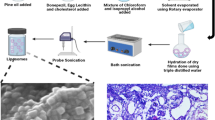
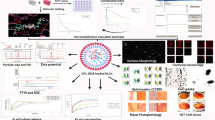
Abstract
Alzheimer disease (AD) is very common among the older people. There are few medications available as oral and suspension dosage forms for the management of AD. Due to the rising cases of AD and the associated risks of the existing line of treatment, oil in water (o/w) nanoemulsion (NE) loaded with donepezil was prepared to explore intranasal route of administration. The NE was prepared using labrasol (10%), cetyl pyridinium chloride (1% in 80% water), and glycerol (10%), with a drug concentration of 1 mg/ml. The developed NE was characterized for particle size, polydispersity index (PDI), and zeta potential. In vitro release studies were conducted to observe the release of drug. Further in vivo studies of developed NE were done on Sprague Dawley rats using technetium pertechnetate (99mTc) labeled formulations to investigate the nose to brain drug delivery pathway. The nanoemulsion showed particle size of 65.36 nm with a PDI of 0.084 and zeta potential of −10.7 mV. In vitro release studies showed maximum release of 99.22% in 4 h in phosphate-buffered saline, 98% in 2 h in artificial cerebrospinal fluid, and 96% in 2 h in simulated nasal fluid. The cytotoxicity and antioxidant activity of the NE showed dose-dependent cytotoxicity and % radical scavenging activity (%RSA). The images of giemsa staining also confirmed that the developed formulation has no impact on the morphology of cells. Scintigrams showed maximum uptake of NE in the brain. The findings suggested that the developed NE loaded with donepezil hydrochloride could serve as a new approach for the treatment of Alzheimer via nose to brain drug delivery.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer disease is a brain disorder which occurs due to the degeneration of neurons. It is characterized by memory loss, agitation, confusions, and lack of daily activities [1]. This disorder occurs due to the development of amyloid plaques [2] and neurofibrillary tangles [3]. It progresses at a gradual rate initially and worsens the condition until the death of the patient. According to WHO report of 2015, annually 7.7 million new patients are reported suffering from dementia, among which Alzheimer is the cause behind two thirds of the reported cases [4].
Cholinesterase inhibitors (ChE) are the compounds that are used for symptomatic treatment by improvement in neural in AD. Due to the decrease in cortical activity of acetylcholine (ACh) levels in AD, acetylcholinesterase (AChE), which is responsible for the metabolism of ACh in synaptic space, is the main drug target for the treatment of AD [5, 6]. The two drug classes currently available for the treatment of AD are uncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist, memantine, and acetylcholinesterase inhibitors (AChEIs: donepezil, galantamine, and rivastigmine, which all raise ACh levels) [7]. Donepezil is an inhibitor of acetylcholinesterase enzyme and is the first choice of drug for the management of AD patients. It enhances the concentration of acetylcholine in the brain (ACh) [8] and possess protective effect on the neuroinflammation occurred in the brains of AD patients [9]. Currently, donepezil is available as oral formulation (5–10 mg) and is associated with major gastrointestinal side effects [10]. Moreover, it is a hydrophilic drug, unable to cross blood brain barrier efficiently and very less drug concentration reached after oral administration [10]. So an effective alternative option which helps in masking the hydrophilic nature of the drug and also enhances the concentration in the brain is required urgently. Few studies have been reported in which nanoparticles of donepezil were prepared and explored via intravenous routes. Bhawna et al. [11] prepared PLGA nanoparticles of donepezil hydrochloride administered via intravenous and oral route. The results confirmed less delivery of drug via intravenous route in the brain (121.68 ± 13.23 ng/ml at 6 h) with higher amount of drug absorption in the peripheral organs like the liver and kidney. These studies suggested that some direct routes of delivery to the brain such as intranasal delivery must be explored further which not only minimize the distribution of drug in the peripheral organs but also enhance the amount of distribution at the target site i.e., the brain via bypassing blood brain barrier.
In the present work, nose to brain delivery of donepezil was attempted by preparing a nanoemulsion (NE) formulation. Nanoemulsions are colloidal particulate system which has a higher solubilization capacity than simple micellar solutions. These are thermodynamically stable which offers advantages over unstable dispersions (emulsions and suspensions), because they can be manufactured with little energy input (heat or mixing) [12]. Moreover, development of nanoparticles loaded with the drug molecule is a difficult and complex process, which requires high-energy methods and costly process due to the use of expensive polymers [12]. Because of lipophilic nature and low globule size of the nanoemulsion, they are widely explored as a delivery system to enhance uptake across the nasal mucosa. Few studies confirmed that nanoemulsions can be effectively delivered through intranasal route and showed higher uptake in the brain and are capable to protect the encapsulated drug from biological and chemical degradation [13]. Nanoemulsions can be administered effortlessly and are cost-effective and convenient to use. Moreover, circumventing blood brain barrier for direct transport of drugs following nasal administration provides an alternate best solution for transport of drugs in the target organ (brain) [11, 13].
