amyloid-beta aggregation, neuroinflammation, oxidative stress, and insulin resistance, all of which are implicated in AD [78].
NMDA pathway: NMDARs which are vital for synaptic transmission and plasticity are implicated in AD [79]. These receptors are essential for memory and learning processes [80]. In the early stages of AD, an increase in oligomeric amyloid-beta peptide is observed, which leads to NMDAR-dependent synaptic depression and elimination of spine [81]. Notch signaling pathway is a key player in vascular development and function that has been linked to AD [82]. Dysfunctional Notch signaling could contribute to the pathophysiology of neurodegenerative diseases like AD [83]. Notch intracellular domain (NICD) is released from the transmembrane by γ-secretase in signal-receiving cells, leading to the activation of canonical Notch target genes [84]. Notch receptor genes and proteins have been associated with aging, cerebrovascular disease, and AD that have potential overlapping between age-related vascular and Alzheimer’s pathophysiology [85]. The GLUT4 is an insulin-regulated glucose transporter found in various tissues including the brain, and plays a crucial role in AD [86]. It facilitates the movement of glucose from the bloodstream to parenchymal cells for metabolism [87]. Alterations in GLUT4 lead to glucose deficiency in the brain that potentially hastens cognitive decline [88]. In the hippocampus, GLUT4 translocates to the plasma membrane post-memory training [89]. Inhibiting GLUT4-mediated glucose transport can impair memory acquisition, with long-term inhibition affecting long-term memory while enhancing short-term memory [90]. This indicates GLUT4’s critical role in hippocampal memory processes [91].
Akt-GSK-3β pathway involving Akt and GSK-3β is significant in AD [92]. This pathway is crucial for neuroprotection as it promotes cell survival by encouraging cell proliferation and inhibiting apoptosis [93]. It is particularly relevant in AD due to its role in facilitating Tau protein hyper-phosphorylation [94]. GSK-3β is instrumental in the neuronal stress response affecting transcriptional activity of the cAMP response element binding [95]. This regulates the transcription of BDNF and other neuropeptides [96]. These elements are vital for long-term memory regulation and maintenance of synaptic plasticity [97]. The mTOR pathway is a key regulator of cell growth, proliferation, and metabolism, and has been linked to AD [98]. This pathway responds to environmental stimuli such as growth factors, energy state, and nutrients [99]. Increased activity of the mTOR signaling pathway is believed to contribute to AD’s major pathological processes [100]. mTOR inhibitors have shown promise in alleviating AD-like pathology and cognitive deficits in numerous animal models suggesting the potential of reducing mTOR activity as a novel therapeutic strategy for AD [101]. Oxidative stress induced by the accumulation of Aβ in AD contributes to neuronal death by damaging lipids, proteins, and DNA [102]. It also triggers apoptosis and interferes with various signaling pathways, including ERK1/2, Nrf2, RCAN1, CREB/ERK, Nrf2, PP2A, NFκB, and PI3K/Akt, leading to changes in GSK-3β expression and PP2A activity [103].
The NF-κB pathway a family of transcription factors that regulate numerous genes associated with inflammation is implicated in AD due to chronic inflammation and overactivation of the NF-κB pathway [104]. This pathway can be activated through two distinct pathways: canonical and noncanonical, with the former playing a crucial role in inflammatory responses seen in AD [105]. Extracellular Aβ induces iNOS, leading to an oxidative stress response and activation of the NF-κB inflammation pathway [106]. The multifactorial nature of AD has led to the exploration of novel targets for AD therapeutics including NF-κB signaling pathway [107]. NLRP1/3 pathway: The NLRP1 and NLRP3 are implicated in AD due to their role in inflammation [108]. In AD, these inflammasomes are activated, leading to an increase in inflammasome components and downstream effectors [109]. NLRP3 inflammasome activated in microglia by Aβ contributes to neuroinflammation [110]. Similarly, the NLRP1 inflammasome responds to Aβ aggregates leading to the activation of caspase-1 and processing of interleukin-1β (IL-1β) and interleukin-18 (IL-18) [111]. The Wnt/β-catenin pathway is crucial for cell survival and death and is implicated in AD [112]. Its loss makes neurons more susceptible to Aβ-induced apoptosis [113]. Activation of this pathway occurs when Wnt proteins bind to the Frizzled (Fzd) receptor family and Wnt co-receptor LRP5 or LRP6, leading to GSK3β inhibition and β-catenin stabilization [114]. Stabilized β-catenin then moves into the nucleus interacts with TCF/LEF and induces the expression of specific target genes [115]. Impaired Wnt signaling pathways are linked to increased Aβ levels, reduced β-catenin levels, and enhanced GSK-3β enzyme expression [116]. Wnt/β-catenin signaling also regulates adult hippocampal neurogenesis with Wnt7a playing a critical role in neurogenesis by activating Wnt/β-catenin signaling and specific downstream target genes [117].
AMPK pathway: The AMPK is a crucial controller of energy balance within cells and has significant role in managing glucose and lipid metabolism [118]. It has been proposed that AMPK may be involved in AD [119]. AMPK influences the generation of Aβ protein is a key factor in AD by adjusting neuronal cholesterol and sphingomyelin levels and controlling APP distribution in lipid rafts [120]. Furthermore, AMPK activity, which is linked to mitochondrial biogenesis and function, is found to be reduced in AD brains [121]. AMPK activation also facilitates autophagy and promotes lysosomal degradation of Aβ [122]. However, AMPK activation can also lead to non-neuroprotective outcomes, including increased Aβ generation and tau phosphorylation [123]. mTOR pathway: mTOR is a serine/threonine kinase that is integral to various cellular processes such as growth, proliferation, metabolism, protein synthesis, and autophagy [124]. mTOR activation is thought to increase Aβ generation and deposition by influencing APP metabolism and upregulating β- and γ-secretases [125]. It also inhibits autophagy, leading to a decrease in Aβ clearance [126]. Furthermore, mTOR is implicated in the pathogenesis of AD by inhibiting insulin signaling and affecting neuronal growth and plasticity as a nutrient sensor [127]. However, mTOR activation also has harmful effects, including inhibiting insulin signalling and autophagic removal of Aβ and tau aggregates [128].
Sirtuin 1 (Sirt1) pathway: SIRT1 a member of the Sirtuin family, plays a crucial role in AD by regulating processing of APP [129]. It enhances the production and activity of α-secretase, an enzyme that prevents the formation of toxic Aβ species [130]. Additionally, regions of the brain with high Aβ deposition also show increased aerobic glycolysis, which can reduce NAD+ levels and potentially affect the Sirtuin pathway [131]. Therefore, therapeutic strategies that increase SIRT1 could potentially reduce AD neuropathology by inhibiting the formation of Aβ [132]. PGC-1α pathway: PGC-1α is a key regulator of mitochondrial biogenesis which is involved in various metabolic processes and could potentially protect against AD [133]. It activates survival pathways such as the MEK/ERK and PI3K/AKT signalling pathways which prevent apoptosis in hippocampal neurons [134]. PI3Ks are a group of enzymes vital for cellular functions that have a significant role in AD through the PI3K/Akt signalling pathway [135]. This pathway regulates numerous biological processes and can inhibit several neurotoxic mechanisms, making it a potential therapeutic target for AD [136]. It influences Tau phosphorylation and amyloid cascade both crucial in Alzheimer’s progression [137]. The pathway is also linked to oxidative stress, neuroinflammation, insulin signalling alterations, and autophagy changes in Alzheimer’s [138]. HIF-1α pathway: HIF-1α is a key regulator that manages cellular reactions to low oxygen levels [139]. It has a crucial role in AD. When oxygen levels are low HIF-1α stabilizes and moves to nucleus to form a complex with HIF-1β [140]. This process is controlled by enzymes like prolyl hydroxylase (PHD) and HIF prolyl hydroxylase (HPH) which modify HIF-1α enabling it to associate with Von Hippel-Lindau (VHL) [141]. Any disruption in the autophagy process can lead to neuroinflammation and neuronal cell death, causing hypoxia and triggering various transcription factors, including HIF-1α [142].
The NRF2-ARE pathway is crucial in AD [143]. NRF2 is a transcriptional regulator that responds to oxidative stress [144]. When oxidative damage is high NRF2 moves to nucleus and binds to Antioxidant Response Element (ARE) which triggers transcription of antioxidant protector genes [145]. This pathway is involved in AD due to its dysfunction and altered localization [146]. It triggers genes that protect cells and detoxify enzyme genes which can prevent AD pathology [147]. However, in AD, buildup of Aβ and tau decreases NRF2 levels, reducing the antioxidant response [148]. This decrease in NRF2 levels leads to further accumulation of Aβ and tau by disrupting their autophagy-mediated turnover [149]. Therefore, NRF2-ARE pathway is considered a potential therapeutic target for AD [150]. PKC pathway: PKC is a group of enzymes that is essential for various cellular functions [151]. In AD, PKC enhances the production of a secretory form of amyloid precursor protein (sAPP α) by activating α-secretase activity, which decreases buildup of harmful Aβ levels in brain [152]. PKC isoforms like PKCα and -ε signalling pathways are closely linked with pathological damage in AD [153]. Activating these PKC isoforms can reduce Aβ production and related dementia in AD by enhancing APP α-processing pathways and Aβ degradation [154]. TGF-β pathway: TGF-β a transcriptional regulator is crucial in AD [155]. Under low oxygen conditions, TGF-β stabilizes and forms a complex with Smad proteins key molecules in TGF-β signalling [156]. This pathway is involved in AD due to its dysfunction and altered localization [157]. It triggers genes that protect cells and detoxify enzyme genes which can prevent AD pathology [158]. However, in AD the buildup of Aβ and tau decreases TGF-β levels reducing the antioxidant response [159]. This decrease in TGF-β levels leads to further accumulation of Aβ and tau by disrupting their autophagy-mediated turnover [160]. Therefore, the TGF-β pathway is considered a potential therapeutic target for AD [161].
JAK-STAT pathway is crucial in neuroinflammatory diseases like AD [162]. It initiates innate immunity, manages adaptive immune mechanisms, and controls the neuroinflammatory response [163]. This pathway transmits signals from receptors on cell membrane to nucleus, regulating cellular activities such as growth, differentiation, and apoptosis [164]. Any imbalance in this pathway leads to severe immunodeficiencies and malignancies, and it also plays a role in neuro-transduction and pro-inflammatory signalling mechanisms [165]. Ras/ MAPK pathway: It transmits signals from receptors on the cell membrane to the nucleus that regulates cellular activities such as growth, differentiation, and apoptosis [166]. In AD, all MAPK pathways, including ERK, JNK, and p38 pathways, are activated in vulnerable neurons, indicating their involvement in the disease’s pathophysiology and pathogenesis [167]. Oxidative stress can trigger intracellular signalling pathways including p38 MAPK signalling pathway which contributes to aggregation of Aβ and hyperphosphorylated tau protein in brain [168]. CDK5 pathway: CDK5 is a crucial member of the cyclin-dependent kinases, playing a significant role in development of a central nervous system and various neuronal activities [169]. In AD, CDK5 is closely linked with the disease’s pathogenesis [170]. When neurons are exposed to pathological stimuli, CDK5 activity increases leading to abnormal hyperphosphorylation of several CDK5 substrates like APP, tau, and neurofilament resulting in AD [171]. The imbalance of CDK5 contributes to numerous pathological events in AD from the creation of senile plaques and NTs to synaptic damage, mitochondrial dysfunction, cell cycle reactivation, and neuronal cell apoptosis [172].
Biomarkers
A biomarker, which is also called a biological marker, is a detectable sign that gives us information about alterations occurring inside our body. These changes can be detected by measuring the increase or decrease in the level of biomarkers present in the blood, urine, or soft tissues. These studies help us to diagnose disease at an early stage [173]. The different biomarkers which are essential in the diagnosis of AD are given in Table 1.
Conventional Treatments
Conventional treatments for AD mainly concentrate on managing the symptoms of the condition. There is currently no cure or synthetic medication available to halt or reverse the disease’s progression [193]. The two main classes of synthetic drugs used for AD are cholinesterase inhibitors and NMDA receptor antagonists [194]. Cholinesterase inhibitors, including medications like Donepezil, Rivastigmine, and Galantamine. They work by elevating acetylcholine levels, a neurotransmitter associated with memory and cognition, in the brain [195]. These drugs aim to enhance communication between nerve cells and temporarily reduce cognitive and behavioral observed in individuals with Alzheimer’s. [196].
Whereas, NMDA receptor antagonists include Memantine which helps to regulate the activity of glutamate, an excitatory neurotransmitter [197]. It is typically used in moderate to severe Alzheimer’s cases and can provide some relief from symptoms. It is important that these medications do not modify the course of the disease [197]. Their effects can vary among individuals. While they may offer temporary improvement in cognitive function and behavior, the progression of AD continues [198]. The various Synthetic drugs used in the management of AD are discussed below in the table (Table II):
In addition to this, there is currently no approved herbal medication or therapy that is commonly accepted as a standard treatment for AD. Hence, the majority of the medications used in traditional treatment of the condition are synthetic. The majority of traditional methods focus on drugs such as NMDA receptor antagonists and cholinesterase inhibitors, which are designed synthetically to target particular components of Alzheimer’s symptoms [203]. Additionally, the use of herbal medicines and other complementary and alternative therapies as possible supplements to traditional medical care is the subject of the remaining research. In small-scale studies, certain herbs and compounds, such as Ginkgo biloba, Curcuma longa, Papaya, Blueberry, and Colostrinin have shown potential for maintaining cognitive function and reducing inflammation, which is linked to AD. The various herbal drugs used in the management of AD are described below in Table III.
The drawbacks of current and conventional treatments for various medical conditions include issues related to pharmacokinetics, bioavailability, patient compliance, and toxicity or side effects [217]. Conventional treatments often suffer from poor pharmacokinetics, leading to inadequate absorption and distribution of the drug within the body. This results in suboptimal bioavailability, where only a small fraction of the administered dose reaches the target site in an effective form [218]. Additionally, the difficulty of patient compliance is a significant concern, as many traditional therapies require frequent dosing or have inconvenient administration routes, making it challenging for patients to adhere to their treatment regimens. Moreover, toxicity and adverse side effects are common problems associated with conventional treatments, which can cause harm to patients and reduce the overall effectiveness of the therapy [219]. These limitations highlight the need for novel delivery systems that can enhance pharmacokinetics, improve bioavailability, simplify administration, and minimize toxicity, thereby offering more effective and safer treatment options.
Need for a Novel drug Delivery System and Their Mechanism of Penetration
There are several conventional treatments which have been explored by the researchers for the management of AD [220]. However, they have some limitations. For instance, they have difficulty crossing the Blood-brain barrier (BBB), which prevents them from reaching the target site [221]. Additionally, conventional treatments are associated with side effects due to their non-specific targeting or toxicity to healthy cells. Furthermore, a major limitation of herbal drugs is their low solubility and metabolism, which can limit their bioavailability and efficacy [222]. In addition, the quality and purity of herbal drugs can vary depending on the sources and preparation methods, which also affects their safety and efficacy. Also, the active ingredients in herbal drugs can interact with other medications or cause side effects such as gastrointestinal upset, dizziness, or headache [223]. Most importantly, At present, the options for treating AD are quite restricted and have shown only modest efficacy. The main classes of drugs used to treat AD are cholinesterase inhibitors and NMDA receptor antagonists [224]. However, these drugs have several limitations that make them less effective in treating AD. Cholinesterase inhibitors have limited efficacy and can cause side effects such as diarrhea, nausea and vomiting. Whereas, NMDA receptor antagonists can cause side effects such as dizziness, headache, and confusion [225]. Furthermore, these drugs do not address the underlying pathophysiology of AD, which involves different pathophysiological events such as buildup of amyloid and tau, neuro-inflammation, and neuronal injury [226].
