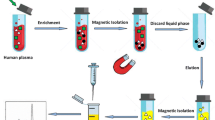
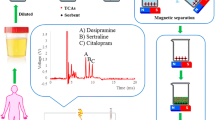
Abstract
Background
In recent days, solid-phase extraction methods are widely utilized for the extraction of drug molecules from plasma samples due to their easy operating procedures and low matrix effect. The trace-level solid-phase extraction of three structurally related antiepileptic drugs brivaracetam (BVC), eslicarbazepine acetate (ESL), and carbamazepine (CBZ) was investigated by using a magnetic porous material graphene oxide-β-cyclodextrin (MGO-CD). Morphology, magnetic properties, and structure of the synthesized MGO-CD were characterized by using FT-IR, SEM, XRD, and VSM.
Results
Solid-phase extraction (SPE) methods were used to extract the analytes from human plasma. Different extraction solvents such as acetonitrile (ACN), methanol (MeOH), acetone, chloroform (CHCl3), tertiary butyl diethyl ether (TBDE), and ethyl acetate (EtOAc) with variable polarities were used to extract drug molecules from MGO-CD. The linearity analysis showed good correlation coefficient values (R2) of 0.9989, 0.9995, and 0.9982 for BVC, ESL, and CBZ respectively. The LOD and LOQ ranges were found to be 6.14–28.32 ng mL−1 and 20.45–94.31 ng mL−1 respectively.
Conclusion
The high accuracy and precision made the developed HPLC method with MGO-CD a suitable alternative for the bioequivalence study of BVC, ESL, and CBZ in human plasma. This developed HPLC-UV method has good efficiency for recoveries and good linearity and is simple to handle. And also, it gave low retention time for the three antiepileptic drugs within 8 min. It provides high efficiency for the extraction of trace-level substances from human plasma.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Epilepsy is a chronic brain disease described by sudden and transient brain dysfunction caused by recurrent episodes of neurons in the brain [1,2,3]. It is identified with symptoms like seizures and should be treated immediately with a quick-acting antiepileptic drug (AED). The treatment results in lowering the potential sequelae, predominantly excitotoxic and ischemic neuronal cell loss, which initiates within minutes of uninterrupted seizure activity [4,5,6,7,8]. Brivaracetam (BVC), eslicarbazepine acetate (ESL), and carbamazepine (CBZ) drugs are widely used drugs for the treatment of this condition (Fig. 1) [9]. The IUPAC name of BVC is ((2S)-2-[(4R)-2-oxo-4-propylpyrrolidin-1-yl]), which is a 4-n-propyl equivalent of racetum and levetiracetam derivative and is primarily employed for partial onset of seizures in adults and adolescents [10, 11]. The mode of action of BVC is through binding to the pervasive synaptic vesicle glycoprotein 2A (SV2A), like levetiracetam but has a 20-fold greater affinity [12,13,14,15]. CBZ is a mood-stabilizing and anticonvulsant tricyclic lipophilic drug. This drug is the first one to treat epilepsy for psychomotor and partial onset of seizures and is also used for variability of indications, including schizophrenia, attention-deficit hyperactivity disorder (ADHD), paroxysmal dangerous pain disorder, phantom limb disease, and post-traumatic stress syndrome [16,17,18].
ESL is a modern and third-generation single enantiomer drug that belongs to the dibenzoazepine family. ESL is an anticonvulsant medication, effectively administrated in the adjunctive therapy for partial-onset seizures [19, 20]. ESL is a prodrug that is intensively transformed to eslicarbazepine, which is an important metabolite in the human body [21,22,23]. ESL was known to wield anticonvulsant activity by inhibiting the repetitive neuronal firing and also by stabilizing the inactive sodium channels [24,25,26].
Considering the importance of these molecules as antiepileptic drugs, it is important to develop an analytical method for the extraction of these drugs from biological fluids. The available literature revealed that the different analytical methods such as fluorescence-based immunoassays, enzyme-linked immunosorbent assays, electrochemical, and spectrophotometric methods are known for extraction of these drugs. These methods give results with high accuracy and sensitivity and also rapidly by using a small amount of blood sample. But the main drawback with these methods is their high operating costs. Solid-phase extraction (SPE) method is the commonly used extraction method compared with liquid-liquid extraction (LLE) due to its advantages such as high preconcentration value, low solvent consumption, and easy handling. Adsorbent plays a key role in the SPE method; various adsorbents such as simple graphene oxide, silica gel, activated carbon, ionic liquids, calixarenes, and chelating resins were reported. But these materials normally showed low absorbance values and made the elution procedure critical. Therefore, the development of a method which overcomes all the limitations mentioned here is of utmost importance for the analysis of drugs in biological samples.