Patient compliance is a big concern in AD patients; this noninvasive delivery system could be administered to supine patients also. Moreover, AD patients can be more compliant to their treatment if the dosing schedule is not too frequent (i.e., a once a day regime) with respect to multiday fractionated administrations. Nose to brain drug delivery ensures targeting of therapeutics to the CNS with rapid achievement of drug level in the target tissue with less dosing schedules and fewer side effects with avoidance of the first pass metabolism. Donepezil-loaded nanoemulsion was prepared by homogenization and ultrasonication method to determine the pharmacokinetic parameters in the blood and brain of Sprague Dawley rats.
Materials and methods
Materials
Donepezil was gifted by Sun Pharmaceuticals private limited, Mumbai, Maharashtra, India. Labrasol was also a gift sample from Gattefosse, Mumbai, and Maharashtra, India. Tween 20, soybean oil, propylene glycol, cetyl pyridinium chloride, and chitosan were purchased from Sigma-Aldrich (Bangalore, India). Technetium pertechnetate was obtained from the Regional Center for Radiopharmaceuticals of the Board of Radiation and Isotope Technology (BRIT), Delhi, India. Glycerol was a product of CDH (P) Ltd., India. Water used was Milli-Q (Millipore, USA). All other solvents were of HPLC grade. Fetal bovine serum was obtained from Himedia Laboratories, India (Cat no. RM1112, gamma irradiated, sterile, cell culture grade supplement, European Union approved).
Animal preparation
All animal experiments were carried out in compliance with the Institute of Nuclear Medicine and Allied Sciences (INMAS) Institutional Animal Ethics Committee (IAEC), New Delhi, India, vide number INM/IAEC/16/10, and their guidelines were followed throughout the study. Sprague Dawley rats (male/female 2–3 months) weighing 180–200 g obtained were from the Central Animal House Facility of INMAS, Delhi, India. All animals were given normal feed and filtered drinking water ad libitum. Rats were kept at room temperature of 25 ± 5 °C.
Ultraviolet spectrophotometric analysis of donepezil
Stock solution of donepezil (50 μg/ml) was prepared in methanol, and dilutions were prepared in the range of 5‑50 μg/ml [14]. Maximum absorbance (λmax) was observed for the stock solution using UV/Vis spectrophotometry, Shimadzu. After finalizing the λmax, standard plot was prepared by observing absorbance of the diluted samples, and regression equation was calculated.
Preparation and optimization of nanoemulsion
For the preparation of nanoemulsion, the solubility of the donepezil was assessed in various oils, surfactants, and co-surfactants. Different combinations were prepared with selected oils, surfactants, and co-surfactants by mixing the ingredients followed by vortexing. The developed combinations were kept overnight to check for their stability on the basis of miscibility and transparency. Finally, the nanoemulsion was prepared by pre-homogenization step followed by ultrasonication. The pre-emulsion was subjected to high-shear homogenization using Tissue Master 125 homogenizer, Omni International, Georgia under ice bath and further subjected to high energy ultrasonication via Bench Top Ultrasonicator, Model UP400S, 24 KHz 400 W, Hielcher, Ultrasound Technology, Germany to prepare the final NE [15].
Measurement of transmittance
Transmittance percentage of the developed NEs was measured to determine the clarity of prepared NEs using UV/Vis spectrophotometer, Shimadzu at 650 nm. mili Q water was used as a blank while measurements were made in triplicate [16].
Characterization of developed NE for particle size and zeta potential
Average particle size, polydispersity index (PDI), and zeta potential were determined by using a Zetasizer, 100 HS, Malvern Instruments, Worcestershire, UK. Experiment was performed in triplicates by diluting the donepezil-loaded nanoemulsion and placebo respectively to 1/50 v/v in water [16].
In vitro drug release study
The in vitro release studies were carried out by using dialysis bag diffusion technique at 100 rpm. Phosphate-buffered saline (PBS) pH 7.4 [17], artificial cerebrospinal fluid (ACSF) [18], and simulated nasal fluid (SNF) pH 5–5.5 [19] (method of preparation given in supplementary file) were used as medias to check the release of drug. Two milliliters of NE sample enclosed in each dialysis bags (cellulose membrane mw cut-off 12,400, sigma) was incubated in 500 ml of simulated medias at 37 °C under agitation in USP II dissolution test apparatus. Samples were collected at predetermined time intervals and analyzed for donepezil content after suitable dilutions by UV spectrophotometric method at absorbance of 316 nm. The volume of fluid was replaced by equivalent amount of media to maintain the sink conditions. The percentage was calculated to check the drug release from NE.