The aforementioned limitations of the conventional treatments can be addressed by using NDDS. Advantages of using NDDS include enhanced drug efficacy, reduced side effects, prolonged drug action, better patient compliance, targeted drug delivery, protection of sensitive drugs from degradation, and overcoming biological barriers [227]. The different mechanism that helps the nanocarriers to cross BBB include the paracellular pathway, adsorption-mediated transcytosis, receptor-mediated transcytosis, and carrier-mediated pathway [228]. In passive diffusion, Nanoparticles (NPs) with high lipophilicity and small size can diffuse through BBB. This is facilitated by the lipid bilayer of the BBB’s endothelial cells, which allows lipophilic substances to dissolve and cross BBB [229]. In adsorption-mediated transcytosis, NPs with a positive charge or hydrophobic surface can adsorb to the luminal surface of the endothelial cells and induce endocytosis, followed by exocytosis at abluminal side [230]. In receptor-mediated transcytosis, NPs are conjugated with ligands that bind to specific receptors on endothelial cells which trigger receptor-mediated endocytosis and exocytosis across the BBB (Fig. 2) [231]. In carrier-mediated transport, NPs are conjugated with molecules that are substrates for transporters on the endothelial cells that utilize carrier-mediated transport to cross the BBB (Fig. 3) [232]. The various nanocarrier explored to treat AD includes VDDS, Nanoparticle (Gold NPs, Silver NPs, Copper NPs), Intranasal, Liposome, Nanoemulsion, Nano Suspension, in situ gel, Nanoparticle and SLN, and PLGA Nanoparticle.
Vesicular Drug Delivery System (VDDS)
Liposomes
Liposomes are defined by the presence of at least one lipid bilayer. This lipid bilayer forms a closed sphere that houses a cavity filled with drug. This arrangement is due to the amphipathic characteristics of phospholipids, which have hydrophilic heads and hydrophobic tails. They are being investigated as a potential method for delivering drugs to treat AD. They can carry various therapeutic molecules and cross the blood-brain barrier. Recent developments have led to liposomes that can better penetrate the blood-brain barrier, enhancing the effectiveness of Alzheimer’s drugs are discussed below [233].
Andrade et al., prepared transferrin-functionalized VB12 liposomes (VB12-Tf-LIP) by thin film hydration technique. Results of the study showed that the prepared formulation exhibited particle size below 200 nm. Thereby helping the liposomes to cross the BBB. This further helped the VB12-Tf-LIP to exhibit a 1.6-fold increase in Aβ1−42 fibril disaggregation as compared to the VB12 alone treated group. In addition to this, the prepared formulation exhibited anti-AD activity by inhibiting the Aβ fibrillation and disaggregation of preformed fibrils [234].
Similarly, Mutu et al., prepared rivastigmine liposomes by thin film hydration technique. Their activity was evaluated in the Balb-C-type mice model. The results of the study showed that rivastigmine liposomes exhibited an increase in anti-cholinesterase activity by 2.8-fold and 2.2-fold as compared to negative control and rivastigmine alone treated group, respectively. Hence, they exhibited better anti-AD activity as compared to other groups [235].
In another study, Vasileva et al., prepared α-tocopherol and donepezil co-loaded liposomes by solvent evaporation technique. Their activity was evaluated in a transgenic AD mice model. The prepared formulation showed a decrease in the number of Aβ plaques by 1.5-fold as compared to the untreated group [236].
Kuedo et al., explore the potential of ethanolic extract shrimp shells (EESS) loaded liposomes against AD. Their activity was evaluated on a thiopental-induced Wistar rat model. The result demonstrated that the prepared formulation exhibited a neuroprotective effect by modulating BDNF/TrkB, GAP-43, and PSD-95 signaling pathways. In addition, upregulated synaptic proteins. Thereby, improved cognition in the AD model [237].
Li et al. prepared Galanthamine hydrobromide-loaded liposomes against the AD model by the thin-film homogenization method. Their in vivo activity was checked on the Male Sprague–Dawley rats. The result of the prepared formulation showed a 7-fold decreased in the acetylcholinesterase activity in the diseased group [238].
Niosomes
Niosomes are vesicles formed by non-ionic surfactants and cholesterol for targeted drug delivery. They are structurally similar to liposomes but offer greater stability. They can encapsulate both water-soluble and fat-soluble drugs making them ideal for various applications in drug delivery systems. They can encapsulate various therapeutic agents and cross the blood-brain barrier [239]. Various niosomes that are being studied as a potential delivery system for AD treatments are mentioned below.
Kulkarni et al., formulated N-Acetyl Cysteine niosomes by ethanol injection method. Their activity was evaluated on Male Wistar rats. Results showed that the prepared formulation exhibited a 1.2-fold increase in nasal permeation as compared to unprocessed N-Acetyl Cysteine. Furthermore, the prepared formulation exhibited a 1.3-fold decrease in AChE level as compared to untreated group [240].
Similarly, Moulahoum et al., prepared carnosine-loaded niosome Against AD. The prepared formulation exhibited a neuroprotective effect by exhibiting antioxidant and anti-AOPP activity. In addition, it was observed from the study that the carnosine-treated group exhibited a 2.1-fold decrease in AoPP level as compared to the carnosine-treated group [241].
In addition, study Ansari et al., explore the potential of Artemisia absinthium loaded niosomes against AD. The formulation was prepared by thin hydration techniques. The result of the study showed that Artemisia absinthium noisome exhibited a neuroprotective effect by decreasing aggregation of Aβ proteins and neurofibril tangles at the desired site [242].
Exosome
Exosomes are tiny vesicles ranging from 30 to 150 nm in diameter. They can carry genetic material and proteins from their parent cells. They are particularly useful in cancer detection as they can contain relevant information from cancer cells. As potential drug delivery tools, exosomes offer low immunogenicity, the ability to cross the blood-brain barrier, and the flexibility to encapsulate various therapeutic agents, thereby extending their half-life and stability [243]. Studies related to exomes for the management of AD are given below.
Chen et al., isolated exosomes from mesenchymal stem cells (MSC-exosomes). Their activity was examined on the J20 mouse model. The result of the study showed that the prepared formulation exhibited a 2-fold improvement in cognition as compared to the disease group. Also, reduce the Aβ plaque to 5-fold in Tg- exosome treated group as compared to the diseased group animals [244].
Similarly, Zaldivar et al., isolated exosomes from Mesenchymal stem cells, and their activity was examined in the C57BL/6 AD mice model. The result of the study showed that the prepared formulation exhibited a 1.6-fold increase in novel object activity as compared to the negative control group. Overall, the results of the aforementioned activity revealed improvement in learning and memory in the MSCs exosome-treated group [245].
Cui et al., isolated rabies viral glycoprotein (RVG) exosomes from the Mesenchymal stem cells (MSCs), and their activity was examined on the APP/PS1 double transgenic mice. The result of the study showed that the prepared formulation exhibited a 1.4-fold increase in the Morris water maze test as compared to the diseased group. Overall, the result showed a 2-fold decrease in the formation of plaques in MSC-RVG-Exo treated group [246].
In another study, Sheykhhasan et al., isolated Q10-loaded exosomes from adipose-derived stem cells (ADSCs-Exo) and their activity was examined on the STZ-induced Wistar rats. The result of the study showed that the prepared formulation exhibited improvement in cognition by learning and memory as compared to the untreated. In addition, exosomes, exhibited anti Amyloid beta effect, antioxidant and anti-inflammatory action in the brain, and produced a neuroprotective effect [247].
Similarly, Jahangard et al., isolated exosomes from the Mesenchymal stem cells that contain miR-29, and their activity was examined in the male Wistar rats. The result shows that the prepared formulation showed a decrease of 1.5-fold in the Aβ as compared to the diseased group. Overall, the study showed increased learning and memory in the AD model [248].
Transferosome
Transferosomes are unique, deformable vesicular structures composed of phospholipids and an edge activator, which allow them to navigate through small pores. They are used for both local and systemic drug delivery due to their high encapsulation efficiency, ability to act as a reservoir for gradual drug release, and protection of the drug from metabolic breakdown. They can also deliver drugs through the nasal route, bypassing the blood-brain barrier and enhancing bioavailability [249]. The several studies reported for the management of AD are discussed below.
Raj et al., prepared curcumin-loaded transferosome-based In-Situ Gel by the thin film hydration method. The in vivo study was performed on the Swiss albino mice. The result of the study showed that the prepared formulation increased the 2-fold concentration of the drug in the brain as compared to the curcumin IV formulation. This study showed that the prepared In-situ gel formulation increased BA in the brain. Hence, transfersome can be used in the management of the AD [250].
In another study, Mishra et al., prepared Berberine and curcumin co-loaded transferosomes by film hydration method against the AD model. Their activity was checked on the Swiss albino mice. The result of the study showed that the prepared formulation decreased the 4-fold AChE in the BBR-CUR-TRANS treated group as compared to the control group. Overall, the study showed an improvement in memory in the AD mice model [251].
Similarly, Nojoki et al., prepared chitosan-transfersulin (CTI) transfersome by the film hydration method against the AD model. An in vivo study of the prepared formulation was performed on the STZ-induced Wistar rats. The result of the study showed that the prepared formulation-treated group exhibited a 1.5-fold increased in latency as compared to the diseased group. Additionally, the Histopathological evaluation of the study indicated a decrease in the level of CA1, CA3, and DG in CTI treated group by 2-fold, 3.6-fold, and 2.5-fold respectively as compared to the control group [252].
Ethosomes
Ethosomes are nanoscale carriers composed of phospholipids, ethanol, and water, designed for delivering substances through the skin. They can encapsulate and deliver both lipophilic and hydrophilic drugs efficiently. They are stable, approved for pharmaceutical use, and can be incorporated into different formulations like gels and creams [253]. Studies that have explored the use of ethosomes as a type of nanoscale carrier in Alzheimer’s treatment are mentioned below.
Shi et al., prepared ligustrazine phosphate-loaded Ethosomes (LP-Ethosomes), and examined its activity in the male Sprague–Dawley rats. The result of the study showed that the LP-Ethosomes treated group indicated a decrease in escape time by 2.5-fold as compared to the control group. the ethosomal-treated group exhibited an increased in MDA activity by 0.93-fold as compared to the control group. Overall, the study showed that prepared formulation is effective in treating AD [254].
Phytosomes
Phytosomes are a type of advanced drug delivery system that encapsulates plant-based bioactive compounds with phospholipids, forming a cell-like structure. This unique structure enhances the pharmacokinetic and pharmacodynamic properties of the herbal extracts leading to increased bioavailability. Phytosomes enhance the potency, quality, and precision of treatments. Additionally, it shields the components of herbal extracts from degradation by digestive fluids and gut bacteria [255]. Studies reported so far for the AD treatment are given below.
Wattanathorn et al., prepared mulberry fruit and ginger (PMG) loaded Phytosome. Their activity was evaluated on the male Wistar rats. The result of the study showed that the prepared formulation decreased 1.5-fold in the Morris Water Maze test which resulted the improved memory as compared to the diseased treated group. Also, PMG Phytosome increased 1.4-fold in the locomotor as compared to the induced group. Thus, the study showed that the prepared formulation will be effective in the neuroprotectant effect [256].
Similarly, Ullah et al., prepared curcumin-loaded phytosomes. Their activity was examined on the GFAP-IL6 mice AD model. The result of the study showed that the prepared formulation decreased 1.4-fold Iba-1 + microglia in the hippocampus as compared to the normal food-fed GFAP-IL6 group. Furthermore, it also decreased 1.3-fold of TSPO + microglial cells in the hippocampus as compared to the normal food-fed group. This indicated the neuroprotective effect of the prepared formulation [257].
in vitroCubosomes
Cubosomes are lipid-based NPs that form a 3D cubic lattice. They can encapsulate and deliver a wide range of therapeutic agents, including both hydrophobic and hydrophilic drugs. Their unique structure provides stability, controlled drug release, and protection against degradation. By adjusting the lipid composition and surface modifications, drug release kinetics can be modulated, enhancing therapeutic efficacy and reducing side effects [258]. Studies related to cubosomes for the management of AD are given below.
Elnaggar et al., prepared monoolein cubosomes co-loaded piperine which are modified by the Tween (T-cubs). Their in vivo activity was examined in male Wistar rats. The result of the study showed that the prepared formulation showed an increase of 4.7-fold in the latency test as compared to the positive group. Further, T-cubs also decreased 3.8-fold in the AChE activity as compared to the diseased group. Overall, the result showed that the prepared formulation is effective against AD [259].
Wu et al., prepared cubosomes which are modified by Odorranalectin (OL-Cubs) against the AD, and examined their anti-AD activity on the Sprague-Dawley rats. The result of the study showed that the prepared formulation showed an improved in escape latency by the 2-fold as compared to the AD group in the water maze learning test. The overall study concluded that the OL-Cubs was effective for the improved learning impairment in AD [260].
Nanoparticles (NPs)
NDDS utilize NPs to enhance the delivery and effectiveness of therapeutic agents. These systems aim to control the size, surface properties, and release of active pharmaceutical ingredients for optimal therapeutic effect. NPs can reduce side effects and are prepared using various techniques. The field of nanomedicine is advancing rapidly, with nano-delivery systems serving as diagnostic tools and delivering therapeutic agents to targeted sites. These nanoparticle-based systems could potentially address the challenges of conventional therapies and contribute to improved clinical outcomes [261].
Gold NPs (AuNPs)
AuNPs are increasingly being used in NDDSs due to their biocompatibility and adaptable surface. These properties allow for the addition of bioactive ligands, enhancing drug stability and efficacy, and enabling drugs to cross the blood-brain barrier [262]. Recent studies showing promises in treating AD are described below.
Zhang et al., prepared tetrapeptide-anchored gold NPs and analysed their effect on the Kun Ming (KM) mice model against the AD. Results of the study showed that the prepared formulation of AuNPs exhibited an antioxidant effect by increasing the level of SOD, GSH, and catalase and increased the level of AChE in the brain. Hence, improved cognition and managed AD [263].
Hou et al., prepared AuNPs of chiral L- and D-glutathione and examined their activity on the KM mice model against the AD. Results of the study showed that prepared formulation improved memory by 2-fold as compared to AD group. Furthermore, the prepared formulation decreased Aβ plaque deposition in the brain. This indicated the effectiveness of the AuNPs against AD [264].
Tramontin et al., prepared AuNPs for the treatment of AD. They examined their activity in the Okadaic acid (OA) induced male Wistar rats model. Results of the study showed that the prepared formulation improved memory by 1-5-fold as compared to the diseased group. Overall, the Study showed that prepared formulation improved cognition and decreased oxidative stress. Hence, effective against the AD mice model [265].
Poly (lactic-co-glycolic acid) NPs (PLGA NPs)
PLGA NPs are a promising area of research in NDDS, particularly for neurodegenerative diseases. Their biocompatibility, non-toxicity, and various benefits such as improved drug solubility, protection from enzymatic digestion, increased targeting efficiency, and enhanced cellular internalization make them an attractive option. Despite their potential, no PLGA NPs are currently on the market or in clinical trials for neurodegenerative diseases and are only at the preclinical stage [266]. Some of the preclinical studies supporting PLGA for management of AD are mentioned below.
Lopez et al., prepared Memantine polylactic-co-glycolic nanoparticle (MEM–PEG–PLGA) NPs and examined its in vivo activity on the Male APPswe/PS1dE9 (APP/PS1) and C57BL/6 mice. Results of study showed that the MEM–PEG–PLGA NPs treated group exhibited an increased in the latency by 2.5-fold as that of the untreated group. Additionally, the prepared formulation exhibited a decreased in the level of Aβ plaques and Tau proteins in the brain as compared to the untreated group. Overall study, indicated the anti-AD potential of the developed formulation in the diseased mice [267].