In the current method, iron oxide-graphene oxide-β-cyclodextrin composite was used as an adsorbent. Β-cyclodextrin is a cyclic oligosaccharide consisting of seven D-glucopyranose units bound by β-1, 4-glycosidic bonds [27]. The usage of β-cyclodextrin in the composite further increases the adsorption capacity due to the presence of a hydrophilic outer shell due to the presence of more hydroxyl groups and hydrophobic cavity due to its corban chain conformation. Owing to this dual character, it acts as the host-guest moiety by capturing compounds with suitable dimensions into its cavity [28,29,30,31]. Enrichment capability and high supramolecular recognitions of β-cyclodextrin are widely used in different analytical aspects especially in separation methods [32, 33]. Graphene oxide (GO), the other component of the composite, is made through the oxidation of graphene. It contains hydroxyl, carboxyl, and epoxide derivative as functional groups, which increases the dispersibility in the solution. GO is known for its exceptional electrical, optical, and mechanical properties due to the high surface area. Thus, the combination of these three components makes this composite an ideal adsorbent for the extraction of drugs from plasma. Iron oxide induces magnetic property to the adsorbent, and this made the extraction process easy and quick with low matrix effect and high recovery percentage of analytes. There is no need to go for high-speed centrifugation; instead, a simple magnet can be used for the separation. The MGO-CD was prepared through the encapsulation of β-cyclodextrin on MGO with the help of linker tetrafluoterthalonitrile. The liker tetrafluoterthalonitrile helped to build a porous structure of MGO and β-cyclodextrin via nucleophilic aromatic substitution reactions [34]. The MGO-CD morphology, structure, and magnetic nature were confirmed by using SEM, VSM, IR, and XRD studies. Simultaneous identification and quantification synergy of BVC, ESL, and CBZ in human blood plasma by using MGO-CD as solid phase has been accomplished with HPLC.
Methods
Materials
All the chemicals and solvents used in the current studies are of analytical grade. Graphene powder, FeCl3·6H2O, and ammonium hydroxide solution (28.0–30% NH3 basis) were procured from Sigma-Aldrich. Acetonitrile, methanol, chloroform, TBDE, acetone, ethanol, ethyl acetate, and DMF were purchased from (Merck) Mumbai, India. Double distilled deionized water is used for the entire analysis. Tetrafluoroterepthalonitrile, β-cyclodextrin (> 98%), sodium acetate, and mono-, di-, and polyethylene glycol were procured from Sigma-Aldrich. CBZ, BVC, and ESL were obtained as gifted samples from Mylan & Hetero laboratories.
Instruments and analytical conditions
HPLC having a binary pump system with a diode array detector and LC-Solutions software (Shimadzu, Japan) was used for developing the method. Luna RP C18 (4.6 × 150 mm, 5 μ) column was used, and column oven temperature was maintained at ambient conditions. Acetonitrile and 0.1% formic acid (65:35) mixture was used as a mobile phase. FT-IR spectra were recorded on Perkin Elmer, USA, using KBr pellets at ambient temperature. Powder X-ray diffractograms of GO, MGO, and synthesized MGO-CD were recorded by using a Bruker AXS D8 diffractometer with Cu Kα radiation (1.5406 A°), step size 2 mdeg, and 0.5 s per step scan speed. The morphology of MGO-CD was analyzed by FEI Apreo LoVac equipped with an Aztec Standard EDX System. The magnetic properties of samples were measured at room temperature by using a vibrational sample magnetometer (VSM, Lakeshore 7400, Westerville, OH, USA) in magnetic fields up to 15 kOe. Raman spectra were taken on a SENTERRA Dispersive Raman microscope (Bruker, Germany) with a wavelength 785.0 nm incident laser light.