Cytotoxicity analysis
Cell viability analysis of developed NE was performed on neuroblastoma cells (Neuro 2a) using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [20]. The cells were maintained in Dulbecco’s modified Eagle’s media (DMEM) supplemented with 10% fetal bovine serum, at 37 °C in humidified 5% CO2/ 95% air incubator (New Brunswick, Germany). Neuro 2a cells were seeded at a concentration of 105 cells/well in 96-well tissue culture plate (HiMedia, India) and allowed to adhere overnight. Different concentrations of optimized donepezil NE (Cmax value (6 ng/ml) of the active ingredient (donepezil) of NE: Cmax/2, Cmax, 2*Cmax, 4*Cmax, and 40*Cmax) at different concentrations and placebo were added in triplicates and incubated under normal conditions for 24 h. After incubation, 20 μl of MTT was added to each well and replaced after 4 h with 200 μl of dimethyl sulfoxide (DMSO) to dissolve the insoluble formazan. The absorbance was measured at 570 nm using an ELISA microplate reader (Bio-Rad, Hercules, CA). Untreated cells and media were taken as positive control and blank, respectively. Percentage cell viability was calculated using Eq. 1.
where Af is the absorbance obtained for cells treated with formulations and Ac is the absorbance obtained for positive control.
Giemsa staining
It is a technique used for identifying the morphology of the cells under the microscope. This stain is composed of three dyes with acidic and basic nature: methylene blue, eosin, and azure dye which possess affinity for the basic and acidic components of cells and stained accordingly. For the staining of cells, the Neuro 2a cells were seeded in the six well plates and cultured in the fresh complete media for overnight. Then, the cells were treated with donepezil NE, placebo, and aqueous drug solution at concentration of Cmax and 10* Cmax and incubated for 24 h. After 24 h treatment period, the cells were washed with PBS and fixed with 100% cold methanol. The freshly prepared giemsa solution (5%) was added to each well and incubated for 15 min at room temperature. After staining, images were taken under microscope at × 40 and × 20 magnifications to check any morphological changes in the structure of cells after treatment [21].
In vivo preliminary analysis
Radiolabeling of the nanoemulsion formulation
Technetium pertechnetate (99mTc) was used for the radiolabeling of donepezil by the simple reducing method using stannous chloride (SnCl2 H2O) as a reducing agent (1 mg/ml in 10% acetic acid) [22]. The pH of the solutions was measured and adjusted accordingly. The solutions were then passed through a 0.22-μm Millipore filter (Billerica, MA) after which 2 mCi of activity (99mTc) was added dropwise in solution, and the mixture was incubated at room temperature for 30 min. Radiolabeling efficiency was determined using instant thin layer chromatography (ITLC-SG) strips with 100% acetone as mobile phase at 15 and 60 min after filtration. This radiolabeled donepezil was further used for the preparation of radiolabeled donepezil nanoemulsion (DNE) and radiolabeled aqueous drug solution (DS). The radiolabeling efficiency of formulation was also determined using Eq. 2.
Gamma scintigraphic analysis
The SD rats weighing between 200 and 250 g were selected for the study. Three rats per group as discussed above were used (n = 12). The following were the group of animals used for the study:
-
Group I - Rats administered 99mTC- DNE intranasally (i.n)
-
Group II - Rats administered 99mTC-DS intranasally (i.n)
-
Group III - Rats administered 99mTC- DNE orally
-
Group IV - Rats administered 99mTC- DNE intravenously (i.v)
99mTc-DNE and 99mTc-DS were injected i.n, orally, and i.v in the SD rats. The rats were anesthetized using 0.4-ml ketamine hydrochloride via intraperitonial injection (50 mg/ml) and placed on the imaging board. The localization of drug was visualized using single-photon emission computerized tomography (SPECT), provided by GE Healthcare System (Hawkeye Millennium VG, GE Medical Systems, Milwaukee, WI), and the scintigraphic images were recorded at predetermined time points 30 min, 1.5 h, 3 h, 6 h, and 24 h using the eNTEGRA software (Hawkeye Millennium VG, Milwaukee, WI).