Abreu et al., prepared PGZ-loaded NPs (PGZ-NPs) by the solvent displacement technique against the AD. They examined their activity in the male APP/PS1 mice. Results of the study showed that the prepared formulation decreased 2.5-fold of the Aβ burden as compared to the diseased group. This indicated that the PGZ-NPs improved cognition and were effective against the AD in mice model [268].
Jeon et al., prepared Vitamin D-binding protein (DBP) PLGA NPs by the emulsion diffusion method and investigated its in vivo activity on a 5XFAD AD mice model. The result of the study showed that the prepared formulation exhibited improvement decreased by 1.3-fold in a cognition-treated group compared to the disease group. Additionally, the DBP-PLGA NPs exhibited treated group decreased Aβ aggregation and reduced neurodegeneration. The overall study indicated that the prepared formulation is effective against AD [269].
Xu et al., prepared rhynchophylline-loaded mPEG-PLGA NPs coated with Tween 80 (T80-NPS-RIN) by nanoprecipitation method against the AD model. They investigate their in vivo activity on the C57BL/6 mice and male Sprague-Dawley rats. Results of the study showed that prepared formulation has a neuroprotective effect against AD by decreasing inflammation, oxidative stress, and tau protein in the brain [270].
Vilella et al., fabricated the Polymeric NPs (g7-NPs-Zn) against the AD model and evaluated their anti-AD activity on the APP23 mice. Results of the study showed that the prepared formulation decreased the Aβ plaques by 1.2-fold as compared to the saline group. Furthermore, g7-NPs-Zn reduced the IL-6 by 3-fold as compared to the diseased group. Thus, the study concluded that the prepared formulation was an effective formulation against AD [271].
Silver NPs (AgNPs)
AgNPs are gaining attention for their potential role in AD treatment. Produced through environmentally friendly methods, these NPs exhibit properties that enable them to cross the blood-brain barrier, a significant hurdle in brain disease treatment. They have demonstrated an ability to improve drug stability and efficacy, making them suitable carriers for Alzheimer’s drugs. Additionally, their antioxidant and anti-diabetic properties could also be beneficial in managing Alzheimer’s [272]. Studies related to AgNPs for the management of AD are given below.
Zhang et al., prepared N. khasiana leaf extract-based (AgNPs) and evaluated its Anti- AD activity in the male Wistar rats. Result of study showed that prepared formulation decreased the Barnes Maze Task by 1.2-fold as compared to the negative control group which improved memory. Finally, this study indicated that the AgNPs will be effective in cognition impairment and managing AD [273].
Ittiyavirah et al., prepared an Ethanolic extract based Boerhaavia diffusa AgNPs (AgNPsBD) against the AD mice model and investigated their anti-AD activity in the male Wistar albino rats. Result of the study showed that prepared formulation exhibited an increase of 1.5-fold Morri’s water maze test activity as compared to the diseased induced group. This indicated the Enhancement in the ability to learn and remember spatial information in rodents. Also, AgNPsBD increased GSH level by 1.4-fold as compared to the AD group. Overall, the study showed that the formulation is effective against AD [274].
In another study, Ramshini et al., explored the AgNPs against the AD in Wistar rat model. Result of the study indicated a decrease in the escape latency by 2.2-fold & and 2.4-fold as compared to scopolamine & and lysozyme-treated group. In addition to this, AgNPs also exhibited improvement in memory and spatial learning by inhibiting amyloid fibrils-induced neurotoxicity. Overall event, indicated the potential of AgNPs against AD [275].
Cerium Oxide NPs (CNPs)
CNPs are a type of nanomaterial with significant potential in various fields. They are known for their biomimetic activities, including acting as superoxide dismutase, catalase, and more. Two forms exist: CeO2 and Ce2O3, with CeO2 being more stable and commonly used. CNPs have antioxidant properties due to the self-regeneration of their surface which involves redox-cycling between cerium’s 3+ and 4+ states. They are used in biomedical applications, such as treating bacterial infections, and have potential in biology and medicine [276].
Danish et al., synthesized Cerium oxide NPs (CNPs) by homogenous precipitation method against the AD and investigated their anti-AD activity on the female Wistar rats. Result of study showed that prepared formulation improved memory in the MWT escapes latency by 1.4-fold as compared to AD-induced group. Also, CNPs increased SOD and GSH activity by 2.7-fold and 4-fold as compared to Scopolamine group respectively [277].
Similarly, Hu et al., synthesized cerium dioxide NPs (LMC) and loaded them with Resveratrol (LMC-RES) against the AD mice model and explored their activity on the 5xFAD mice. Result of study showed that prepared formulation increased GSH level by 3-fold and SOD level by 4-fold as compared to Aβ induced group. LMC-RES also decreased the Aβ 1–42 concentration by 1.3-fold as compared to diseased group. Overall, study concluded that LMC-RES have antioxidant properties, reduced ROS, protected neurons, and improved cognition in AD [278].
In another study, Wahle et al., synthesized cerium dioxide (CeO2 NPs) against the AD and examined their anti-AD activity on the 5xFAD transgenic mice. Result of study showed that prepared formulation decreased the plaque load percentage by the 1.3-fold as compared to control group in hippocampus. Overall study concluded that CeO2 NPs was effective against the AD [279].
Zinc Oxide NPs (ZnO NPs)
ZnO NPs are unique nanomaterials with diverse applications. They are known for their distinct optical and chemical properties, which can be adjusted by changing the NPs’ morphology. ZnO NPs are commonly used in electronics and optoelectronics. They also have potential in biomedicine and biotechnology, including enhancing plant growth and productivity, managing diseases, and serving as an antimicrobial agent [280].
Abdulmalek et al., prepared zinc oxide NPs (ZnONP), and evaluated their activity on the male Wistar rats. Result of study showed that the prepared formulation decreased Aβ-42 by 4.2-fold as compared to the STZ-induced group. Also, it improved AChE activity by 7.4-fold as compared to diseased group. Overall study indicated the effectiveness of ZnOPN against neurodegenerative disorders [281].
Similarly, Kesmati et al., explored cognitive potential of ZnO NPs was evaluated on male NMRI mice. Results of study showed that prepared formulation exhibited improvement in locomotor activity by 1.2-fold as that of the untreated group. In addition to this, the ZnO NPs treated group also showed an increase in step-down latency time by 1.3-fold as compared to untreated group. Hence, it indicated the effectiveness of the developed formulation against AD [282].
Selenium NPs (SeNPs)
SeNPs are nanomaterials that have attracted attention due to their biocompatibility, bioavailability, and minimal toxicity. They are derived from selenium salts using reducing agents. SeNPs are recognized for their distinct optical and chemical properties and have applications in electronics and optoelectronics. In biomedicine and biotechnology, they show potential in promoting plant growth, disease management, and as antimicrobial agents [283].
Gholamigeravand et al., prepared Selenium NPs (SeNPs) by chemical Precipitation method against the AD model. They examined their activity on the male Wistar rats. Result of study showed that prepared formulation decreased Aβ plaques in brain by 1.1-fold as compared to STZ-induced group. Furthermore, SeNPs improved memory by 1.2-fold as compared to diseased group. Thus, the study concluded that prepared formulation was effective against AD [284].
In another study, Ji et al., prepared Se-loaded chondroitin sulphate (CS@Se) NPs against the AD and evaluated their anti-AD activity on the SPF-grade male C57BL/6 mice. Result of study showed that prepared formulation improved memory by 1.5-fold in MWT as compared to AD model group. Furthermore, CS@Se NPs treated group showed an increase in GSH level by 13-fold as compared to the diseased group [285].
Sun et al., prepared chiral penicillamine Se-NPs (L-/D-Pen@Se NPs) against AD and investigated their in vivo activity on the APP/PS1 transgenic mice. Result of study showed that prepared formulation improved memory in MWT by 1.4-fold as compared to AD group. Overall, study showed that the L-/D-Pen@Se NPs improved the cognitive impairment in AD [286].
Micelles
Micelles are colloidal particles formed from surfactant molecules in a liquid. They are typically spherical, with hydrophilic heads facing the solvent and hydrophobic tails in the center. This formation occurs spontaneously when the surfactant concentration exceeds the critical micelle concentration. Micelles have diverse applications, including in electronics, optoelectronics, and biomedicine. They can enhance plant growth, manage diseases, and serve as antimicrobial agents [287].
In another study, Hagl et al., evaluated anti-AD effect of curcumin-loaded micelles (CMI) on Male C57BJ/6-Thy1-APP751SL mice. Result of the study showed a decrease in Aβ40 level in the CMI-treated group by 2.8-fold as that of placebo group. Further, CMI treated group exhibited an increase in the level of ATP by 1.2-fold as compared to placebo group [288].
Yang et al., prepared the micelles that targeted the neuronal mitochondria (CT-NM) against AD and investigated their anti-AD activity on the ICR mice, nude mice, and SD rats. Result of study showed that CT-NM decreased the level of Aβ by 3-fold as compared to the diseased group. Further, the prepared formulation increased the level of the SOD and GSH by 2.1-fold and 2-fold respectively. Hence, the prepared formulation was a potential method for the treatment of AD [289].
Dendrimers
Dendrimers are highly structured, branched polymers with a typically spherical 3D shape. They are symmetric around the core and are also known as arborols or cascade molecules. Dendrimers are unique due to their structural perfection, usually being monodisperse and highly symmetric. They have diverse applications, including in biomedicine and biotechnology, where they can enhance plant growth, manage diseases, and serve as antimicrobial agents [290].
Gothwal et al., prepared rivastigmine (RIV) loaded dendrimeric (PAMAM-Lf-RIV) against the AD mice model. Their activity was examined on the Wistar rats. Result of study showed that prepared formulation improved memory by 1.2-fold as compared to control group. Overall, the study concluded that PAMAM-Lf-RIV was an effective formulation against AD [291].
In another study, Gothwal et al. prepared PAMAM-LF-loaded memantine (MEM-PAMAM-Lf) dendrimers. Their in vivo activity was evaluated against AD-induced mice. The result of study showed that prepared formulation decreased 1.4-fold AChE activity as compared to AL-induced group. Overall, the study concluded that the MEM-PAMAM-Lf improved memory, and a promising approach against AD [292].
Klementieva et al., prepared Poly (propylene imine) (PPI) glycodendrimers against AD mice model and investigated their anti-AD activity in APP/PS1 transgenic mice AD model. Result of study showed that G4mDS treated group exhibited a decreased in total amyloid burden, fibrillar amyloid burden, aggregation index, and soluble Aβ level by 1.5-fold, 1.3-fold, 1.7-fold, and 1.8-fold of that of untreated group, respectively [293].
Nanoemulsion
Nanoemulsions, also known as mini emulsions, are stable dispersions of liquid within another liquid, with droplet sizes around 100 nm. Their small size results in properties such as high surface area, stability, transparency, and adjustable rheology. They are formed by shearing a mixture of two immiscible liquids (like oil and water), surfactants, and possibly co-surfactants. They can be oil-in-water or water-in-oil, depending on the core particle. Nanoemulsions are used in areas like drug delivery, food, cosmetics, pharmaceuticals, and material synthesis [294].
Song et al., prepared the Osthole loaded nano emulsion (OST-NE) against the AD mice model. Their anti-AD activity was examined in the Scopolamine-Induced Kunming mice. The result of study showed that prepared formulation improved memory by 3.7-fold as compared to the diseased group. The OST-NE also increased the SOD activity by 1.1-fold as compared to the Scopolamine-induced group. Thus, OST-NE was an effective AD [295].
Furthermore, Alaqeel et al., prepared the quercetin-loaded nano emulsion (QCNE) against the AD. They investigated their anti-AD activity against the albino male rats. The result of study showed that prepared formulation increased SOD and GSH activity by 1.4-fold and 1.9-fold as compared to AD group respectively. Furthermore, QCNE decreased the level of IL-1β & TNF-α by 1.8-fold and 2-fold as compared to the diseased group. Thus, QCNE indicated the potential against neuronal disease [296].
Ismail et al., prepared the thymoquinone-rich fraction nano emulsion (TQRFNE) against the AD. They examined their anti-AD activity on the Sprague-Dawley rats. Result of study showed that prepared formulation decreased the BACE1 and RAGE levels by 2-fold and 1.7-fold in hippocampus respectively. This indicated the reduction of Aβ secretion in brain. Furthermore, TQRFNE also decreased the level of Aβ40 and Aβ42 in hippocampus and was effective against AD [297].
Beniwal et al. fabricated the citral Nanoemulsion (N-Citral) against the AD model and examined their anti-AD activity on the male rats model. The result of study showed that prepared formulation improved memory by 4-fold as compared to the AD-induced group. While N-Citral decreased the level of MDA by 1.2-fold as compared to diseased group. It indicated that the N-Citral was effective against the neurodegenerative disease [298].
Nanostructured Lipid Carriers (NLCs)
Nanostructured Lipid Carriers (NLCs) are lipid NPs made from a blend of solid and liquid lipids. They remain solid at room and body temperatures. As the second generation of lipid NPs, NLCs were developed to address the limitations of the first generation, such as restricted drug loading and drug leakage during storage. NLCs have diverse applications due to their unique properties. They have potential in biomedicine and biotechnology, including enhancing plant growth, disease management [299].
Shehatae et al., prepared Donepezil and Astaxanthin co-loaded NLCs (DPL/AST–NLCs) by the hot high-shear homogenization technique against AD rats model examined their anti-AD activity on the male albino rats. Result of the study showed that prepared formulation improved memory in the MWT by 3.6-fold as compared to the AD group. Furthermore, DPL/AST–NLCs decreased Aβ1–42 by 4.3-fold as compared to diseased group. In addition to this, DPL/AST–NLCs also decreased inflammation, and oxidative stress, and improved cognitive impairment [300].
In another study, Shehata et al., fabricated Astaxanthin loaded NLCs (AST–NLCs) by the hot high-pressure homogenization (HPH) technique against AD rat model. They investigated their activity on male albino rats. The result of study showed that prepared formulation improved memory in MWT by 2.6-fold as compared to AD group. Furthermore, the prepared formulation decreased Aβ1–42 content by 3-fold as compared to the diseased group. Overall, the study concluded that AST–NLCs indicated the potential against AD [301].
Anand et al., prepared NLC loaded with the rivastigmine hydrogen tartrate (RHT-NLCs). They examined their In-vivo activity on the albino Wistar rats. The result of study showed that prepared formulation improved memory by 2.9-fold as compared to the negative control group. Overall, study concluded that RHT-NLCs were an effective formulation against AD [302].
Solid Lipid NPs (SLNs)
Solid Lipid NPs (SLNs) are lipid NPs composed of a solid lipid core that can encapsulate both water-soluble and fat-soluble active pharmaceutical ingredients. Typically spherical, their size ranges from 10 to 1000 nm. SLNs have unique properties that make them useful in various fields. They have potential in biomedicine and biotechnology, including enhancing plant growth, disease management [303].
Shivananjegowda et al., prepared Memantine Hydrochloride (MeHCl) and Tramiprosate (TMPS) co-loaded solid lipid NPs (SLNs) against the AD. Their activity was examined on the Albino Wister Rats. Result of study showed that prepared formulation improved memory by 3-fold in escape latency test as compared to AlCl3-induced group. Also, M + T SLN reduced the Aβ level in AD mice model as compared to diseased group. Overall, study shows the M + T SLN was effective the AD [304].
Similarly, Saini et al., demonstrated ferulic acid SLNs coated with chitosan by the hot homogenization technique against AD and investigated their activity in the STZ-induced Wistar rats. The result of the study showed that prepared formulation promoted an increase in level of GSH, SOD, and Catalase. Furthermore, it reduced the level of AChE. Thereby managed the neurodegeneration and cognition in the AD [305].
Dara et al., fabricated Erythropoietin SLN (EPO-SLN) by the double-emulsion method and examined the activity on the Albino Wistar male rats. Result of study showed that prepared formulation exhibited a reduction in Aβ plaques by 6.5-fold as compared to diseased Aβ induced group. Also, EPO-SLN decreased ROS by 1.5-fold as compared to the diseased group. Overall, study shows that EPO-SLN was effective against neurodegenerative disorders [306].