Synthetic procedure for MGO-CD
Synthesis of graphene oxide
Modified Hummer’s method was used for the synthesis of graphene oxide (GO) from graphite nanopowder with a set of modifications (Fig. 2) [35]. In brief, graphite powder (2 g) was added slowly into 50 mL of concentrated sulfuric acid, and for this black mixture, 2 g of sodium nitrate was added for initiating the reaction. For the above reaction mixture, potassium permanganate was added by maintaining the temperature below 20 °C. The resultant mixture was stirred at 60 °C for 4 h. At this stage, the temperature was raised up to 90 °C after adding the 100 mL of deionized water and stirred for 15.0–20.0 min. The addition of 20 mL of 30% H2O2 and 200 mL of warm water resulted in a color change to bright yellow which indicates the formation of GO. The solid was collected and washed several times with 5% hydrochloric acid and water and then dried for 12 h.
Synthesis of MGO
MGO was prepared with the help of a simple and ecofriendly solvothermal route [36]. The GO (0.2 g) was dispersed into a 250-mL round bottom flask containing the mixture of mono- (20 mL) and di-ethylene glycol (60 mL) and sonicated for about 2 h. To this, 0.68 g of FeCl3·6H2O was added and the dispersion was stirred for 25 min. Thereafter, polyethylene glycol (2.25 g) and sodium acetate (8.10 g) were added slowly and sonicated for 30 min. The reaction mixture was transferred into a stainless-steel autoclave and the reaction is carried out at 180 °C for 12 h. Finally, the formed product was collected by using centrifugation and washed several times with water and ethanol and then dried for 12 h at 50 °C in a vacuum oven.
Preparation of MGO-CD composite
MGO (0.0615 g), tetrafluoroterphthalonitrile (0.4 g), cyclodextrin (0.615 g), and potassium carbonate (0.96 g) were taken in a three-neck round bottom flask containing the solvent mixture of tetrahydrofuran (THF, 4 mL) and dimethylformamide (DMF, 26 mL). The reaction mixture was deoxygenated by purging nitrogen gas for about 30 min. After that, the reaction mixture was stirred mechanically at 85 °C with constant speed for about 24 h. After the completion of reaction time, the formed product was collected by using centrifugation for 5.0 min at 5000 RPM. Then, the product was washed with deionized water and HCl until the supernatant is neutralized. Further, the product was washed with THF and dichloromethane for the removal of unbounded cyclodextrin and dried at 50 °C in the oven for 10 h [34].
Preparation of standard and spiked human plasma sample solutions
The standard stock solutions of BVC, ESL, and CBZ (1000 μg mL−1) were prepared by dissolving in acetonitrile and stored at 4 °C in the refrigerator. The drug-free human plasma was collected from our institute dispensary and stored at −20 °C. The protein content from the plasma was removed by adding 1 mL acetonitrile 1:4 (v/v) ratio to 250 μL of plasma 2-mL Eppendorf tube. The sample was vortexed for 5 min and then centrifuged for 4 min at 4000 rpm. We repeated this procedure to getting 4 mL of supernatant. The 4 mL of supernatant was taken in a 5-mL glass vial and dried with N2 gas at 40 °C in a vacuum oven. For the reconstitution of the solution, phosphate buffer (pH 7.2) solution was added to the residue. In order to construct the calibration curve, eight spiked plasma sample solutions containing the abstained final concentrations of three antiepileptic drugs of BVC, ESL, and CBZ (0.5–50.0, 0.1–40.0, and 0.25–60.0 μg mL−1 respectively) were prepared. For QC analysis, three-level spiked sample solutions (low: BVC, ESL, and CBZ is 1.0, 0.5, and 0.75 μg mL−1; middle: 10.0, 5.0, and 7.5 μg mL−1; high: 20.0, 10.0, and 15.0 μg mL−1) were prepared. Eighteen milligrams of MGO-CD was added and then the solutions were mixed up to 25.0 min. The MGO-CD was separated from the solution by using a strong magnet. The analytes are extracted with 1.0 mL acetonitrile through ultrasound, and the solution was evaporated and reconstituted with acetonitrile. From this, 20 μL solution was directly injected into HPLC for analysis.