Biodistribution studies of intranasal nanoemulsion formulation
Sprague Dawley (SD) rats (aged 4–5 months) weighing between 180 and 200 g were selected for the study. Three rats for each formulation per time points were used in the study. Radiolabeled drug aqueous solution (99mTc-DS) and radiolabeled donepezil nanoemulsion formulation (99mTc-DNE) (200 mCi/100 ml) containing 0.09 mg donepezil (equivalent to 0.45 mg/kg body weight) were administered [22] according to following protocol (total rats used = 60):
-
Group I - Rats administered 99mTC- DNE intranasally (i.n)
-
Group II - Rats administered 99mTC-DS intranasally (i.n)
-
Group III - Rats administered 99mTC- DNE orally
-
Group IV - Rats administered 99mTC- DNE intravenously (i.v)
The intranasal administration was done with the help of 2–20-μl micropipette, and rats were placed in supine position for 30–40 s after administration. The blood samples were collected by retro orbital puncture and rats were sacrificed at respective time intervals of 30 min, 1.5 h, 3 h, 6 h, and 24 h. Subsequently, brains were dissected, washed twice using normal saline, made free from adhering tissue/fluid, and weighed. Radioactivity present in each tissue/organ was measured using shielded well type gamma scintillation counter. Radio pharmaceutical uptake per gram in each tissue/organ was calculated as a fraction of administered dose using Eq. 3:
The various pharmacokinetic parameters, such as Cmax, Tmax, area under the curve (AUC0–24), area under the first moment curve (AUMC0–24), mean residence time (MRT), elimination rate constant (Kel), and clearance (CL), were determined [23]. The organ targeting efficiency was calculated using two equations (Eqs. 4 and 5) mentioned below [24]. Drug targeting efficiency (DTE%) which represents time average partitioning ratio was calculated as follows:
Direct transport percentage (DTP %) of target organ was calculated using equation:
where Bx = (Boral/Poral) × Pi.n Bx is the target organ AUC fraction contributed by systemic circulation following nasal administration. Boral is the AUC0–24h (brain) following oral administration. Poral is the AUC0–24h (blood) following oral administration. Bi.n is the AUC0–24h (brain) following intranasal administration. Pi.n is the AUC0–24h (blood) following intranasal administration. AUC is the area under the curve.
Statistical analysis
Statistical analysis was carried out using Graph Pad prism 5.0 (Graph Pad software, San Diego, CA). All results were expressed as mean ± SD. Groups of data were compared with the analysis of variance (ANOVA) followed by Dunnett’s t test. The values were considered statistically significant, when a value of p was less than 0.05.
Results
Development of donepezil nanoemulsion
Before preparing nanoemulsion, the donepezil was analyzed by UV spectrophotometric analysis, and standard plot was obtained with R2 = 0.9944 (Fig. 1). Besides stability, the selection of excipients for the final preparation of the NE should be such that maximum amount of drug gets dissolved in them without any precipitation. Based upon the solubility studies, maximum solubility of the donepezil was observed in labrasol as oil (1000 ± 100 μg/ml). Among surfactants and co-surfactants, maximum solubility was seen in 1% CPC (2500 ± 250 μg/ml) and in glycerol (30,000 ± 800 μg/ml) (Table S1). To develop the final formulation, labrasol 10% as oil, 1% CPC in water 80% as surfactant, and glycerol 10% as co-surfactant were selected. To reduce the size of particles of the formulation in nano range, the formulation was subjected to homogenization at 1000 rpm for 20 min and ultrasonication for 150 s at 40% amplitude with 10 s on/off cycles. The final formulation was selected on the basis of clarity of the NE and its transmittance analysis. The % transmittance was obtained as 100% when the NE was observed at 650 nm (Table 1).
Determination of particle size and zeta potential
The prepared nanoemulsion was analyzed for the measurement of size of particles and their nature of dispersity. The findings showed the particle size of 65.36 nm, PDI of 0.084, and zeta potential of − 10.7 mV for the nanoemulsion which was close to the observed values of placebo formulation with particle size of 62.4 nm, PDI of 0.147, and zeta potential of − 9.03 mV, respectively (Figs. 2 and 3).
In vitro drug release studies
The in vitro drug release was seen in different simulated medias such as PBS, ACSF, and SNF using dissolution test apparatus for agitation at 100 rpm for 24 h. In PBS media, maximum release of donepezil from the nanoemulsion observed was 99.22% ± 4.82 at 4 h, whereas donepezil showed optimum burst release of 96% ± 5.21 at 2 h in SNF and 98% ± 4.90 within 2 h in ACSF media, respectively (Fig. 4). It was inferred from the results of in vitro permeation that the optimized NE showed fast release in all the studied simulated medias.
Cytotoxicity analysis
Viability assays are vital steps in toxicity studies that determine the cellular response to a toxicant. The MTT assay is a quick and effective method for testing mitochondrial activity, which correlates quite well with cell viability [18]. The cell viability (%) of different test agents i.e., aqueous donepezil, its NE, and placebo (PNE), at their respective Cmax values in cerebrospinal fluid was determined after being incubated with Neuro 2a cell lines for 24 h in a MTT assay. Figure 5 indicates the results of cell viability (%) in the order of placebo > aq. Donepezil > NE > i.e., cell viability (85%) in presence of aqueous drug solution compared with its NE (76.3%). This could be due to the presence of surfactants in the formulation.
Giemsa staining was performed to visualize the morphology of cells at two concentration (Cmax and 10*Cmax). (Figure 6 shows a comparative cellular morphology between untreated cells, cells treated with aqueous drug solution, and donepezil NE treated cells at Cmax and 10*Cmax values. Staining results were validated from the results obtained in MTT assay. High fold toxicity and nuclear/cell disintegration were observed at 10*Cmax values of cells treated with aqueous donepezil. Cells treated with donepezil NE showed negligible toxicity and cellular disintegration at both Cmax and 10*Cmax value which can be seen in Fig. 6. Both giemsa staining and cell viability assay provide preliminary effect of donepezil NE on Neuro 2a cell lines.