In another study, Campisi et al., prepared the curcumin SLNs-loaded (SLNs-CUR) by solvent evaporation method against the AD model and examined their activity on the TgCRND8 (Tg) mice. The result of the study showed that the prepared formulation improved memory by 9-fold in Morris water maze test as compared to untreated group [307].
Carbon Nanotubes (CNTs)
Carbon nanotubes (CNTs) are minuscule tubular structures made of carbon atoms, with a diameter much smaller than a strand of human hair. Their small size, strength, and ability to be functionalized with various biomolecules make them ideal for targeted delivery to specific cells or tissues. They possess a high surface area and robust adsorption capabilities, which allow for high drug-loading capacity. This makes them a key component in nano-drug delivery systems. They can also penetrate cells, delivering drugs directly to the cytoplasm or nucleus. In the case of neurovascular disorders, CNTs can potentially deliver drugs across the blood-brain barrier [308].
Yang et al., evaluated the potential of single-walled carbon nanotubes (SWCNTs) against AD in the Kunming mice. Result of study showed that prepared formulation exhibited improvement in memory by 1.1-fold as compared to AD group. Furthermore, it reduced level of AChE and effective against AD [309].
Xue et al., evaluated potential of single-walled carbon nanotubes (SWNT) against AD and examined the activity on the CRND8 mice. Result of study showed that prepared formulation decreased p-ULK1/t-ULK1 by 1.7-fold as compared to untreated group. Overall, the study shows that SWNT has a neuroprotective effect against AD therapy [310].
Ranjan et al., evaluated potential of carbon nanotubes (CNTs) against AD model and examined their activity in Male Wistar rats. The result of study showed that the prepared formulation decreased the level of Ascorbic Acid by 2.1-fold, 1.8-fold, and 1.3-fold in brain which enhanced memory. Thus, CNTs are an effective approach for the treatment of the animal model of AD [311].
Hydrogel
Hydrogels are three-dimensional polymeric networks capable of absorbing large amounts of water and biological fluids are increasingly used in drug delivery due to their unique properties and biocompatibility. They can encapsulate a wide range of drug molecules and control their release over time. Their responsiveness to specific triggers such as pH, temperature, or enzymes allows for targeted drug delivery, reducing potential systemic toxicity. Hydrogels can be formed into various shapes and sizes, enhancing their versatility [312].
Ribeiro et al., prepared Curcumin-loaded mesoporous silica NPs (MSN-CCM) Hydrogel (HG@MSN-CCM) against the AD and examined their anti-AD activity on the STZ-induced mice female Swiss albino mice. Result of study showed that prepared formulation showed that prepared formulation improved memory in open field test by 4.5-fold as compared to negative control group. Thus, Study shows the HG@MSN-CCM improved cognition and acted as a potential formulation against AD [313].
Similarly, Ou et al. examined the therapeutic effect of Timosaponin BII-loaded hydrogel (ISGs) on scopolamine-induced AD mice. Result of the study showed that ISGs treated group increased cholinergic M1 receptor in hippocampus of mice by 2-fold as compared to untreated group [314].
Chen et al., prepared timosaponin BII loaded in situ hydrogel (ISG) against the AD and evaluated their in vivo activity against the C57BL/6J mice. Result of study showed that prepared formulation improved memory in the platform crossing test by 1.4-fold as compared to model group. Further, T BII-ISG improved the distance covered in open field test by 1.1-fold as compared to AD model group. Thus, study concluded the effectiveness of the developed formulation against AD [315].
In another study, anti-AD effect of galantamine loaded hydrogel (Gal) was evaluated on streptozotocin-induced AD Wistar rats. The result of study showed increased in the body weight of rat in Gal treated group by 1.4-fold as that of untreated group. Further, increased escape latency was also observed in Gal treated group by 1.3-fold as compared to untreated group [316].
Few other NDDS formulation studies for the management of AD are discussed in Tables IV and V. Industrial applications and clinical applications for the treatment of AD for various delivery systems are discussed in Table IV. Additionally, Clinical trials, ongoing clinical trials, and patents related to AD are mentioned in Tables VI and VII, and Table VIII, respectively.
Future prospective
Research on AD is advancing across multiple fronts. In terms of early detection and diagnosis, ongoing efforts are focused on developing more accurate and accessible methods, including biomarkers, imaging techniques, and blood tests, to identify signs of the disease before symptoms appear. Researchers are exploring the concept of precision medicine, tailoring Alzheimer’s treatments based on individual genetic and molecular profiles to optimize effectiveness and minimize side effects. In drug development, numerous trials are underway with a focus on medications capable of decelerating or stopping the progression of disease by targeting specific biological processes such as accumulation of beta-amyloid plaques and tau tangles. Non-pharmacological studies, such as lifestyle modifications, cognitive training, and physical exercise, are being investigated for their potential in preventing or delaying Alzheimer’s onset and managing symptoms. Technology, such as wearable devices and smartphone apps, is being explored to monitor and assess cognitive function, providing valuable data for early detection and disease management. Global collaboration among researchers, healthcare professionals, and organizations is deemed crucial, fostering initiatives and partnerships to pool resources, share data, and accelerate progress. There is a growing emphasis on public awareness and advocacy to reduce the stigma associated with AD with governments and organizations working on policies to support research funding, caregiver support, and improved healthcare access. Staying updated on the latest research findings and breakthroughs is essential, given the dynamic and continuously evolving nature of the field, with reliable sources like scientific journals, health organizations, and research institutions recommended for the most current information.
Data Availability
None
Abbreviations
- AD:
-
Alzheimer's disease
- EoAD:
-
Early-onset AD
- LoAD:
-
Late-onset AD
- FAD:
-
Family AD
- Aβ:
-
Amyloid-β
- NTs:
-
Neurofibrillary tangles
- NP:
-
Neuritic plaques
- NPs:
-
Nanoparticles
- NDDS:
-
Novel drug delivery systems
- APP:
-
Amyloid precursor protein
- NMDA:
-
N-methyl-D-aspartate receptors
- BBB:
-
Blood-brain barrier
- ELISA:
-
Enzyme-Linked Immunosorbent Assay
- CSF:
-
Cerebrospinal fluids
- PET:
-
Positron Emission Tomography
- MRI:
-
Magnetic Resonance Imaging
- FDG:
-
F-18 fluorodeoxyglucose
- NFL:
-
Neurofilament Light
- APOE ε4:
-
Apolipoprotein E
- PSEN1:
-
Presenilin 1
- PCR:
-
Polymerase chain reaction
- TNF-α:
-
Tumor Necrosis Factor-Alpha
- CRP:
-
C-reactive protein
- IL-6:
-
Interleukin-6
- CLU:
-
Clusterin
- CRP:
-
C-reactive protein
- MiRNAs:
-
MicroRNAs
- RT-qPCR:
-
Quantitative reverse transcription polymerase chain reaction
References
Angelucci F, et al. Alzheimer’s disease (AD) and mild cognitive impairment (MCI) patients are characterized by increased BDNF serum levels. Curr Alzheimer Res. 2010;7(1):15–20.
Gulisano W, et al. Role of amyloid-β and tau proteins in Alzheimer’s disease: confuting the amyloid cascade. J Alzheimers Dis. 2018;64(s1):S611–31.
Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s Disease Lancet. 2006;368(9533):387–403.
Gallardo G, Holtzman DM. Amyloid-β and Tau at the crossroads of alzheimer's disease. Adv Exp Med Biol. 2019;1184:187–203. https://doi.org/10.1007/978-981-32-9358-8_16.
Sirkis DW, et al. Dissecting the clinical heterogeneity of early-onset Alzheimer’s disease. Mol Psychiatry. 2022;27(6):2674–88.
Drachman DA. Aging of the brain, entropy, and Alzheimer disease. Neurology. 2006;67(8):1340–52.
Dorszewska J, et al. Molecular basis of familial and sporadic Alzheimer’s disease. Curr Alzheimer Res. 2016;13(9):952–63.
Guerreiro RJ, Gustafson DR, Hardy J. The genetic architecture of Alzheimer’s disease: beyond APP, PSENs and APOE. Neurobiol Aging. 2012;33(3):437–56.
Sadigh-Eteghad S, et al. Amyloid-beta: a crucial factor in Alzheimer’s disease. Med Principles Pract. 2015;24(1):1–10.
Tarawneh R, Holtzman DM. The clinical problem of symptomatic Alzheimer disease and mild cognitive impairment. Cold Spring Harbor Perspect Med. 2012;2(5):a006148.
Di Santo SG, et al. A meta-analysis of the efficacy of donepezil, rivastigmine, galantamine, and memantine in relation to severity of Alzheimer’s disease. J Alzheimers Dis. 2013;35(2):349–61.
Ravindranath V, Sundarakumar JS. Changing demography and the challenge of dementia in India. Nat Rev Neurol. 2021;17(12):747–758. https://doi.org/10.1038/s41582-021-00565-x.
Li X, et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2019. Front Aging Neurosci. 2022;14:937486.
Niotis K, et al. Dementia prevention in clinical practice. In seminars in neurology. Stuttgart: Thieme Medical Publishers, Inc.; 2022.
Nandi A et al. Global and regional projections of the economic burden of Alzheimer’s disease and related dementias from 2019 to 2050: A value of statistical life approach. EClinicalMedicine. 2022;51:101580.
Fonseca ACR, et al. Cholesterol and statins in Alzheimer’s disease: current controversies. Exp Neurol. 2010;223(2):282–93.
Kumar A, Sidhu J, Goyal A, Tsao JW. Alzheimer disease. In: StatPearls. StatPearls Publishing, Treasure Island (FL). 2023.
Abbas M. Potential role of nanoparticles in treating the accumulation of amyloid-beta peptide in Alzheimer’s patients. Polymers. 2021;13(7):1051.
Vassar R. Bace 1: the β-secretase enzyme in alzheimer’s disease. J Mol Neurosci. 2004;23:105–13.
Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nat Rev Mol Cell Biol. 2007;8(2):101–12.
Penke B, Bogár F, Fülöp L. β-Amyloid and the pathomechanisms of Alzheimer’s disease: a comprehensive view. Molecules. 2017;22(10):1692.
Kolarova M et al. Structure and pathology of tau protein in Alzheimer disease. Int J Alzheimers Dis. 2012;2012:731526.
Staessen JA, Richart T, Birkenhäger WH. Less atherosclerosis and lower blood pressure for a meaningful life perspective with more brain. Hypertension. 2007;49(3):389–400.
Cheraghzadeh M, et al. Amyloid Beta sharply increases HMG-CoA reductase protein levels in astrocytes isolated from C57BL/6 mice. Gene Rep. 2021;23:101070.
Mohamed A, Smith K, de Chaves EP. The mevalonate pathway in Alzheimer’s disease—cholesterol and non-sterol isoprenoids. Alzheimer’s Disease-Challenges for the Future; 2015. pp. 167–222.
Kang N, Ji Z, Li Y, Gao J, Wu X, Zhang X, et al. Metabolite-derived damage-associated molecular patterns in immunological diseases. FEBS J. 2024;291(10):2051–2067. https://doi.org/10.1111/febs.16902.
Bordt EA, Polster BM. NADPH oxidase-and mitochondria-derived reactive oxygen species in proinflammatory microglial activation: a bipartisan affair? Free Radic Biol Med. 2014;76:34–46.
Pimenova AA, Raj T, Goate AM. Untangling genetic risk for Alzheimer’s disease. Biol Psychiatry. 2018;83(4):300–10.
Gessel MM, et al. Familial Alzheimer’s disease mutations differentially alter amyloid β-protein oligomerization. ACS Chem Neurosci. 2012;3(11):909–18.
Podleśny-Drabiniok A, Marcora E, Goate AM. Microglial phagocytosis: a disease-associated process emerging from Alzheimer’s disease genetics. Trends Neurosci. 2020;43(12):965–79.
Foster EM, et al. Clusterin in Alzheimer’s disease: mechanisms, genetics, and lessons from other pathologies. Front NeuroSci. 2019;13:164.
Tan M-S, Yu J-T, Tan L. Bridging integrator 1 (BIN1): form, function, and Alzheimer’s disease. Trends Mol Med. 2013;19(10):594–603.
Tu S, et al. Oligomeric Aβ-induced synaptic dysfunction in Alzheimer’s disease. Mol Neurodegeneration. 2014;9:1–12.
Lee H, Aylward AJ, Pearse RV, Hsieh YC, Augur ZM, Benoit CR, et al. Cell-type-specific regulation of APOE levels in human neurons by the Alzheimer's disease risk gene SORL1. Cell Rep. 2023;42(8):112994. https://doi.org/10.1016/j.celrep.2023.112994.
Shi Y, Holtzman DM. Interplay between innate immunity and Alzheimer disease: APOE and TREM2 in the spotlight. Nat Rev Immunol. 2018;18(12):759–72.
Gadhave K, et al. A multitude of signaling pathways associated with Alzheimer’s disease and their roles in AD pathogenesis and therapy. Med Res Rev. 2021;41(5):2689–745.
Hampel H, et al. The amyloid-β pathway in Alzheimer’s disease. Mol Psychiatry. 2021;26(10):5481–503.
Sun X, Chen W-D, Wang Y-D. β-Amyloid: the key peptide in the pathogenesis of Alzheimer’s disease. Front Pharmacol. 2015;6:221.
Finder VH, Glockshuber R. Amyloid-β aggregation. Neurodegenerative Dis. 2007;4(1):13–27.
Walsh DM, Selkoe DJ. Amyloid β-protein and beyond: the path forward in Alzheimer’s disease. Curr Opin Neurobiol. 2020;61:116–24.
Bignante EA, et al. Amyloid β precursor protein as a molecular target for amyloid β–induced neuronal degeneration in Alzheimer’s disease. Neurobiol Aging. 2013;34(11):2525–37.
Pryor NE, Moss MA, Hestekin CN. Unraveling the early events of amyloid-β protein (Aβ) aggregation: techniques for the determination of Aβ aggregate size. Int J Mol Sci. 2012;13(3):3038–72.
Ono K, Watanabe-Nakayama T. Aggregation and structure of amyloid β-protein. Neurochem Int. 2021;151:105208.
Muralidar S, et al. Role of tau protein in Alzheimer’s disease: the prime pathological player. Int J Biol Macromol. 2020;163:1599–617.
Medeiros R, Baglietto-Vargas D, LaFerla FM. The role of tau in Alzheimer’s disease and related disorders. Volume 17. CNS neuroscience & therapeutics; 2011. pp. 514–24. 5.
Rajmohan R, Reddy PH. Amyloid-beta and phosphorylated tau accumulations cause abnormalities at synapses of Alzheimer’s disease neurons. J Alzheimers Dis. 2017;57(4):975–99.
Billingsley ML, Kincaid RL. Regulated phosphorylation and dephosphorylation of tau protein: effects on microtubule interaction, intracellular trafficking and neurodegeneration. Biochem J. 1997;323(3):577–91.
Wang J-Z, Liu F. Microtubule-associated protein tau in development, degeneration and protection of neurons. Prog Neurobiol. 2008;85(2):148–75.
Mietelska-Porowska A, et al. Tau protein modifications and interactions: their role in function and dysfunction. Int J Mol Sci. 2014;15(3):4671–713.
Rawat P, et al. Phosphorylated tau in Alzheimer’s disease and other tauopathies. Int J Mol Sci. 2022;23(21):12841.
Gao L, et al. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl Neurodegener. 2022;11(1):1–34.
Gao L, et al. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl Neurodegener. 2022;11(1):4.
Rajasekhar K, Govindaraju T. Current progress, challenges and future prospects of diagnostic and therapeutic interventions in Alzheimer’s disease. RSC Adv. 2018;8(42):23780–804.