The study of MGO-CD properties
Adsorption capacity of MGO-CD
Different amounts of MGO-CD (1.0–12.0 mg) in different centrifuge tubes were taken and then three antiepileptic drugs at a concentration of each 100.0 μg/mL were added. After continuous shaking of this mixture up to 120.0 min, the supernatant was collected by a strong external magnet and then directly injected into HPLC (Fig. 3a).
The study of adsorption kinetics
Ten milliliters of three antiepileptic drug solution containing concentrations of each BVC, ESL, and CBZ (100.0 μg/mL) was taken and added 18.0 mg of MGO-CD for the study of adsorption kinetics. The mixture was shaken continuously with different time ranges from 5 to 180.0 min. The unbounded solution was separated by using a strong external magnet and directly injected into HPLC analysis (Fig. 3b).
Study of adsorption isotherm
For the study of adsorption isotherm, 2 mg of MGO-CD in 1-mL drug solutions with different concentrations (5.0–700.0 μg mL−1) was taken. The solutions are kept for 30.0 min and then supernates were collected from MGO-CD by using a strong magnet. 20.0 μL of each supernate was directly injected into HPLC-UV and the concentrations are determined (Fig. 3c).
Results
Characterization of synthesized MGO-CD
The magnetic graphene oxide and cyclodextrin (MGO-CD) composite was synthesized as depicted in the experimental details. The MGO-CD composite was characterized by FT-IR, powder XRD, SEM, and VSM. The MGO showed one specific band at 591 cm−1 in the infra-red spectrum which corresponds to the Fe–O vibration in Fe3O4. The MGO-CD exhibited an additional band at 2255 cm−1 attributed to the stretching frequency of -C ≡ N in the linker (Fig. S1). The IR spectra also exhibited a weak band at 1156 cm−1, and strong peaks at 1422 and 1580 cm−1 correspond to C–F and aromatic C=C stretching frequencies respectively. The stretching frequencies of O–H, C–H, and C–O were also found at 3453, 2945, and 1032 cm−1. These results suggested the formation of magnetic graphene oxide cyclodextrin composite (MGO-CD).
Powder X-ray diffractogram of GO exhibited a peak at 10.56° which corresponds to the characteristic diffraction from graphitic carbon in exfoliated GO. The peak was disappeared in both MGO and MGO-CD due to the disturbance in stacking of GO sheets after loading with iron oxide (Fe3O4) and CD. The diffraction peaks observed at 30.0°, 35.9°, 43.0°, 53.2°, 57.3°, and 62.4° were assigned to (220), (311), (400), (422), (511), and (440) planes, which corresponds to the cubic phase of Fe3O4 with a face-centered cubic structure as found in the literature reports. The intensity of XRD peaks was found to be slightly decreased on loading with cyclodextrin. These results supported the incorporation of CD onto MGO (Fig. 4).
There will be irregularity that occurred in the sp2 carbon network in GO, due to the incorporation of CD and Fe3O4. Hence, the ordered and disordered crystal structures of fabricated MGO-CD need to be investigated using Raman spectroscopy. The obtained Raman spectra of GO and MGO-CD are plotted in Fig. 5. D-band and G-band are the parameters corresponding to the structural defects and imperfections, and first-order scattering of the E2g phonon of the sp2 carbon domains, respectively. From the spectrum of GO, it can be observed that D-band and G-band are assigned at 1345 and 1585 cm−1, respectively. The disordering in the structure of GO is measured using the intensity ratio of ID/IG. It can also be observed that there is an increase in the intensity ratio of MGO-CD than that of GO due to the incorporation of CD and Fe3O4, which indicates a significant amount of structural defects of the sp2 carbon network in GO.
The morphology of the synthesized composite was examined by SEM. Figure 6a shows a sheet-like structure of the exfoliated GO. This was further employed for the synthesis of MGO-CD composites. There was clear diffraction observed from Fig. 6b that shows the formation of Fe3O4 composites on the GO sheets. The particles have been observed clearly along with the stacking of the GO sheets because of the adherence of Fe3O4 and CD.
The vibrational sample magnetometer (VSM) data was collected to understand the magnetic properties of MGO-CD; the hysteresis loops of MGO-CD are shown in Fig. 7. S-like curves of magnetic hysteresis loops observed indicate the paramagnetic nature of MGO-CD because of no remnant magnetization or coercivity at room temperature. The specific saturation magnetization (Ms) of the composite was observed to be 43.96 emu g−1, which is appropriate for the separation of composites with a magnet. The MGO-CD could be easily separated from the mixture of composites in the solution by placing a permanent magnet.