In vivo studies
Donepezil was radiolabeled using technetium pertechnetate (99mTc) by direct labeling method, and radiolabeling efficiency of 98 ± 0.9% was observed. The stability of labeling efficiency in blood plasma and normal saline was assessed, and it was observed that radiolabeled formulation was stable in normal saline and blood plasma up to 24 h (Table 2). To visualize the uptake of radiolabeled formulation in the brain via intranasal route of administration, gamma scintigraph images of rats at 0 h, 0.5 h, 1.5 h, 3 h, 6 h, and 24 h were taken after administration of radiolabeled formulation via intranasal (i.n), intravenous (i.v), and oral route. The images (Fig. 7) of rats administered with nanoemulsion formulation via i.n route showed maximum distribution in the brain region and remained in the target site until 24 h, whereas no uptake of formulation in the brain was seen in the rats administered via oral routes. The rats administered with formulation i.v and aqueous formulation i.n showed only trace amount of drug distribution in the brain. Further, to explore pharmacokinetic parameters, biodistribution studies were carried out. The radioactivity in counts per gram and % age per gram of the organ were estimated at predetermined time points up to 24 h in blood and brain tissues of the rats (Table 3). The rats administered with radiolabeled formulation via i.n route showed maximum percentage per gram of radioactivity (3.42 ± 0.5%/g) at 1.5 h in the brain, which was significantly higher than the rats administered with aqueous formulation i.n (2.34 ± 0.19%/g), radiolabeled formulation i.v (1.88 ± 0.24%/g) and orally (0.58 ± 0.10%/g) (Fig. 8). On the other hand, the blood samples of the rats administered with radiolabeled formulation i.v showed significantly higher uptake of drug (2.97 ± 0.35%/g) at 30 min as compared to the rats administered orally (1.55 ± 0.15%/g) and intranasally (2.04 ± 0.22%/g) (Fig. 9). The pharmacokinetic parameters were also calculated (Table 4) with Cmax of (3.42%/g) at Tmax (1.5 h) in the brain tissues of rats administered with radiolabeled formulation intranasally, which was found to be significantly higher than the Cmax (1.88%/g and 0.58%/g) at Tmax (3 h) of rats administered via i.v and oral route, respectively The blood samples of 99mTc-DNE i.n administered rats also showed Cmax of (2.04%/g) at Tmax of 1.5 h. AUC of 48.55% h/g, AUMC of 619.17% h2/g, MRT of 12.75 h, kel’ of 0.078 h−1, and CL of 0.082%/g h−1 were also calculated for brain tissues of the rats administered with 99mTC-DNE i.n, and all the parameters were found higher than the tissues of the rats administered i.v and orally. DTE% and DTP% were found to be 360.59% and 72.23% for the rats administered radiolabeled formulation i.n (Table 5). From the observations, it was confirmed that the developed formulation reached successfully with higher percentage at the target site i.e., brain via the nasal route of administration.
Determination of %/g radiolabeled drug in brain of the different rats administered intranasally (i.n), orally, and intravenously (i.v). Only statistically significant outcomes at p < 0.05 have been reported, where 99m-Tc is the technetium pertechnetate, DNE is the donepezil nanoemulsion, and DS is the donepezil aqueous solution
Determination of %/ g radiolabeled drug in blood of the different rats administered intranasally, orally, and intravenously. Only statistically significant outcomes at p < 0.05 have been reported, where 99m-Tc is the technetium pertechnetate, DNE is the donepezil nanoemulsion, and DS is the donepezil aqueous solution
Discussion
The rising cases of the AD, its associated risk factors, and various adverse effects of the drugs currently available in the market necessitate the development of alternative therapies for the treatment of AD. Nanoemulsions are the lipid-based carrier systems which are extensively helpful for the transportation of drug to the CNS via intranasal route of administration by enhancing its absorption in the nasal epithelial membrane [7]. These are made up of two immiscible liquids, i.e., oil and water which were mixed homogenously in the presence of surfactants and co-surfactants to create an oil‑water interface and to prepare a stabilized formulation [12]. Donepezil hydrochloride was selected for its encapsulation in a nanocarrier system because it is a drug of choice for the first line of treatment and its very low availability in the central nervous system (Cmax in brain, 11 ng/ml) after oral administration [25]. Currently, this drug is administered only via oral route due to which most of the drug gets distributed in the peripheral body (100% bioavailability) and causes many adverse effects like nausea, diarrhea, malaise, dizziness, and insomnia. To consider all these limitations of the current treatment, it was aimed to prepare an o/w nanoemulsion for its administration through the nasal cavity to enhance the transportation of NE to the brain via the olfactory nerves. The use of nanotechnology in the intranasal systems not only enhances the therapeutic effects of the drugs at the target site but also minimizes the side effects by minimizing the distribution of drug in the rest of the body [26].