Song J-H, Yu J-T, Tan L. Brain-derived neurotrophic factor in Alzheimer’s disease: risk, mechanisms, and therapy. Mol Neurobiol. 2015;52:1477–93.
Miranda M, et al. Brain-derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Front Cell Neurosci. 2019;13:363.
Tower J. Programmed cell death in aging. Ageing Res Rev. 2015;23:90–100.
Ismail NA, et al. A narrative review of brain-derived neurotrophic factor (BDNF) on cognitive performance in Alzheimer’s disease. Growth Factors. 2020;38(3–4):210–25.
Bathina S, Das UN. Brain-derived neurotrophic factor and its clinical implications. Archives Med Sci. 2015;11(6):1164–78.
Zhang F, et al. Roles of brain-derived neurotrophic factor/tropomyosin-related kinase B (BDNF/TrkB) signalling in Alzheimer’s disease. J Clin Neurosci. 2012;19(7):946–9.
Benarroch EE. Brain-derived neurotrophic factor: regulation, effects, and potential clinical relevance. Neurology. 2015;84(16):1693–704.
Numakawa T, Odaka H, Adachi N. Actions of brain-derived neurotrophin factor in the neurogenesis and neuronal function, and its involvement in the pathophysiology of brain diseases. Int J Mol Sci. 2018;19(11):3650.
Lu B, et al. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci. 2013;14(6):401–16.
Ho Y-S, et al. Endoplasmic reticulum stress induces tau pathology and forms a vicious cycle: implication in Alzheimer’s disease pathogenesis. J Alzheimers Dis. 2012;28(4):839–54.
Salminen A, et al. ER stress in Alzheimer’s disease: a novel neuronal trigger for inflammation and Alzheimer’s pathology. J Neuroinflamm. 2009;6(1):1–13.
Ansari N, Khodagholi F. Molecular mechanism aspect of ER stress in Alzheimer’s disease: current approaches and future strategies. Curr Drug Targets. 2013;14(1):114–22.
Uddin MS, Yu WS, Lim LW. Exploring ER stress response in cellular aging and neuroinflammation in Alzheimer’s disease. Ageing Res Rev. 2021;70:101417.
Resende R, et al. ER stress is involved in Aβ-induced GSK‐3β activation and tau phosphorylation. J Neurosci Res. 2008;86(9):2091–9.
Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006;13(3):385–92.
Unterberger U, et al. Endoplasmic reticulum stress features are prominent in Alzheimer disease but not in prion diseases in vivo. J Neuropathology Experimental Neurol. 2006;65(4):348–57.
Akhtar A, Sah SP. Insulin signaling pathway and related molecules: role in neurodegeneration and Alzheimer’s disease. Neurochem Int. 2020;135:104707.
Sędzikowska A, Szablewski L. Insulin and insulin resistance in Alzheimer’s disease. Int J Mol Sci. 2021;22(18):9987.
Bedse G, et al. Aberrant insulin signaling in Alzheimer’s disease: current knowledge. Front NeuroSci. 2015;9:204.
Tumminia A, et al. Type 2 diabetes mellitus and Alzheimer’s disease: role of insulin signalling and therapeutic implications. Int J Mol Sci. 2018;19(11):3306.
Dineley KT, Jahrling JB, Denner L. Insulin resistance in Alzheimer’s disease. Neurobiol Dis. 2014;72:92–103.
De la Fuente M. The role of the microbiota-gut-brain axis in the health and illness condition: a focus on Alzheimer’s disease. J Alzheimers Dis. 2021;81(4):1345–60.
Giovannini MG, et al. The microbiota–gut–brain axis and alzheimer disease. From dysbiosis to neurodegeneration: focus on the central nervous system glial cells. J Clin Med. 2021;10(11):2358.
Doifode T, et al. The impact of the microbiota-gut-brain axis on Alzheimer’s disease pathophysiology. Pharmacol Res. 2021;164:105314.
Kesika P, et al. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci. 2021;264:118627.
Mota SI, Ferreira IL, Rego AC. Dysfunctional synapse in Alzheimer’s disease–A focus on NMDA receptors. Neuropharmacology. 2014;76:16–26.
Wang R, Reddy PH. Role of glutamate and NMDA receptors in Alzheimer’s disease. J Alzheimers Dis. 2017;57(4):1041–8.
Liu J, et al. The role of NMDA receptors in Alzheimer’s disease. Front NeuroSci. 2019;13:43.
Polychronidou E, et al. Notch signaling and ageing. In GeNeDis 2014: neurodegeneration. Springer; 2015.
Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci. 2016;19(6):771–83.
Woo H-N, et al. Alzheimer’s disease and notch signaling. Biochem Biophys Res Commun. 2009;390(4):1093–7.
Kapoor A, Nation DA. Role of Notch signaling in neurovascular aging and Alzheimer’s disease. In Seminars in cell & developmental biology. Amsterdam: Elsevier; 2021.
Pearson-Leary J, McNay EC. Novel roles for the insulin-regulated glucose transporter-4 in hippocampally dependent memory. J Neurosci. 2016;36(47):11851–64.
McNay EC, Pearson-Leary J. GluT4: a central player in hippocampal memory and brain insulin resistance. Exp Neurol. 2020;323:113076.
Ramírez-Expósito MJ, et al. Putative involvement of endocrine disruptors in the alzheimer’s disease Via the insulin-regulated Aminopeptidase/GLUT4 pathway. Curr Neuropharmacol. 2021;19(7):939–56.
Wang T, et al. Current understanding of glucose transporter 4 expression and functional mechanisms. World J Biol Chem. 2020;11(3):76.
Karnieli E, Armoni M. Transcriptional regulation of the insulin-responsive glucose transporter GLUT4 gene: from physiology to pathology. Am J Physiology-Endocrinology Metabolism. 2008;295(1):E38–45.
Sayem ASM, et al. Action of phytochemicals on insulin signaling pathways accelerating glucose transporter (GLUT4) protein translocation. Molecules. 2018;23(2):258.
Kitagishi Y, et al. Dietary regulation of PI3K/AKT/GSK-3β pathway in Alzheimer’s disease. Alzheimers Res Ther. 2014;6:1–7.
Limantoro J, de Liyis BG, Sutedja JC. Akt signaling pathway: a potential therapy for Alzheimer’s disease through glycogen synthase kinase 3 beta inhibition. Egypt J Neurol Psychiatry Neurosurg. 2023;59(1):147.
Huang H-C, et al. Curcumin attenuates amyloid-β-induced tau hyperphosphorylation in human neuroblastoma SH-SY5Y cells involving PTEN/Akt/GSK-3β signaling pathway. J Recept Signal Transduction. 2014;34(1):26–37.
Zhao R et al. RETRACTED: Implication of phosphatidylinositol-3 kinase/Akt/glycogen synthase kinase-3β pathway in ginsenoside Rb1’s attenuation of beta-amyloid-induced neurotoxicity and tau phosphorylation. Amsterdam: Elsevier; 2011.
Salem MA, et al. Tadalafil and bergapten mitigate streptozotocin-induced sporadic Alzheimer’s disease in mice via modulating neuroinflammation, PI3K/Akt, Wnt/β-catenin, AMPK/mTOR signaling pathways. Toxicol Appl Pharmcol. 2021;429:115697.
Zhang X, et al. Ginsenoside Rd attenuates tau protein phosphorylation via the PI3K/AKT/GSK-3β pathway after transient forebrain ischemia. Neurochem Res. 2014;39:1363–73.
Perluigi M, Di Domenico F, Butterfield DA. mTOR signaling in aging and neurodegeneration: at the crossroad between metabolism dysfunction and impairment of autophagy. Neurobiol Dis. 2015;84:39–49.
Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168(6):960–76.
Perluigi M, et al. mTOR in Alzheimer disease and its earlier stages: links to oxidative damage in the progression of this dementing disorder. Free Radic Biol Med. 2021;169:382–96.
Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–93.
Misonou H, Morishima-Kawashima M, Ihara Y. Oxidative stress induces intracellular accumulation of amyloid β-protein (Aβ) in human neuroblastoma cells. Biochemistry. 2000;39(23):6951–9.
Manczak M, et al. Mitochondria are a direct site of Aβ accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15(9):1437–49.
Sivamaruthi BS, et al. NF-κB pathway and its inhibitors: a promising Frontier in the management of Alzheimer’s Disease. Biomedicines. 2023;11(9):2587.
Ju Hwang C et al. NF-κB as a key mediator of brain inflammation in Alzheimer’s disease. CNS & Neurol Dis-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders). 2019;18(1):3–10.
Valerio A, et al. NF-κB pathway: a target for preventing β‐amyloid (Aβ)‐induced neuronal damage and Aβ42 production. Eur J Neurosci. 2006;23(7):1711–20.
Srinivasan M, Lahiri DK. Significance of NF-κB as a pivotal therapeutic target in the neurodegenerative pathologies of Alzheimer’s disease and multiple sclerosis. Expert Opin Ther Targets. 2015;19(4):471–87.
Saresella M, et al. The NLRP3 and NLRP1 inflammasomes are activated in Alzheimer’s disease. Mol Neurodegeneration. 2016;11(1):1–14.
Hanslik KL, Ulland TK. The role of microglia and the Nlrp3 inflammasome in Alzheimer’s disease. Front Neurol. 2020;11:570711.
Fusco R, et al. Focus on the role of NLRP3 Inflammasome in diseases. Int J Mol Sci. 2020;21(12):4223.
Duan Y, Kelley N, He Y. Role of the NLRP3 inflammasome in neurodegenerative diseases and therapeutic implications. Neural Regeneration Res. 2020;15(7):1249.
Jia L, Piña-Crespo J, Li Y. Restoring Wnt/β-catenin signaling is a promising therapeutic strategy for Alzheimer’s disease. Mol Brain. 2019;12:1–11.
Inestrosa NC, Toledo EM. The role of wnt signaling in neuronal dysfunction in Alzheimer’s Disease. Mol Neurodegeneration. 2008;3:1–13.
Tapia-Rojas C, Inestrosa NC. Loss of canonical wnt signaling is involved in the pathogenesis of Alzheimer’s disease. Neural Regeneration Res. 2018;13(10):1705.
Zheng H, et al. TREM2 promotes microglial survival by activating Wnt/β-catenin pathway. J Neurosci. 2017;37(7):1772–84.
Boonen RA, van Tijn P, Zivkovic D. Wnt signaling in Alzheimer’s disease: up or down, that is the question. Ageing Res Rev. 2009;8(2):71–82.
Serafino A, et al. Targeting the Wnt/β-catenin pathway in neurodegenerative diseases: recent approaches and current challenges. Expert Opin Drug Discov. 2020;15(7):803–22.
Cai Z, et al. Roles of AMP-activated protein kinase in Alzheimer’s disease. Neuromol Med. 2012;14:1–14.
Yang L, et al. AMPK: potential therapeutic target for Alzheimer’s disease. Curr Protein Pept Sci. 2020;21(1):66–77.
Chen M, et al. AMPK: a bridge between diabetes mellitus and Alzheimer’s disease. Behav Brain Res. 2021;400:113043.
Du L-L, et al. AMPK activation ameliorates Alzheimer’s disease-like pathology and spatial memory impairment in a streptozotocin-induced Alzheimer’s disease model in rats. J Alzheimers Dis. 2015;43(3):775–84.
Salminen A, et al. AMP-activated protein kinase: a potential player in Alzheimer’s disease. J Neurochem. 2011;118(4):460–74.
Sun P, et al. Protective role of Dihydromyricetin in Alzheimer’s disease rat model associated with activating AMPK/SIRT1 signaling pathway. Biosci Rep. 2019;39(1):BSR20180902.
Yates SC, et al. Dysfunction of the mTOR pathway is a risk factor for Alzheimer’s disease. Acta Neuropathol Commun. 2013;1(1):1–15.
Oddo S. The role of mTOR signaling in Alzheimer disease. Front Biosci. (Scholar edition), 2012;4:941.
Rosner M, et al. The mTOR pathway and its role in human genetic diseases. Mutat Research/reviews Mutat Res. 2008;659(3):284–92.
Kou X, Chen D, Chen N. Physical activity alleviates cognitive dysfunction of Alzheimer’s disease through regulating the mTOR signaling pathway. Int J Mol Sci. 2019;20(7):1591.
Franco R, et al. Potential of GPCRs to modulate MAPK and mTOR pathways in Alzheimer’s disease. Prog Neurobiol. 2017;149:21–38.
Bonda DJ, et al. The sirtuin pathway in ageing and Alzheimer disease: mechanistic and therapeutic considerations. Lancet Neurol. 2011;10(3):275–9.
Kumar R, et al. Sirtuin1: a promising serum protein marker for early detection of Alzheimer’s disease. PLoS ONE. 2013;8(4):e61560.
Qin W, et al. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem. 2006;281(31):21745–54.
Julien C, et al. Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J Neuropathology Experimental Neurol. 2009;68(1):48–58.
Mota BC, Sastre M. The role of PGC1α in Alzheimer’s disease and therapeutic interventions. Int J Mol Sci. 2021;22(11):5769.
Sheng B, et al. Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer’s disease. J Neurochem. 2012;120(3):419–29.
Long H-Z, et al. PI3K/AKT signal pathway: a target of natural products in the prevention and treatment of Alzheimer’s disease and Parkinson’s disease. Front Pharmacol. 2021;12:648636.
Gabbouj S, et al. Altered insulin signaling in Alzheimer’s disease brain–special emphasis on PI3K-Akt pathway. Front NeuroSci. 2019;13:629.
Li H, et al. Neuroprotective effects of ginseng protein on PI3K/Akt signaling pathway in the hippocampus of D-galactose/AlCl3 inducing rats model of Alzheimer’s disease. J Ethnopharmacol. 2016;179:162–9.
Razani E, et al. The PI3K/Akt signaling axis in Alzheimer’s disease: a valuable target to stimulate or suppress? Cell Stress Chaperones. 2021;26(6):871–87.
Salminen A, Kauppinen A, Kaarniranta K. Hypoxia/ischemia activate processing of amyloid precursor protein: impact of vascular dysfunction in the pathogenesis of Alzheimer’s disease. J Neurochem. 2017;140(4):536–49.
Iyalomhe O, et al. The role of hypoxia-inducible factor 1 in mild cognitive impairment. Cell Mol Neurobiol. 2017;37:969–77.
Guo C, et al. Intranasal lactoferrin enhances α-secretase-dependent amyloid precursor protein processing via the ERK1/2-CREB and HIF-1α pathways in an Alzheimer’s disease mouse model. Neuropsychopharmacology. 2017;42(13):2504–15.
Zhang Z, et al. Hypoxia inducible factor-1 as a target for neurodegenerative diseases. Curr Med Chem. 2011;18(28):4335–43.
Bahn G et al. NRF2/ARE pathway negatively regulates BACE1 expression and ameliorates cognitive deficits in mouse Alzheimer’s models. Proc Natl Acad Sci. 2019;116(25):12516–12523.
Fao L, Mota SI, Rego AC. Shaping the Nrf2-ARE-related pathways in Alzheimer’s and Parkinson’s diseases. Ageing Res Rev. 2019;54:100942.
Joshi G, Johnson JA. The Nrf2-ARE pathway: a valuable therapeutic target for the treatment of neurodegenerative diseases. Recent Pat CNS Drug Discovery (Discontinued). 2012;7(3):218–29.
Gan L, Johnson JA. Oxidative damage and the Nrf2-ARE pathway in neurodegenerative diseases. Biochim Biophys Acta (BBA)-Mol Basis Dis. 2014;1842(8):1208–18.
Calkins MJ, et al. The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid Redox Signal. 2009;11(3):497–508.
Johnson JA, et al. The Nrf2–ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann N Y Acad Sci. 2008;1147(1):61–9.
Zgorzynska E, Dziedzic B, Walczewska A. An overview of the Nrf2/ARE pathway and its role in neurodegenerative diseases. Int J Mol Sci. 2021;22(17):9592.