Optimization of extraction conditions
In this method, parameters such as extraction solvent (eluent) (ACN, MeOH, acetone, CHCl3, TBDE, and EtOAc), sorbent amount (2.0–22.0 mg), extraction time (intermissions from 5.0–30.0 min), and eluent amount (0.5 to 2.5 mL) were optimized. The mixture of three antiepileptic drug solution contains the concentrations of BVC (10.0 μg mL−1), ESL (10.0 μg mL−1), and CBZ (20.0 μg mL−1) respectively.
Rate of adsorption
In order to get the amount of adsorbent required for better extraction, the effect of adsorbent quantity on the extraction of these drugs was studied. These studies revealed that at 2.0 mg of absorbent, the adsorption rates were found to be 33.50% (ESL), 17.95% (CBZ), and 8.19% (BVC). Increasing in the four-unit volume of the adsorbent amount, the adsorption rate for ESL gradually increased and it reaches equilibrium (97.62%) at 18.0 mg. In the case of BVC, the rate of adsorption was slow up to 10.0 mg and it reaches maximum (83.08%) at 18 mg whereas the adsorption rate for CBZ was gradually increased (87.98%) and reaches maximum at 18.0 mg (Fig. 8a). These studies suggest that the maximum rate of adsorption was found to be at 18.0 mg in all the three drugs.
Effect of eluent solvent
The choice of desorption solvent plays a significant role in the extraction of analytes from the adsorbent. In the previous methods of extraction of these three drugs, acetonitrile, methanol, acetone, and a mixture of solvents under acidic conditions were used. In the present method, methanol, TBDE, acetone, acetonitrile, chloroform, and ethyl acetates were used as desorption solvents. Based on the polarity scale, acetonitrile is expected to give high desorption efficiency for these three antiepileptic drugs among all solvent used and chloroform is of low eluting efficiency. The same pattern has been found in our experimental results (Fig. 8b). The percentage recovery of these three drugs was found to be higher in the case of acetonitrile and lower with chloroform as eluent. From the HPLC chromatograms, the polarity sequence of the three antiepileptic drugs is BVC > ESL > CBZ respectively (Fig. 9).
Effect of eluent amount
The rate of desorption of analytes from any adsorbent is known to depend not only on the nature of the eluent but also on the amount. To study the effect of eluent amount on desorption rate, different volumes of eluent were used from 0.5 to 2.5 mL. The studies clearly suggested that the 1.5 mL of eluent gives maximum desorption of drugs (Fig. 8c). The variation was found to be in the range of 10% between 0.5 and 2.5 mL. Based on these studies, 1.5-mL eluent volume was found to be the optimal condition to get the best desorption efficiency.
Effect of time on the extraction
Time also showed a significant effect on the desorption of three antiepileptic drugs from MGO-CD. The effect of time on extraction has been studied between 5.0 and 30.0 min. From Fig. 8d, it is clear that the extraction increases with the increase in time from 5.0 to 20.0 min (up to 30%) and becomes saturated after 25.0 min. Based on this study, the optimized desorption was found to be 20.0 min for effective desorption of these drugs from the MGO-CD.
Adsorption capacity
For the evolution and determination of adsorption capacity of MGO-CD, different initial concentrations of drug solutions (5.0–700.0 μg mL−1) were prepared. The equilibrium adsorption capacity Qe (mg/g) of MGO-CD was measured by changing the concentrations of sample solutions and the values were calculated by using the subsequent equation.
where Qe (mg/g) is the amount of three antiepileptic drugs adsorbed per unit weight of adsorbent at equilibrium, C0 (μg mL−1) is the initial concentration, Ce (μg mL−1) is the equilibrium concentrations of drugs in the solution, m (g) is the mass of MGO-CD, and V (L) is the volume of the sample solution.
The apparent binding amount of the MGO-CD was calculated by using the following Langmuir isotherm model.
where K is the constant coefficient, Qm is the maximum sorption capacity of the MGO-CD, Qe is the equilibrium sorption capacity, and Ce is the equilibrium concentration. This transformation (Ce/Qe versus Ce) gives information about the binding characteristics of the equilibrium adsorption. The Qm values can be obtained from the plot of Ce/Qe versus Ce. By using this transformation, the maximum sorption values for BVC, ESL, and CBZ were found to be 36.38, 106.86, and 54.49 mg g−1 respectively.