To prepare nanoemulsion loaded with donepezil hydrochloride, solubility studies were carried out to select the excipients. Labrasol (10%) as oil, 1% CPC as surfactant in water (80%), and glycerol (10%) as co-surfactant were selected for the formulation of finalized nanoemulsion. The formulation was optimized on the basis of transmittance and clarity studies. The optimized nanoemulsion was characterized with a particle size of 65 nm, PDI of 0.084, and zeta potential of − 10.7 mV. The nanosize of the particles has greater surface area and enhanced the transit through the nasal membrane leading to better release of drug from nanoemulsion [26]. The small droplet size can also result in uniform dispersity of the particles due to low interfacial tension and provides mono dispersity. This is reflective by the low PDI value of 0.084. The higher its value, the lower is the uniformity of droplet size in the formulation and vice versa. Moreover, zeta potential is the indicator of surface charge of the droplets, which is responsible for the stability of colloidal dispersions. For a physically stable nanoemulsion, a charge value of ± 30 mV is required [27]. Our findings were in agreement with the study of Sood et al. [28], in which donepezil- and curcumin-loaded nanoemulsions were prepared for intranasal administration with particle size of 25 nm, PDI of 0.22, and zeta potential of – 12‑− 28 mV. In another study, Pandey et al. [29] developed paroxetine nanoemulsion for the treatment of AD for its administration via intranasal route and also found particle size 58.47 nm, PDI 0.33, and zeta potential of − 33 mV of the developed nanoemulsion. Md. et al. [30] also prepared nanoparticles of donepezil hydrochloride with particle size ranges from 90 to 182 nm, which was higher than our developed donepezil nanoemulsion (65 nm).
To assess the rate of drug release, in vitro release studies were performed and maximum release was observed in SNF (96%) and ACSF (100%) media within 1 h. The release of drug in PBS was also comparable with maximum release of 62.8% until 6 h. The high release of the drug from the nanoemulsion system might be due to the nanosize of the particles and presence of surfactants, which enhances the solubilization of the drug in the NE system, and drug release was found to be high in different mediums. The maximum release obtained in SNF and ACSF would lead to enhanced permeability of the drug through the nasal mucosa and distribution in the brain. Cytotoxicity analysis of the developed NE was carried out to check the toxicity of the nanoemulsion against neuroblastoma (Neuro 2a) cells. The results indicated approximately similar cell viability in case of aqueous drug solution and NE formulation. This showed that the developed donepezil nanoemulsion and excipients used for the preparation of NE were nontoxic to the N2a cells, and the formulation could be safe for further drug delivery studies.
To observe the uptake of donepezil nanoemulsion (DNE) in the target site i.e., brain via nose to brain drug delivery, in vivo studies on Sprague Dawley rats were carried out. The developed DNE and donepezil drug solution were radiolabeled using technetium pertechnetate, and radiolabeling efficiency of 98% was observed. The radiolabeled formulation and aqueous drug formulation were also found to be stable for 24 h. The gamma images of the rats administered with 99mTc-DNE i.n showed enhanced uptake of drug in the brain as compared with the rats administered with aqueous 99mTc-D i.n, 99mTc-DNE orally, and i.v. In a study by Mahajan et al. [26], it was observed that the gamma images of the rat brains showed transport of drug in the CNS at larger extent after intranasal administration of nanoemulsion loaded with saquinavir. Gamma images showed very less amount of drug distributed in the peripheral regions of the rats after i.n administration of 99mTc-DNE. From this, it is suggestive that due to less peripheral distribution of drug through nasal administration, peripheral side effects of the drug can be minimized. To observe the percentage of radioactivity per gram of the target organ, biodistribution studies were performed and significant percentage per gram (%/g) of radioactivity was observed in the brain of rats administered with 99mTc-DNE i.n. The Cmax and Tmax values were also higher in the rats administered 99mTc-DNE i.n. Further DTE% and DTP% showed higher direct nose to brain transportation of radiolabeled formulation via intranasal route of administration. In a study by Bhawna et al. [11], radiolabeled donepezil nanoparticles were administered via i.n route, and Cmax showed significantly higher concentration of donepezil in the brain and plasma as 147.54 ± 25.08 and 183.451 ± 13.45 ng/ml, respectively, for nanosuspension when administered intranasally. Md. et al. [30] also prepared PLGA nanoparticles of donepezil hydrochloride and administered these via intravenous route of administration and found maximum concentration of (121. 68‑13.23 ng/ml) in the brain at 6 h, whereas our developed nanoemulsion showed maximum concentration in the brain at 1.5 h only. In another study by Yadav et al. [31] showed that i.n delivery of cyclosporine- A (CsA) NE was an effective way of brain targeting compared with that of other treatment strategies. Hanafy et al. [32] prepared galantamine hydrobromide-loaded cationic chitosan NPs by ionic gelation for intranasal delivery. Complexation was investigated as an approach to enhance the galantamine hydrobromide entrapment. The prepared NPs were delivered successfully to different brain regions shortly after administration suggesting the potential of this delivery system for AD management. Our results were also found to be in agreement with Kumar et al. [23] and Vyas et al. [33]. From the results, it was concluded that the developed nanoemulsion loaded with donepezil hydrochloride could enhance the transportation of drug to the brain via intranasal drug delivery.