Saha S, et al. A perspective on Nrf2 signaling pathway for neuroinflammation: a potential therapeutic target in Alzheimer’s and Parkinson’s diseases. Front Cell Neurosci. 2022;15:787258.
Lucke-Wold BP, et al. Common mechanisms of Alzheimer’s disease and ischemic stroke: the role of protein kinase C in the progression of age-related neurodegeneration. J Alzheimers Dis. 2015;43(3):711–24.
Hongpaisan J, Sun M-K, Alkon DL. PKC ε activation prevents synaptic loss, Aβ elevation, and cognitive deficits in Alzheimer’s disease transgenic mice. J Neurosci. 2011;31(2):630–43.
Sun M-K, Alkon DL. The memory kinases: roles of PKC isoforms in signal processing and memory formation. Prog Mol Biol Transl Sci. 2014;122:31–59.
Garrido JL, et al. Protein kinase C inhibits amyloid β-peptide neurotoxicity by acting on members of the wnt pathway. FASEB J. 2002;16(14):1982–4.
Caraci F, et al. TGF-β1 pathway as a new target for neuroprotection in Alzheimer’s disease. CNS Neurosci Ther. 2011;17(4):237–49.
Smith AL, Robin TP, Ford HL. Molecular pathways: targeting the TGF-β pathway for cancer therapy. Clin Cancer Res. 2012;18(17):4514–21.
Hu Y, et al. TGF-β1 restores hippocampal synaptic plasticity and memory in Alzheimer model via the PI3K/Akt/Wnt/β-catenin signaling pathway. J Mol Neurosci. 2019;67:142–9.
Zheng C, Zhou X-W, Wang J-Z. The dual roles of cytokines in Alzheimer’s disease: update on interleukins, TNF-α, TGF-β and IFN-γ. Transl Neurodegener. 2016;5(1):1–15.
Tesseur I, et al. Deficiency in neuronal TGF-β signaling promotes neurodegeneration and Alzheimer’s pathology. J Clin Investig. 2006;116(11):3060–9.
Caraci F, et al. Dysfunction of TGF-β1 signaling in Alzheimer’s disease: perspectives for neuroprotection. Cell Tissue Res. 2012;347:291–301.
Wyss-Coray T. TGF-β pathway as a potential target in neurodegeneration and Alzheimer’s. Curr Alzheimer Res. 2006;3(3):191–5.
Rusek M, et al. The role of the JAK/STAT signaling pathway in the pathogenesis of Alzheimer’s Disease: new potential treatment target. Int J Mol Sci. 2023;24(1):864.
Tsiogka A, et al. The JAK/STAT pathway and its selective inhibition in the treatment of atopic dermatitis: a systematic review. J Clin Med. 2022;11(15):4431.
Huang I, et al. JAK–STAT signaling pathway in the pathogenesis of atopic dermatitis: an updated review. Front Immunol. 2022;13:1068260.
Nevado-Holgado AJ, et al. Genetic and real-world clinical data, combined with empirical validation, nominate Jak-Stat signaling as a target for Alzheimer’s disease therapeutic development. Cells. 2019;8(5):425.
Molina JR, Adjei AA. The ras/raf/mapk pathway. J Thorac Oncol. 2006;1(1):7–9.
Kim EK, Choi E-J. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta (BBA)-Mol Basis Dis. 2010;1802(4):396–405.
Kirouac L, et al. Activation of Ras-ERK signaling and GSK-3 by amyloid precursor protein and amyloid beta facilitates neurodegeneration in Alzheimer’s disease. Eneuro. 2017;4(2). https://doi.org/10.1523/ENEURO.0149-16.2017.
Shukla V, Skuntz S, Pant HC. Deregulated Cdk5 activity is involved in inducing Alzheimer’s disease. Arch Med Res. 2012;43(8):655–62.
Shah K, Lahiri DK. Cdk5 activity in the brain–multiple paths of regulation. J Cell Sci. 2014;127(11):2391–400.
Crews L, et al. Modulation of aberrant CDK5 signaling rescues impaired neurogenesis in models of Alzheimer’s disease. Cell Death Dis. 2011;2(2):e120–120.
Maitra S, Vincent B. Cdk5-p25 as a key element linking amyloid and tau pathologies in Alzheimer’s disease: Mechanisms and possible therapeutic interventions. Life Sci. 2022;308:120986.
Hampel H, et al. Total and phosphorylated tau protein as biological markers of Alzheimer’s disease. Exp Gerontol. 2010;45(1):30–40.
Andreasen N, Blennow K. β-Amyloid (Aβ) protein in cerebrospinal fluid as a biomarker for Alzheimer’s disease. Peptides. 2002;23(7):1205–1214.
Sergeant N, Delacourte A, Buée L. Tau protein as a differential biomarker of tauopathies. Biochim Biophys Acta (BBA)-Mol Basis Dis. 2005;1739(2–3):179–97.
Islam K, et al. Development of a label-free immunosensor for clusterin detection as an Alzheimer’s biomarker. Sensors. 2018;18(1):308.
Yarchoan M, et al. Association of plasma C-reactive protein levels with the diagnosis of Alzheimer’s disease. J Neurol Sci. 2013;333(1–2):9–12.
Park J-C, Han S-H, Mook-Jung I. Peripheral inflammatory biomarkers in Alzheimer’s disease: a brief review. BMB Rep. 2020;53(1):10.
Hansson O, et al. The Alzheimer’s Association appropriate use recommendations for blood biomarkers in Alzheimer’s disease. Alzheimer’s Dement. 2022;18(12):2669–86.
Zetterberg H, Blennow K. Moving fluid biomarkers for Alzheimer’s disease from research tools to routine clinical diagnostics. Mol Neurodegeneration. 2021;16(1):1–7.
Omar SH, Preddy J. Advantages and pitfalls in fluid biomarkers for diagnosis of Alzheimer’s disease. J Personalized Med. 2020;10(3):63.
Forgrave LM, et al. The diagnostic performance of neurofilament light chain in CSF and blood for Alzheimer’s disease, frontotemporal dementia, and amyotrophic lateral sclerosis: a systematic review and meta-analysis. Volume 11. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring,; 2019. pp. 730–43.
Chételat G, et al. Amyloid-PET and 18F-FDG-PET in the diagnostic investigation of Alzheimer’s disease and other dementias. Lancet Neurol. 2020;19(11):951–62.
Blennow K, et al. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer’s disease. Alzheimer’s Dement. 2015;11(1):58–69.
Jack CR Jr, et al. Steps to standardization and validation of hippocampal volumetry as a biomarker in clinical trials and diagnostic criterion for Alzheimer’s disease. Alzheimer’s Dement. 2011;7(4):474–85. e4.
Ou Y-N, et al. FDG-PET as an independent biomarker for Alzheimer’s biological diagnosis: a longitudinal study. Alzheimers Res Ther. 2019;11:1–11.
Lautner R, et al. Apolipoprotein E genotype and the diagnostic accuracy of cerebrospinal fluid biomarkers for Alzheimer disease. JAMA Psychiatry. 2014;71(10):1183–91.
Lanoiselée H-M, et al. PSEN1, and PSEN2 mutations in early-onset Alzheimer disease: a genetic screening study of familial and sporadic cases. PLoS Med. 2017;14(3):e1002270.
Zetterberg H, Blennow K, Hanse E. Amyloid β and APP as biomarkers for Alzheimer’s disease. Exp Gerontol. 2010;45(1):23–9.
Zhao Y, et al. microRNA-based biomarkers in Alzheimer’s disease (AD). Front NeuroSci. 2020;14:585432.
Herrmann W, Obeid R. Homocysteine: a biomarker in neurodegenerative diseases. Clin Chem Lab Med. 2011;49(3):435–41.
Su XQ, Wang J, Sinclair AJ. Plasmalogens and Alzheimer’s disease: a review. Lipids Health Dis. 2019;18:1–10.
Yiannopoulou KG, Papageorgiou SG. Current and future treatments for Alzheimer’s disease. Ther Adv Neurol Disord. 2013;6(1):19–33.
Wollen KA. Alzheimer’s disease: the pros and cons of pharmaceutical, nutritional, botanical, and stimulatory therapies, with a discussion of treatment strategies from the perspective of patients and practitioners. Altern Med Rev. 2010;15(3):223–44.
Mendiola-Precoma J et al. Therapies for prevention and treatment of Alzheimer’s disease. BioMed Res Int. 2016;2016:1–17.
Folch J et al. Current research therapeutic strategies for Alzheimer’s disease treatment. Neural Plast 2016;2016:8501693.
Danysz W, Parsons CG. Alzheimer’s disease, β-amyloid, glutamate, NMDA receptors and memantine–searching for the connections. Br J Pharmacol. 2012;167(2):324–52.
Parsons CG, Stöffler A, Danysz W. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system-too little activation is bad, too much is even worse. Neuropharmacology. 2007;53(6):699–723.
Adlimoghaddam A, et al. A review of clinical treatment considerations of donepezil in severe Alzheimer’s disease. CNS Neurosci Ther. 2018;24(10):876–88.
Kalola UK, Nguyen H. Galantamine 2021.
Kuns B, Rosani A, Varghese D. Memantine. In: StatPearls. StatPearls Publishing, Treasure Island (FL). 2023.
Deardorff WJ, Grossberg GT. A fixed-dose combination of memantine extended-release and donepezil in the treatment of moderate-to-severe Alzheimer’s disease. Drug Des Dev Ther. 2016;10:3267–3279.
Zanforlin E, Zagotto G, Ribaudo G. An overview of new possible treatments of Alzheimer’s disease, based on natural products and semi-synthetic compounds. Curr Med Chem. 2017;24(34):3749–73.
Liu X, et al. Long-term treatment with Ginkgo biloba extract EGb 761 improves symptoms and pathology in a transgenic mouse model of Alzheimer’s disease. Brain Behav Immun. 2015;46:121–31.
Rao A, Balachandran B. Role of oxidative stress and antioxidants in neurodegenerative diseases. Nutr Neurosci. 2002;5(5):291–309.
McKenna DJ, Jones K, Hughes K. Efficacy, safety, and use of ginkgo biloba in clinical and preclinical applications. Altern Ther Health Med. 2001;7(5):70.
El Tabaa MM, et al. Neuroprotective role of Ginkgo biloba against cognitive deficits associated with Bisphenol A exposure: an animal model study. Neurochem Int. 2017;108:199–212.
Chang D et al. Herbal medicine for the treatment of vascular dementia: an overview of scientific evidence. Evid Based Complementary Altern Med. 2016;2016:7293626.
Al-Snafi AE. Hypotensive and vascular activities of medicinal plants. GSC Biol Pharm Sci. 2022;19(3):044–63.
Farkhondeh T, Yazdi HS, Samarghandian S. The protective effects of green tea catechins in the management of neurodegenerative diseases: a review. Curr Drug Discov Technol. 2019;16(1):57–65.
Ifegwu NO, Agbai JU, Mbanaso EL, Njoku-Oji NN, Aligwekwe AU. Combined effect of ethanolic leaf extracts of Carica papaya and Newbouldia laevis on the histology of testes of Alloxan-induced diabetic rats. World Journal of Biology Pharmacy and Health Sciences. 2023;13(1):001–13.
Uritu CM et al. Medicinal plants of the family Lamiaceae in pain therapy: A review. Pain Res Manag. 2018;2018:7801543.
Burki S et al. GC/MS assisted phytochemical analysis of Ajuga parviflora leaves extract along with anti-hepatotoxic effect against anti-tubercular drug induced liver toxicity in rat. Pak J Pharm Sci. 2020;33:325-331.
Sultana N. Plants of genus Rubus as a source of pharmaceuticals. CPQ Nutr. 2018;3(1):1–71.
Bashir MI, Aziz NHKA, Noor DAM. Polygonum minus aqueous extract supplement reduces stress-induced anorexia and anhedonia in mice. https://doi.org/10.53350/pjmhs22161696
Boldogh I, Kruzel ML. Colostrinin™: an oxidative stress modulator for prevention and treatment of age-related disorders. J Alzheimers Dis. 2008;13(3):303–21.
Wen H, Jung H, Li X. Drug delivery approaches in addressing clinical pharmacology-related issues: opportunities and challenges. AAPS J. 2015;17:1327–40.
Kumar V. Target-oriented drug delivery systems. In Modern Pharmaceutics, vol 2. Boca Raton: CRC Press; 2016. pp. 347–416.
Lee S, Glendenning P, Inderjeeth C. Efficacy, side effects and route of administration are more important than frequency of dosing of anti-osteoporosis treatments in determining patient adherence: a critical review of published articles from 1970 to 2009. Osteoporos Int. 2011;22:741–53.
Wong KH, et al. Review of current strategies for delivering Alzheimer’s disease drugs across the blood-brain barrier. Int J Mol Sci. 2019;20(2):381.
Xie J, et al. Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials. 2019;224:119491.
Das SK, et al. Gene therapies for cancer: strategies, challenges and successes. J Cell Physiol. 2015;230(2):259–71.
Bandaranayake WM. Quality control, screening, toxicity, and regulation of herbal drugs. In: Ahmad I, Aqil F, Owais M, editors. Modern Phytomedicine. 2006. https://doi.org/10.1002/9783527609987.ch2
Geerts H, Grossberg GT. Pharmacology of acetylcholinesterase inhibitors and N-methyl‐D‐aspartate receptors for combination therapy in the treatment of Alzheimer’s disease. J Clin Pharmacol. 2006;46(S1):S8–16.
Haake A, et al. An update on the utility and safety of cholinesterase inhibitors for the treatment of Alzheimer’s disease. Exp Opin Drug Saf. 2020;19(2):147–57.
García-Morales V et al. Current understanding of the physiopathology, diagnosis and therapeutic approach to Alzheimer’s disease. Biomedicines. 2021;9(12):1910.
Choonara YE, et al. Improving drug delivery technology for treating neurodegenerative diseases. Expert Opin Drug Deliv. 2016;13(7):1029–43.
Zhou Y, et al. Crossing the blood-brain barrier with nanoparticles. J Controlled Release. 2018;270:290–303.
Furtado D, et al. Overcoming the blood–brain barrier: the role of nanomaterials in treating neurological diseases. Adv Mater. 2018;30(46):1801362.
Zhu X et al. Brain drug delivery by adsorption-mediated transcytosis, in Brain targeted drug delivery system. Amsterdam: Elsevier; 2019. pp. 159–183.
Tashima T. Smart strategies for therapeutic agent delivery into brain across the blood–brain barrier using receptor-mediated transcytosis. Chem Pharm Bull. 2020;68(4):316–25.
Grabrucker AM, et al. Nanoparticle transport across the blood brain barrier. Tissue Barriers. 2016;4(1):e1153568.
Fonseca-Santos B, Gremião MPD, Chorilli M. Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. Int J Nanomed. 2015;10:4981–5003.
Andrade S, et al. Transferrin-functionalized liposomes loaded with vitamin VB12 for Alzheimer’s disease therapy. Int J Pharm. 2022;626:122167.
Mutlu NB, et al. New perspective for the treatment of Alzheimer diseases: liposomal rivastigmine formulations. Drug Dev Ind Pharm. 2011;37(7):775–89.
Vasileva L, et al. Mitochondria-targeted delivery strategy of dual-loaded liposomes for Alzheimer’s Disease Therapy. Int J Mol Sci. 2023;24(13):10494.
Kuedo Z, et al. Oral administration of ethanolic extract of shrimp shells-loaded Liposome protects against Aβ-Induced memory impairment in rats. Foods. 2022;11(17):2673.
Li W, et al. Pharmacokinetic behavior and efficiency of acetylcholinesterase inhibition in rat brain after intranasal administration of galanthamine hydrobromide loaded flexible liposomes. Environ Toxicol Pharmacol. 2012;34(2):272–9.