Method validation
Linearity
For the determination of linearity, six different concentrated solutions of three antiepileptic drugs were prepared in the range of 0.5–50.0, 0.1–40.0, and 0.25–60.0 μg mL−1 respectively for BVC, ESL, and CBZ. The calibration curves give good correlation coefficient (R2) values ≥ 0.9982 with acceptable linearity (see Table 1).
LOD and LOQ
The sensitivity of the method was assessed by measuring the limit of determination (LOD) and limit of quantification (LOQ). LOD and LOQ of the developed method were evaluated by using the signal to noise ratio (S/N) method. The LOD was set as the lowest concentration that can be distinguished with signal to noise ratio over 3, whereas the lower limit of quantification of the analytes was assessed by using a signal to noise ratio of 10. The LOD for BVC, ESL, and CBZ were 28.32, 6.14, and 14.86 ng mL−1 and LOQ values for BVC, ESL, and CBZ were 94.31, 20.45, and 49.48 ng mL−1 respectively.
Precision and accuracy
Precision and accuracy of the method were analyzed by preparing three different QC level samples as described in Table 2 (low, middle, and high) of three antiepileptic drugs BVC, ESL, and CBZ. Each level of QC samples was repeatedly injected in triplicate and evaluated the recovery percentage. Inter- and intraday recoveries of analytes results are shown in Table 2. The results clearly showed the low interference effect on target analyte peaks and this demonstrates the good specificity of the method. From the results, it is clear that the recovery ranges in both intra- and interday are 80.25–101.11% and CV ranges are 1.86–5.50%. From these test results, it is clear that the developed HPLC method will be useful for the analysis of these three drugs in human plasma samples.
Robustness
The robustness of the current method was studied by preparing standard solutions of drugs (10.0 μg mL−1) and by varying conditions such as flow rate, column oven temperature, and mobile phase ratio. From Table 3, it is clear that no substantial difference in the results was observed at different chromatographic conditions and the RSD was found to vary between 5.84 and 1.42%. These results indicate the reliability and the good performance of the developed HPLC method.
Discussion
The LC-MS method has been quite widely employed for the analysis of drugs in the plasma due to its high sensitivity and selectivity. But the method is expensive in terms of solvent usage and maintenance that increase the burden for institutions and patients which in turn limits the applicability of the method. In the case of liquid-liquid extractions, the method needs a high amount of organic solvents and it causes pollution. Therefore, the solid-phase extraction method is considered as an alternative method for the analysis of drugs in the plasma due to its good extraction ability, convenient operation, and consumption of a low amount of organic solvents and it needs a low amount of adsorbent. Compared with earlier reported methods by using different equipments, our method showed high precision, wider linear range, and comparable detection limit (see Table 4) [16, 37,38,39,40,41,42,43,44]. These results demonstrate the advantages of the current method over the available methods, and in addition to this, there were no studies that reported on the simultaneous extraction of these three antiepileptic drugs using the solid-phase extraction method.
Conclusions
Magnetic graphene oxide composite of β-cyclodextrin with good water dispersibility was synthesized and characterized by FT-IR, SEM, and powder XRD, and the magnetic property of the material was established by VSM. The material was found to be paramagnetic with sufficient magnetization for the separation of composite with a conventional magnet. The developed material was used as MSPE sorbent for the extraction of three antiepileptic drugs from the human plasma. The method showed high precision with wider linear range and good detection limits. This developed HPLC-UV method has good efficiency for recoveries and good linearity and is simple to handle. And also, it gave low retention time for the three antiepileptic drugs within 8 min. It provides high efficiency for the extraction of trace-level substances from the human plasma.
Availability of data and materials
All data and materials are available upon request.