Conclusion
In the present investigation, nanoemulsion formulation loaded with donepezil hydrochloride was developed on the basis of preliminary solubility, transparence, and clarity studies. The developed NE showed particle size in nano range and was mono dispersed in nature. The in vitro release studies of NE were carried out in three mediums, and maximum release up to 100% was observed in PBS, SNF, and ACSF media. To explore the nose to brain uptake of developed NE, in vivo studies were performed after radiolabeling the formulation and its drug solution. As hypothesized, the pharmacokinetic studies of the 99 m-Tc-DNE when administered intranasally showed higher uptake of the drug in the brain than the drug solution and formulation administered orally and intravenously in Sprague Dawley rats. Moreover, the gamma scintigraphy images obtained were also in agreement with the biodistribution findings and showed maximum uptake in the brain via intranasal route of administration. From the observed findings, it can be concluded that the developed NE loaded with donepezil hydrochloride was nontoxic to the neuro N2 cells and its intranasal administration delivered the drug more effectively at the target site than the other routes.
Abbreviations
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- AChE:
-
Acetylcholinesterase
- AD:
-
Alzheimer’s disease
- ACSF:
-
Artificial cerebrospinal fluid
- ChE:
-
Cholinesterase inhibitors
- DMSO:
-
Dimethyl sulfoxide
- DMEM:
-
Dulbecco’s modified Eagle’s media
- GIS:
-
Gastrointestinal
- i.n:
-
Intranasal
- i.v:
-
Intravenous
- NMDA:
-
N-methyl-D-aspartate
- NE:
-
Nanoemulsion
- Neuro 2a:
-
Neuroblastoma cells
- PBS:
-
Phosphate-buffered saline
- SNF:
-
Simulated nasal fluid
- 99mTc-DS:
-
Technetium pertechnetate labeled donepezil aqueous solution
- 99mTc-DNE:
-
Technetium pertechnetate labeled donepezil nanoemulsion formulation
References
Clark CM, Karlawish JH. Alzheimer disease: current concepts and emerging diagnostic and therapeutic strategies. Ann Intern Med. 2003;138:400–10.
Selkoe DJ. The origins of Alzheimer disease: a is for amyloid. Jama. 2000;283:1615–7.
Spillantini MG, Goedert M. Tau protein pathology in neurodegenerative diseases. Trends Neurosci. 1998;21:428–33.
Prince MJ. World Alzheimer Report 2015: the global impact of dementia: an analysis of prevalence, incidence, cost, and trends. Alzheimer's Dis Int. 2015.
Etienne P, Robitaille Y, Wood P, Gauthier S, Nair NP, Quirion R. Nucleus basalis neuronal loss, neuritic plaques and choline acetyltransferase activity in advanced Alzheimer’s disease. Neuroscience. 1986;19:1279–91.
Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, et al. BACE1 is the major β-secretase for generation of Aβ peptides by neurons. Nat Neurosci. 2001;4:233.
Di Stefano A, Iannitelli A, Laserra S, Sozio P. Drug delivery strategies for Alzheimer’s disease treatment. Expert Opin Drug Deliv. 2011;8:581–603.
Rogers SL, Doody RS, Pratt RD, Ieni JR. Long-term efficacy and safety of donepezil in the treatment of Alzheimer’s disease: final analysis of a US multicentre open-label study. Eur Neuropsychopharmacol. 2000;10:195–203.
Herrmann N, Chau SA, Kircanski I, Lanctot KL. Current and emerging drug treatment options for Alzheimer’s disease. Drugs. 2011;71:2031–65.
Christodoulou C, Melville P, Scherl WF, MacAllister WS, Elkins LE, Krupp LB. Effects of donepezil on memory and cognition in multiple sclerosis. J Neurol Sci. 2006;245:127–36.
Bhavna MS, Ali M, Baboota S, Sahni JK, Bhatnagar A, Ali J. Preparation, characterization, in vivo biodistribution and pharmacokinetic studies of donepezil-loaded PLGA nanoparticles for brain targeting. Drug Dev Ind Pharm. 2014;40:278–87.
Singh Y, Meher JG, Raval K, Khan FA, Chaurasia M, Jain NK, et al. Nanoemulsion: concepts, development and applications in drug delivery. J Control Release. 2017;252:28–49.
Bonferoni MC, Rossi S, Sandri G, Ferrari F, Gavini E, Rassu G, et al. Nanoemulsions for “nose-to-brain” drug delivery. Pharmaceutics. 2019;11(2):84.
Yasui-Furukori N, Furuya R, Takahata T, Tateishi T. Determination of donepezil, an acetylcholinesterase inhibitor, in human plasma by high-performance liquid chromatography with ultraviolet absorbance detection. J Chromatogr B. 2002;768:261–5.