Keerthana D, Kavitha R, Damodharan N. Niosome as a promising carrier targeting Alzheimer’s disease across the Blood Brain Barrier. NeuroQuantology. 2022;20(9):2453.
Kulkarni P, Rawtani D, Barot T. Design, development and in-vitro/in-vivo evaluation of intranasally delivered Rivastigmine and N-Acetyl Cysteine loaded bifunctional niosomes for applications in combinative treatment of Alzheimer’s disease. Eur J Pharm Biopharm. 2021;163:1–15.
Moulahoum H, et al. Potential effect of carnosine encapsulated niosomes in bovine serum albumin modifications. Int J Biol Macromol. 2019;137:583–91.
Ansari M, Eslami H. Preparation and study of the inhibitory effect of nano-niosomes containing essential oil from artemisia absinthium on amyloid fibril formation. Studies. 2020;12:21–5.
Sun K, et al. Exosomes as CNS drug delivery tools and their applications. Pharmaceutics. 2022;14(10):2252.
Chen Y-A, et al. Mesenchymal stem cell-derived exosomes ameliorate Alzheimer’s disease pathology and improve cognitive deficits. Biomedicines. 2021;9(6):594.
Reza-Zaldivar EE, et al. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer’s disease. Neural Regeneration Res. 2019;14(9):1626.
Cui G-h, et al. RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer’s disease. Immun Ageing. 2019;16(1):1–12.
Sheykhhasan M, et al. Neuroprotective effects of coenzyme Q10-loaded exosomes obtained from adipose-derived stem cells in a rat model of Alzheimer’s disease. Biomed Pharmacother. 2022;152:113224.
Jahangard Y, et al. Therapeutic effects of transplanted exosomes containing miR-29b to a rat model of Alzheimer’s disease. Front NeuroSci. 2020;14:564.
Fonseca LC, et al. Intranasal drug delivery for treatment of Alzheimer’s disease. Drug Delivery Translational Res. 2021;11:411–25.
Arun Raj R, Murali A. Formulation and evaluation of curcumin loaded transferosomal nasal in-situ gel for Alzheimer’s disease. Res Rev AJ Drug Formul Dev Prod. 2019;6(2):19–31.
Mishra G, et al. Intranasally co-administered Berberine and Curcumin Loaded in Transfersomal vesicles Improved inhibition of amyloid formation and BACE-1. ACS Omega. 2022;7(47):43290–305.
Nojoki F, et al. Design and development of chitosan-insulin-transfersomes (transfersulin) as effective intranasal nanovesicles for the treatment of Alzheimer’s disease: in vitro, in vivo, and ex vivo evaluations. Biomed Pharmacother. 2022;153:113450.
Mbah CC, Builders PF, Attama AA. Nanovesicular carriers as alternative drug delivery systems: ethosomes in focus. Expert Opin Drug Deliv. 2014;11(1):45–59.
Shi J, Wang Y, Luo G. Ligustrazine phosphate ethosomes for treatment of Alzheimer’s disease, in vitro and in animal model studies. AAPS PharmSciTech. 2012;13:485–92.
Barani M et al. Phytosomes as innovative delivery systems for phytochemicals: a comprehensive review of literature. Int J Nanomed. 2021;16:6983–7022.
Wattanathorn J et al. Memory-enhancing effect of a phytosome containing the combined extract of mulberry fruit and ginger in an animal model of ischemic stroke with metabolic syndrome. Oxidative Med Cell Longev. 2020;2020:3096826.
Ullah F, et al. Evaluation of phytosomal curcumin as an anti-inflammatory agent for chronic glial activation in the GFAP-IL6 mouse model. Front NeuroSci. 2020;14:170.
Barriga HM, Holme MN, Stevens MM. Cubosomes: the next generation of smart lipid nanoparticles? Angew Chem Int Ed. 2019;58(10):2958–78.
Elnaggar YS et al. Novel piperine-loaded tween-integrated monoolein cubosomes as brain-targeted oral nanomedicine in Alzheimer’s disease: pharmaceutical, biological, and toxicological studies. Int J Nanomed. 2015;10:5459–73.
Wu H, et al. A novel small odorranalectin-bearing cubosomes: preparation, brain delivery and pharmacodynamic study on amyloid-β25–35-treated rats following intranasal administration. Eur J Pharm Biopharm. 2012;80(2):368–78.
Yetisgin AA, et al. Therapeutic nanoparticles and their targeted delivery applications. Molecules. 2020;25(9):2193.
Rout GK et al. Current advances in nanocarriers for biomedical research and their applications. Artif Cells Nanomed Biotechnol. 2018;46(sup2):1053–1062.
Zhang J, et al. Neuroprotective effects of maize tetrapeptide-anchored gold nanoparticles in Alzheimer’s disease. Colloids Surf B. 2021;200:111584.
Hou K, et al. Chiral gold nanoparticles enantioselectively rescue memory deficits in a mouse model of Alzheimer’s disease. Nat Commun. 2020;11(1):4790.
dos Santos Tramontin N, et al. Gold nanoparticles treatment reverses brain damage in Alzheimer’s disease model. Mol Neurobiol. 2020;57:926–36.
Cunha A, et al. PLGA-based nanoparticles for neuroprotective drug delivery in neurodegenerative diseases. Pharmaceutics. 2021;13(7):1042.
Sánchez-López E, et al. Memantine loaded PLGA PEGylated nanoparticles for Alzheimer’s disease: in vitro and in vivo characterization. J Nanobiotechnol. 2018;16:1–16.
Silva-Abreu M, Calpena AC, Andrés-Benito P, Aso E, Romero IA, Roig-Carles D, et al. PPARγ agonist-loaded PLGA-PEG nanocarriers as a potential treatment for Alzheimer's disease: in vitro and in vivo studies. Int J Nanomedicine. 2018;13:5577–5590. https://doi.org/10.2147/IJN.S171490.
Jeon SG et al. Vitamin D-binding protein-loaded PLGA nanoparticles suppress Alzheimer’s disease-related pathology in 5XFAD mice. Nanomedicine. 2019;17:297–307.
Xu R et al. Rhynchophylline loaded-mPEG-PLGA nanoparticles coated with Tween-80 for preliminary study in Alzheimer’s disease. Int J Nanomed. 2020;15:1149–60.
Vilella A, et al. Reduced plaque size and inflammation in the APP23 mouse model for Alzheimer’s disease after chronic application of polymeric nanoparticles for CNS targeted zinc delivery. J Trace Elem Med Biol. 2018;49:210–21.
Scarpa E, et al. Gold and silver nanoparticles in Alzheimer’s and Parkinson’s diagnostics and treatments. Ibrain. 2023;9(3):298–315.
Zhang X, Li Y, Hu Y. Green synthesis of silver nanoparticles and their preventive effect in deficits in recognition and spatial memory in sporadic Alzheimer’s rat model. Colloids Surf a. 2020;605:125288.
Ittiyavirah SP, Ghosh R. Nootropic potential of silver nanoparticles of Boerhaavia diffusa and its ethanolic extract in high Fat Diet Model of Dementia in rats. Manipal J Pharm Sci. 2015;1(1):3.
Ramshini H, et al. Silver nano particles ameliorate learning and spatial memory of male Wistar rats by prevention of amyloid fibril-induced neurotoxicity. Arch Ital Biol. 2017;155:131–41.
Xu C, Qu X. Cerium oxide nanoparticle: a remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater. 2014;6(3):e90–90.
Danish SM, et al. Intranasal cerium oxide nanoparticles ameliorate cognitive function in rats with Alzheimer’s via anti-oxidative pathway. Pharmaceutics. 2022;14(4):756.
Hu Y et al. Functionalized Cerium Dioxide nanoparticles with Antioxidative Neuroprotection for Alzheimer’s Disease. Int J Nanomed. 2023;18:6797–812.
Wahle T, et al. Evaluation of neurological effects of cerium dioxide nanoparticles doped with different amounts of zirconium following inhalation exposure in mouse models of Alzheimer’s and vascular disease. Neurochem Int. 2020;138:104755.
Akhtar N, et al. ZnO nanoflower based sensitive nano-biosensor for amyloid detection. Mater Sci Engineering: C. 2017;78:960–8.
Abdulmalek S et al. Protective effect of natural antioxidant, curcumin nanoparticles, and zinc oxide nanoparticles against type 2 diabetes-promoted hippocampal neurotoxicity in rats. Pharmaceutics. 2021;13(11):1937.
Kesmati M, Sargholi Notarki Z, Issapareh N, Torabi M. Comparison the effect of zinc oxide and magnesium oxide nano particles on long term memory in adult male mice. Zahedan J Res Med Sci. 2016;18(9):e3473. https://doi.org/10.17795/zjrms-3473.
Huo X, et al. A novel synthesis of selenium nanoparticles encapsulated PLGA nanospheres with curcumin molecules for the inhibition of amyloid β aggregation in Alzheimer’s disease. J Photochem Photobiol B. 2019;190:98–102.
Gholamigeravand B, et al. Synergistic effects of adipose-derived mesenchymal stem cells and selenium nanoparticles on streptozotocin-induced memory impairment in the rat. Life Sci. 2021;272:119246.
Ji D, et al. Protective effects of chondroitin sulphate nano-selenium on a mouse model of Alzheimer’s disease. Int J Biol Macromol. 2020;154:233–45.
Sun D, et al. Chiral penicillamine-modified selenium nanoparticles enantioselectively inhibit metal-induced amyloid β aggregation for treating Alzheimer’s disease. J Colloid Interface Sci. 2017;505:1001–10.
Kaur J, et al. Advances in designing of polymeric micelles for biomedical application in brain related diseases. Chemico-Biol Interact. 2022;361:109960.
Hagl S, et al. Curcumin micelles improve mitochondrial function in a mouse model of Alzheimer’s disease. J Prev Alzheimers Dis. 2014;1:80–3.
Yang P, et al. Neuronal mitochondria-targeted micelles relieving oxidative stress for delayed progression of Alzheimer’s disease. Biomaterials. 2020;238:119844.
Wasiak T, et al. Cationic phosphorus dendrimers and therapy for Alzheimer’s disease. New J Chem. 2015;39(6):4852–9.
Gothwal A, et al. Behavioral and biochemical implications of dendrimeric rivastigmine in memory-deficit and Alzheimer’s induced rodents. ACS Chem Neurosci. 2019;10(8):3789–95.
Gothwal A, et al. Lactoferrin coupled lower generation PAMAM dendrimers for brain targeted delivery of memantine in aluminum-chloride-induced Alzheimer’s disease in mice. Bioconjug Chem. 2019;30(10):2573–83.
Klementieva O, et al. Effect of poly (propylene imine) glycodendrimers on β-amyloid aggregation in vitro and in APP/PS1 transgenic mice, as a model of brain amyloid deposition and Alzheimer’s disease. Biomacromolecules. 2013;14(10):3570–80.
Kaur A, et al. Memantine nanoemulsion: a new approach to treat Alzheimer’s disease. J Microencapsul. 2020;37(5):355–65.
Song Y et al. Osthole-loaded nanoemulsion enhances brain target in the treatment of Alzheimer’s disease via intranasal administration. Oxidative Med Cell Longev. 2021;2021:8844455.
Alaqeel NK, AlSheikh MH, Al-Hariri MT. Quercetin nanoemulsion ameliorates neuronal dysfunction in experimental Alzheimer’s disease model. Antioxidants. 2022;11(10):1986.
Ismail N, et al. Thymoquinone-rich fraction nanoemulsion (TQRFNE) decreases Aβ40 and Aβ42 levels by modulating APP processing, up-regulating IDE and LRP1, and down-regulating BACE1 and RAGE in response to high fat/cholesterol diet-induced rats. Biomed Pharmacother. 2017;95:780–8.
Beniwal SS, et al. Evaluation of the neuroprotective activity of citral nanoemulsion on Alzheimer’s disease-type dementia in a preclinical model: the assessment of cognitive and neurobiochemical responses. Life. 2023;1(1):9–17.
Sadegh Malvajerd S, et al. Neuroprotective potential of curcumin-loaded nanostructured lipid carrier in an animal model of Alzheimer’s disease: behavioral and biochemical evidence. J Alzheimers Dis. 2019;69(3):671–86.
Shehata MK, Ismail AA, Kamel MA. Combined donepezil with astaxanthin via nanostructured lipid carriers effective delivery to brain for Alzheimer’s disease in rat model. Int J Nanomed. 2023;18:4193–227.
Shehata MK, Ismail AA, Kamel MA. Nose to brain delivery of astaxanthin-loaded nanostructured lipid carriers in rat model of alzheimer's disease: preparation, in vitro and in vivo evaluation. Int J Nanomedicine. 2023;18:1631-1658. https://doi.org/10.2147/IJN.S402447.
Anand A, et al. Sucrose stearate as a biosurfactant for development of rivastigmine containing nanostructured lipid carriers and assessment of its activity against dementia in C. Elegans model. J Drug Deliv Sci Technol. 2019;49:219–26.
Salve P, Pise S, Bali N. Formulation and evaluation of solid lipid nanoparticle based Transdermal Drug Delivery System for Alzheimer’s Disease. Res J Pharm Dosage Forms Technol. 2016;8(2):73–80.
Shivananjegowda MG, et al. Development and evaluation of solid lipid nanoparticles for the clearance of Aβ in Alzheimer’s disease. Pharmaceutics. 2023;15(1):221.
Saini S, et al. Systematically designed chitosan-coated solid lipid nanoparticles of ferulic acid for effective management of Alzheimer’s disease: a preclinical evidence. Colloids Surf B. 2021;205:111838.
Dara T, et al. Improvement of memory deficits in the rat model of Alzheimer’s disease by erythropoietin-loaded solid lipid nanoparticles. Neurobiol Learn Mem. 2019;166:107082.
Campisi A, et al. Effect of unloaded and curcumin-loaded solid lipid nanoparticles on tissue transglutaminase isoforms expression levels in an experimental model of Alzheimer’s disease. Antioxidants. 2022;11(10):1863.
Kumar S, et al. Carbon nanotubes: a novel material for multifaceted applications in human healthcare. Chem Soc Rev. 2017;46(1):158–96.
Yang Z, et al. Pharmacological and toxicological target organelles and safe use of single-walled carbon nanotubes as drug carriers in treating Alzheimer disease. Volume 6. Nanomedicine: Nanotechnology, Biology and Medicine; 2010. pp. 427–41. 3.
Xue X, et al. Single-walled carbon nanotubes alleviate autophagic/lysosomal defects in primary glia from a mouse model of Alzheimer’s disease. Nano Lett. 2014;14(9):5110–7.
Ranjan P, Khan R. Electrochemical Immunosensor for early detection of β-amyloid alzheimer’s disease biomarker based on aligned carbon nanotubes gold nanocomposites. Biosensors. 2022;12(11):1059.
Ho T-C, et al. Hydrogels: Properties and applications in biomedicine. Molecules. 2022;27(9):2902.
Ribeiro TdC et al. Curcumin-loaded mesoporous silica nanoparticles dispersed in thermo-responsive hydrogel as potential Alzheimer disease therapy. Pharmaceutics. 2022;14(9):1976.
Ou G, et al. Preventive effect of nasal timosaponin BII-loaded temperature-/ion-sensitive in situ hydrogels on Alzheimer’s disease. J Traditional Chin Med Sci. 2021;8(1):59–64.
Chen W, et al. Nasal timosaponin BII dually sensitive in situ hydrogels for the prevention of Alzheimer’s disease induced by lipopolysaccharides. Int J Pharm. 2020;578:119115.
Rajkumar M, et al. Galantamine tethered hydrogel as a novel therapeutic target for streptozotocin-induced Alzheimer’s disease in Wistar rats. Curr Res Pharmacol Drug Discovery. 2022;3:100100.
Hernandez C, Shukla S. Liposome based drug delivery as a potential treatment option for Alzheimer’s disease. Neural Regeneration Res. 2022;17(6):1190–8.