Abbreviations
- BVC:
-
Brivaracetam
- ESL:
-
Eslicarbazepine acetate
- CBZ:
-
Carbamazepine
- GO:
-
Graphene oxide
- MGO:
-
Magnetic graphene oxide
- MGO-CD:
-
Material graphene oxide-β-cyclodextrin
- LLE:
-
Liquid-liquid extraction
- SPE:
-
Solid-phase extraction
- ACN:
-
Acetonitrile
- MeOH:
-
Methanol
- CHCl3 :
-
Chloroform
- TBDE:
-
Tertiary butyl diethyl ether
- EtOAc:
-
Ethyl acetate
- THF:
-
Tetrahydrofuran
- DMF:
-
Dimethylformamide
- SEM:
-
Scanning electron microscopy
- XRD:
-
X-ray diffraction
- VSM:
-
Vibrational sample magnetometer
- LOD:
-
Limit of determination
- LOQ:
-
Limit of quantification
- AED:
-
Antiepileptic drug
- SV2A:
-
Synaptic vesicle glycoprotein 2A
- RP:
-
Reverse phase
- μg:
-
Microgram
- ng:
-
Nanogram
- mL:
-
Milliliter
- v/v:
-
Volume/volume
- HPLC:
-
High-performance liquid chromatography
- M.W:
-
Molecular weight
- M.F:
-
Molecular formulae
References
Pitkänen A, Sutula TP (2002) Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol 1(3):173–181. https://doi.org/10.1016/S1474-4422(02)00073-X
Sabu J, Regeti K, Mallappallil M, Kassotis J, Islam H, Zafar S, Khan R, Ibrahim H, Kanta R, Sen S, Yousif A, Nai Q (2016) Convulsive syncope induced by ventricular arrhythmia masquerading as epileptic seizures: case report and literature review. J Clin Med Res 8(8):610–615. https://doi.org/10.14740/jocmr2583w
Marchi N, Granata T, Janigro D (2014) Inflammatory pathways of seizure disorders. Trends Neurosci 37(2):55–65. https://doi.org/10.1016/j.tins.2013.11.002
Santhosh NS, Sinha S, Satishchandra P (2014) Epilepsy: Indian perspective. Ann Indian Acad Neurol 17: 3-11
Wallin MT, Culpepper WJ, Nichols E, Bhutta ZA, Gebrehiwot TT, Hay SI, Khalil IA, Krohn KJ, Liang X, Naghavi M, Mokdad AH (2019) Global, regional, and national burden of multiple sclerosis 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18(3):269–285. https://doi.org/10.1016/S1474-4422(18)30443-5
Trinka E, Kwan P, Lee BI, Dash A (2019) Epilepsy in Asia: disease burden, management barriers, and challenges. Epilepsia 60:7–21. https://doi.org/10.1111/epi.14458
Vaughan KA, Ramos CL, Buch VP, Mekary RA, Amundson JR, Shah M, Rattani A, Dewan MC, Park KB (2019) An estimation of global volume of surgically treatable epilepsy based on a systematic review and meta-analysis of epilepsy. J Neurosurg 130(4):1127–1141. https://doi.org/10.3171/2018.3.JNS171722
Amico RD (2018) Depression in epilepsy: etiology, diagnosis and treatment. Psicol della Salut 2018:24–43
Matagne A, Margineanu DG, Kenda B, Michel P, Klitgaard H (2008) Anti-convulsive and anti-epileptic properties of brivaracetam (ucb 34714), a high-affinity ligand for the synaptic vesicle protein, SV2A. Br J Pharmacol 154(8):1662–1671. https://doi.org/10.1038/bjp.2008.198
Rogawski MA (2008) Brivaracetam: a rational drug discovery success story. Br J Pharmacol 154(8):1555–1557. https://doi.org/10.1038/bjp.2008.221
Tai KK, Truong DD (2007) Brivaracetam is superior to levetiracetam in a rat model of post-hypoxic myoclonus. J Neural Transm 114(12):1547–1551. https://doi.org/10.1007/s00702-007-0788-3
French JA, Costantini C, Brodsky A, Von Rosenstiel P (2010) Adjunctive brivaracetam for refractory partial-onset seizures: a randomized, controlled trial. Neurology 75(6):519–525. https://doi.org/10.1212/WNL.0b013e3181ec7f7f
Malawska B, Kulig K (2008) Brivaracetam: a new drug in development for epilepsy and neuropathic pain. Expert Opin Investig Drugs 17(3):361–369. https://doi.org/10.1517/13543784.17.3.361
Gillard M, Fuks B, Leclercq K, Matagne A (2011) Binding characteristics of brivaracetam, a selective, high affinity SV2A ligand in rat, mouse and human brain: relationship to anti-convulsant properties. Eur J Pharmacol 664(1-3):36–44. https://doi.org/10.1016/j.ejphar.2011.04.064
Klitgaard H, Matagne A, Nicolas JM, Gillard M, Lamberty Y, De Ryck M, Kaminski RM, Leclercq K, Niespodziany I, Wolff C, Wood M (2016) Brivaracetam: rationale for discovery and preclinical profile of a selective SV2A ligand for epilepsy treatment. Epilepsia 57(4):538–548. https://doi.org/10.1111/epi.13340
Ghoraba Z, Aibaghi B, Soleymanpour A (2017) Application of cation-modified sulfur nanoparticles as an efficient sorbent for separation and preconcentration of carbamazepine in biological and pharmaceutical samples prior to its determination by high-performance liquid chromatography. J Chromatogr B 1063:245-52.