Bonferoni MC, Ferraro L, Pavan B, Beggiato S, Cavalieri E, Giunchedi P, et al. Uptake in the central nervous system of geraniol oil encapsulated in chitosan oleate following nasal and oral administration. Pharmaceutics. 2019;11(3):106.
Kaur A, Saxena Y, Bansal R, Gupta S, Tyagi A, Sharma RK, et al. Intravaginal delivery of polyphenon 60 and curcumin nanoemulsion gel. AAPS PharmSciTech. 2017;18:2188–202.
Phosphate-buffered saline (PBS). Cold Spring Harb Protoc. 2006;1:8247.
Tauck DL, Nadler JV. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J Neurosci. 1985;5:1016–22.
Nigam K, Kaur A, Tyagi A, Nematullah M, Khan F, Gabrani R, et al. Nose-to-brain delivery of lamotrigine-loaded PLGA nanoparticles. Drug Deliv Transl Res. 2019;9(5):879–90.
Sobhani H, Tarighi P, Ostad SN, Shafaati A, Nafissi-Varcheh N, Aboofazeli R. Formulation development and toxicity assessment of triacetin mediated nanoemulsions as novel delivery systems for rapamycin. Iran J Pharm Res. 2015;14:3.
Atale N, Chakraborty M, Mohanty S, Bhattacharya S, Nigam D, Sharma M, et al. Cardioprotective role of Syzygium cumini against glucose-induced oxidative stress in H9C2 cardiac myocytes. Cardiovasc Toxicol. 2013;13:278–89.
Md S, Ali M, Ali R, Bhatnagar A, Baboota S, Ali J. Donepezil nanosuspension intended for nose to brain targeting: in vitro and in vivo safety evaluation. Int J Biol Macromol. 2014;67:418–25.
Kumar M, Misra A, Babbar AK, Mishra AK, Mishra P, Pathak K. Intranasal nanoemulsion based brain targeting drug delivery system of resperidone. Int J Pharm Sci Res. 2008;58:285–91.
Wang Y, Aun R, Tse FL. Brain uptake of dihydroergotamine after intravenous and nasal administration in the rat. Biopharm Drug Dispos. 1998;19:571–5.
Jann MW, Shirley KL, Small GW. Clinical pharmacokinetics and pharmacodynamics of cholinesterase inhibitors. Clin Pharmacokinet. 2002;41:719–39.
Mahajan HS, Mahajan MS, Nerkar PP, Agrawal A. Nanoemulsion-based intranasal drug delivery system of saquinavir mesylate for brain targeting. Drug Deliv. 2014;21:148–54.
Attwood D, Mallon C, Ktistis G, Taylor CJ. A study on factors influencing the droplet size in nonionic oil-in-water microemulsions. Int J Pharm. 1992;88:417–22.
Sood S, Jain K, Gowthamarajan K. Intranasal delivery of curcumin–/INS; donepezil nanoemulsion for brain targeting in Alzheimer’s disease. J Neurol Sci. 2013;333:316–7.
Pandey YR, Kumar S, Gupta BK, Ali J, Baboota S. Intranasal delivery of paroxetine nanoemulsion via the olfactory region for the management of depression: formulation, behavioural and biochemical estimation. Nanotechnology. 2015;27:025102.
Md S, Ali M, Bhatnagar A, Baboota S, Sahni JK, Ali J. Design, development, optimization and characterization of donepezil loaded chitosan nanoparticles for brain targeting to treat Alzheimer’s disease. Sci Adv Mater. 2014;6:720–35.
Yadav S, Gattacceca F, Panicucci R, Amiji MM. Comparative biodistribution and pharmacokinetic analysis of cyclosporine-a in the brain upon intranasal or intravenous administration in an oil-in-water nanoemulsion formulation. Mol Pharm. 2015;12:1523–33.
Hanafy AS, Farid RM, Helmy MW, ElGamal SS. Pharmacological, toxicological and neuronal localization assessment of galantamine/chitosan complex nanoparticles in rats: future potential contribution in Alzheimer’s disease management. Drug Deliv. 2016;23:3111–22.
Vyas TK, Babbar AK, Sharma RK, Singh S, Misra A. Intranasal mucoadhesive microemulsions of clonazepam: preliminary studies on brain targeting. J Pharm Sci. 2006;95:570–80.
Acknowledgments
The authors are grateful to the Jaypee Institute of Information Technology, Noida, UP (India) for the infrastructural support.
Funding
This work was funded by the Department of Biotechnology, Government of India (DBT Grant No. BT/PR19580/BIC/101/865/2016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Kaur, A., Nigam, K., Bhatnagar, I. et al. Treatment of Alzheimer’s diseases using donepezil nanoemulsion: an intranasal approach. Drug Deliv. and Transl. Res. 10, 1862–1875 (2020). https://doi.org/10.1007/s13346-020-00754-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-020-00754-z