Ravouru N, Kondreddy P, Korakanchi D. Formulation and evaluation of niosomal nasal drug delivery system of folic acid for brain targeting. Curr Drug Discov Technol. 2013;10(4):270–82.
Kandimalla R, et al. Exosome-based approaches in the management of Alzheimer’s disease. Neurosci Biobehavioral Reviews. 2023;144:104974.
Rajamma SS, et al. Geophila repens phytosome-loaded intranasal gel with improved nasal permeation for the effective treatment of Alzheimer’s disease. J Drug Deliv Sci Technol. 2022;69:103087.
Roney C, et al. Targeted nanoparticles for drug delivery through the blood–brain barrier for Alzheimer’s disease. J Controlled Release. 2005;108(2–3):193–214.
Sivanesan S, Rajeshkumar S. Gold nanoparticles in diagnosis and treatment of alzheimer’s disease. In: Rai M, Yadav A, editors. Nanobiotechnology in neurodegenerative diseases. Springer, Cham; 2019. https://doi.org/10.1007/978-3-030-30930-5_12
Zeng H, et al. The impacts of curcumin on learning memory function in Alzheimer’s disease under the poly lactic-co-glycolic acid nanoparticle drug carrier. Appl Nanosci. 2023;13(5):3483–91.
El-Hawwary SS, et al. Green-synthesized zinc oxide nanoparticles, anti-alzheimer potential and the metabolic profiling of Sabal Blackburniana grown in Egypt supported by molecular modelling. RSC Adv. 2021;11(29):18009–25.
Sun J, et al. Progressive release of mesoporous nano-selenium delivery system for the multi-channel synergistic treatment of Alzheimer’s disease. Biomaterials. 2019;197:417–31.
Poudel P, Park S. Recent advances in the treatment of Alzheimer’s disease using nanoparticle-based drug delivery systems. Pharmaceutics. 2022;14(4):835.
Singh A, et al. Dendrimers: a neuroprotective lead in alzheimer disease: a review on its synthetic approach and applications. Drug Res. 2022;72(08):417–23.
Kaur A, et al. Treatment of Alzheimer’s diseases using donepezil nanoemulsion: an intranasal approach. Drug Delivery Translational Res. 2020;10:1862–75.
Cunha S et al. Improving drug delivery for Alzheimer’s disease through nose-to-brain delivery using nanoemulsions, nanostructured lipid carriers (NLC) and in situ hydrogels. Int J Nanomed. 2021;16:4373–90.
Mohajeri M, et al. Carbon nanomaterials and amyloid-beta interactions: potentials for the detection and treatment of Alzheimer’s disease? Pharmacol Res. 2019;143:186–203.
Nunes D, Loureiro JA, Pereira MC. Drug delivery systems as a strategy to improve the efficacy of FDA-approved Alzheimer’s drugs. Pharmaceutics. 2022;14(11):2296.
Lohan S, et al. Anti-alzheimer’s potential of berberine using surface decorated multi-walled carbon nanotubes: a preclinical evidence. Int J Pharm. 2017;530(1–2):263–78.
Sorokina SA, et al. Disruption of amyloid prion protein aggregates by cationic pyridylphenylene dendrimers. Macromol Biosci. 2016;16(2):266–75.
Ali T, et al. Anthocyanin-loaded PEG-gold nanoparticles enhanced the neuroprotection of anthocyanins in an Aβ 1–42 mouse model of Alzheimer’s disease. Mol Neurobiol. 2017;54:6490–506.
Ruff J, et al. The effects of gold nanoparticles functionalized with ß-amyloid specific peptides on an in vitro model of blood–brain barrier. Nanomed Nanotechnol Biol Med. 2017;13(5):1645–52.
Conti E, et al. Multifunctional liposomes interact with Abeta in human biological fluids: therapeutic implications for Alzheimer’s disease. Neurochem Int. 2017;108:60–5.
Loureiro JA, et al. Cellular uptake of PLGA nanoparticles targeted with anti-amyloid and anti-transferrin receptor antibodies for Alzheimer’s disease treatment. Colloids Surf B. 2016;145:8–13.
Kuo Y-C et al. Wheat germ agglutinin-conjugated liposomes incorporated with cardiolipin to improve neuronal survival in Alzheimer’s disease treatment. Int J Nanomed. 2017;12:1757–74.
Karimzadeh M, Rashidi L, Ganji F. Mesoporous silica nanoparticles for efficient rivastigmine hydrogen tartrate delivery into SY5Y cells. Drug Dev Ind Pharm. 2017;43(4):628–36.
Yang L, et al. Gold nanoparticle-capped mesoporous silica-based H2O2-responsive controlled release system for Alzheimer’s disease treatment. Acta Biomater. 2016;46:177–90.
Mirsadeghi S, et al. Effect of PEGylated superparamagnetic iron oxide nanoparticles (SPIONs) under magnetic field on amyloid beta fibrillation process. Mater Sci Engineering: C. 2016;59:390–7.
Liu Y, et al. Brain-targeted co-delivery of therapeutic gene and peptide by multifunctional nanoparticles in Alzheimer’s disease mice. Biomaterials. 2016;80:33–45.
Elnaggar YS, et al. Intranasal piperine-loaded chitosan nanoparticles as brain-targeted therapy in Alzheimer’s disease: optimization, biological efficacy, and potential toxicity. J Pharm Sci. 2015;104(10):3544–56.
Liu H, et al. Iminodiacetic acid-conjugated nanoparticles as a bifunctional modulator against Zn2+-mediated amyloid β-protein aggregation and cytotoxicity. J Colloid Interface Sci. 2017;505:973–82.
Liu H, et al. Synergistic effects of negatively charged hydrophobic nanoparticles and (–)-epigallocatechin-3-gallate on inhibiting amyloid β-protein aggregation. J Colloid Interface Sci. 2017;491:305–12.
Kwon HJ, et al. Mitochondria-targeting ceria nanoparticles as antioxidants for Alzheimer’s disease. ACS Nano. 2016;10(2):2860–70.
Rassu G, et al. Nose-to-brain delivery of BACE1 siRNA loaded in solid lipid nanoparticles for Alzheimer’s therapy. Colloids Surf B. 2017;152:296–301.
Loureiro JA, et al. Resveratrol and grape extract-loaded solid lipid nanoparticles for the treatment of Alzheimer’s disease. Molecules. 2017;22(2):277.
Misra S, et al. Galantamine-loaded solid–lipid nanoparticles for enhanced brain delivery: Preparation, characterization, in vitro and in vivo evaluations. Drug Delivery. 2016;23(4):1434–43.
Kuo Y-C, Rajesh R. Nerve growth factor-loaded heparinized cationic solid lipid nanoparticles for regulating membrane charge of induced pluripotent stem cells during differentiation. Mater Sci Engineering: C. 2017;77:680–9.
Polchi A, et al. Rapamycin loaded solid lipid nanoparticles as a new tool to deliver mTOR inhibitors: formulation and in vitro characterization. Nanomaterials. 2016;6(5):87.
Sims JR, et al. Donanemab in early symptomatic Alzheimer Disease: the TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA. 2023;330(6):512–27.
Galasko DR, et al. Antioxidants for Alzheimer Disease: a Randomized Clinical Trial with Cerebrospinal Fluid Biomarker measures. Arch Neurol. 2012;69(7):836–41.
Coric V, et al. Targeting Prodromal Alzheimer Disease with Avagacestat: a Randomized Clinical Trial. JAMA Neurol. 2015;72(11):1324–33.
Geldmacher DS, et al. A Randomized Pilot Clinical Trial of the safety of Pioglitazone in treatment of patients with Alzheimer Disease. Arch Neurol. 2011;68(1):45–50.
Porsteinsson AP, et al. Effect of Citalopram on Agitation in Alzheimer Disease: the CitAD Randomized Clinical Trial. JAMA. 2014;311(7):682–91.
van Dyck CH, et al. Effect of AZD0530 on cerebral metabolic decline in Alzheimer Disease: a Randomized Clinical Trial. JAMA Neurol. 2019;76(10):1219–29.
Galasko D, et al. Clinical trial of an inhibitor of RAGE-Aβ interactions in Alzheimer disease. Neurology. 2014;82(17):1536–42.
Rogers J, et al. Clinical trial of indomethacin in Alzheimer’s disease. Neurology. 1993;43(8):1609–1609.
Tuszynski MH, et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;11(5):551–5.
Craft S, et al. Intranasal Insulin Therapy for Alzheimer Disease and Amnestic mild cognitive impairment: a Pilot Clinical Trial. Arch Neurol. 2012;69(1):29–38.
Sperling R et al. Findings of Efficacy, Safety, and Biomarker Outcomes of Atabecestat in Preclinical Alzheimer Disease: A truncated randomized phase 2b/3 clinical trial. JAMA Neurology. 2021;78(3):293–301.
Atri A, et al. Effect of Idalopirdine as Adjunct to Cholinesterase inhibitors on change in cognition in patients with Alzheimer Disease: three randomized clinical trials. JAMA. 2018;319(2):130–42.
Mintzer J, et al. Risperidone in the treatment of psychosis of Alzheimer Disease: results from a prospective clinical trial. Am J Geriatric Psychiatry. 2006;14(3):280–91.
https://clinicaltrials.gov/study/NCT05686044?cond=alzheimer&aggFilters=status:act&rank=2 (Accessed 20 Dec 2023).
https://clinicaltrials.gov/study/NCT05696483?cond=alzheimer&aggFilters=status:act&rank=6 (Accessed 20 Dec 2023).
https://clinicaltrials.gov/study/NCT05181475?cond=alzheimer&aggFilters=status:act&rank=10 (Accessed 20 Dec 2023).
https://clinicaltrials.gov/study/NCT05063539?cond=alzheimer&aggFilters=status:act&page=2&rank=12 (Accessed 20 Dec 2023).
https://clinicaltrials.gov/study/NCT05058040?cond=alzheimer&aggFilters=status:act&page=2&rank=13 (Accessed 20 Dec 2023).
https://clinicaltrials.gov/study/NCT05026177?cond=alzheimer&aggFilters=status:act&page=2&rank=14 (Accessed 20 Dec 2023).
https://clinicaltrials.gov/study/NCT04994483?cond=alzheimer&aggFilters=status:act&page=2&rank=15 (Accessed 20 Dec 2023).
https://clinicaltrials.gov/study/NCT04592874?cond=alzheimer&aggFilters=status:act&page=2&rank=19 (Accessed 20 Dec 2023).
https://clinicaltrials.gov/study/NCT04437511?cond=alzheimer&aggFilters=status:act&page=2&rank=20 (Accessed 20 Dec 2023).
https://clinicaltrials.gov/study/NCT04388254?cond=alzheimer&aggFilters=status:act&page=3&rank=21 (Accessed 20 Dec 2023).
https://clinicaltrials.gov/study/NCT04381468?cond=alzheimer&aggFilters=status:act&page=3&rank=22 (Accessed 22 Dec 2023).
https://clinicaltrials.gov/study/NCT03821857?cond=alzheimer&aggFilters=status:act&page=3&rank=27 (Accessed 22 Dec 2023).
https://clinicaltrials.gov/study/NCT03748303?cond=alzheimer&aggFilters=status:act&page=3&rank=30 (Accessed 22 Dec 2023).
https://clinicaltrials.gov/study/NCT03402503?cond=alzheimer&aggFilters=status:act&page=4&rank=31 (Accessed 22 Dec 2023).
https://clinicaltrials.gov/study/NCT04451408?cond=alzheimer&aggFilters=status:act&page=4&rank=40 (Accessed 22 Dec 2023).
https://clinicaltrials.gov/study/NCT05015374?cond=alzheimer&aggFilters=status:act&page=5&rank=41 (Accessed 22 Dec 2023).
https://clinicaltrials.gov/study/NCT06019117?cond=alzheimer&aggFilters=status:act&page=5&rank=42 (Accessed 22 Dec 2023).
https://clinicaltrials.gov/study/NCT04777409?cond=alzheimer&aggFilters=status:act&page=5&rank=43 (Accessed 23 Dec 2023).
https://clinicaltrials.gov/study/NCT04552795?cond=alzheimer&aggFilters=status:act&page=5&rank=48 (Accessed 23 Dec 2023).
https://clinicaltrials.gov/study/NCT04795466?cond=alzheimer&aggFilters=status:act&page=6&rank=53 (Accessed 23 Dec 2023).
https://clinicaltrials.gov/study/NCT04619420?cond=alzheimer&aggFilters=status:act&page=6&rank=57 (Accessed 23 Dec 2023).
https://clinicaltrials.gov/study/NCT04200911?cond=alzheimer&aggFilters=status:act&page=6&rank=58 (Accessed 23 Dec 2023).
https://clinicaltrials.gov/study/NCT05328115?cond=alzheimer&aggFilters=status:act&page=7&rank=64 (Accessed 23 Dec 2023).
https://clinicaltrials.gov/study/NCT03185208?cond=alzheimer&aggFilters=status:act&page=8&rank=74 (Accessed 23 Dec 2023).
https://clinicaltrials.gov/study/NCT03282916?cond=alzheimer&aggFilters=status:act&page=8&rank=78 (Accessed 23 Dec 2023).
https://patents.google.com/patent/US20190338363A1/en?q=(Alzheimer)&oq=Alzheimer (Accessed 27 Dec 2023).
https://patents.google.com/patent/AU2009202023B2/en?q=(alzheimer)&oq=alzheimer (Accessed 27 Dec 2023).
https://patents.google.com/patent/US7640062B2/en?q=(alzheimer)&oq=alzheimer (Accessed 27 Dec 2023).
https://patents.google.com/patent/JP7182316B2/en?q=(Alzheimer)&oq=Alzheimer (Accessed 27 Dec 2023).
https://patents.google.com/patent/EP2086538B1/en?q=(Alzheimer)&oq=Alzheimer (Accessed 27 Dec 2023).
https://patents.google.com/patent/US8921321B2/en?q=(Alzheimer)&oq=Alzheimer (Accessed 27 Dec 2023).
https://patents.google.com/patent/JP6784646B2/en?q=(Alzheimer)&oq=Alzheimer (Accessed 27 Dec 2023).
https://patents.google.com/patent/JP7210620B2/en?q=(Alzheimer)&oq=Alzheimer&page=1 (Accessed 29 Dec 2023).
https://patents.google.com/patent/AU2019202459B2/en?q=(Alzheimer)&oq=Alzheimer&page=1. Accessed 29 Dec 2023.
https://patents.google.com/patent/JP6864763B2/en?q=(Alzheimer)&oq=Alzheimer&page=1 (Accessed 29 Dec 2023).
https://patents.google.com/patent/US9248104B2/en?q=(Alzheimer)&oq=Alzheimer&page=2 (Accessed 29 Dec 2023).
https://patents.google.com/patent/CA2722314C/en?q=(Alzheimer)&oq=Alzheimer&page=2 (Accessed 29 Dec 2023).
https://patents.google.com/patent/US20220406435A1/en?q=(Alzheimer)&oq=Alzheimer&page=2 (Accessed 30 Dec 2023).
https://patents.google.com/patent/CN103842362B/en?q=(Alzheimer)&oq=Alzheimer&page=2 (Accessed 30 Dec 2023).
https://patents.google.com/patent/EP2001503B1/en?q=(Alzheimer)&oq=Alzheimer&page=2 (Accessed 30 Dec 2023).
Author information
Authors and Affiliations
Contributions
Devank Shekho and Ritika Mishra wrote the manuscript; Raj Kamal prepared diagram and edited the manuscript; and Ankit Awasthi conceptualized the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shekho, D., Mishra, R., Kamal, R. et al. Breaking Barriers in Alzheimer’s Disease: the Role of Advanced Drug Delivery Systems. AAPS PharmSciTech 25, 207 (2024). https://doi.org/10.1208/s12249-024-02923-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-024-02923-6