Queiroz RH, Bertucci C, Malfará WR, Dreossi SA, Chaves AR, Valério DA, Queiroz ME (2008) Quantification of carbamazepine, carbamazepine-10,11-epoxide, phenytoin and phenobarbital in plasma samples by stir bar-sorptive extraction and liquid chromatography. J Pharm Biomed Anal 48(2):428–434. https://doi.org/10.1016/j.jpba.2008.03.020
Mohiuddin I, Berhanu AL, Malik AK, Aulakh JS, Lee J, Kim KH (2019) Preparation and evaluation of a porous molecularly imprinted polymer for selective recognition of the antiepileptic drug carbamazepine. Environ Res 176: 108580
Weissinger F, Losch F, Winter Y, Brecht S, Lendemans D, Kockelmann E (2019) Effectiveness of eslicarbazepine acetate in dependency of baseline anticonvulsant therapy: results from a German prospective multicenter clinical practice study. Epilepsy Behav 101(Pt A):106574. https://doi.org/10.1016/j.yebeh.2019.106574
Thomas S, Bharti A, Maddhesia PK, Shandilya S, Agarwal A, Biswas S, Bhansal V, Gupta AK, Tewari PK, Mathela CS (2012) Highly efficient, selective, sensitive and stability indicating RP-HPLC-UV method for the quantitative determination of potential impurities and characterization of four novel impurities in eslicarbazepine acetate active pharmaceutical ingredient by LC/ES. J Pharm Biomed Anal 61:165–175. https://doi.org/10.1016/j.jpba.2011.11.024
Boto REF, Almeida P, Queiroz JA (2008) Thiacarbocyanine as ligand in dye-affinity chromatography. Biomed Chromatogr 288:278–288
Alves G, Fortuna A, Sousa J, Direito R, Almeida A, Rocha M, Falcão A, Soares-da-Silva P (2010) Enantioselective assay for therapeutic drug monitoring of eslicarbazepine acetate: no interference with carbamazepine and its metabolites. Ther Drug Monit 32(4):512–516. https://doi.org/10.1097/FTD.0b013e3181e5c855
Fortuna A, Sousa J, Alves G, Falcão A, Soares-da-Silva P (2010) Development and validation of an HPLC-UV method for the simultaneous quantification of carbamazepine, oxcarbazepine, eslicarbazepine acetate and their main metabolites in human plasma. Anal Bioanal Chem 397(4):1605–1615. https://doi.org/10.1007/s00216-010-3673-0
Almeida L, Soares-da-silva P (2007) Eslicarbazepine acetate ( BIA 2-093 ). Neurotherapeutics 4(1):88–96. https://doi.org/10.1016/j.nurt.2006.10.005
Bialer M, Johannessen SI, Kupferberg HJ, Levy RH, Perucca E, Tomson T (2007) Progress report on new antiepileptic drugs: a summary of the Eigth Eilat Conference (EILAT VIII). Epilepsy Res 73(1):1–52. https://doi.org/10.1016/j.eplepsyres.2006.10.008
Keating GM (2014) Eslicarbazepine acetate: a review of its use as adjunctive therapy in refractory partial-onset seizures. CNS Drugs 28(7):583–600. https://doi.org/10.1007/s40263-014-0182-2
Szejtli J (1988) Cyclodextrin technology Kluwer Academic Publishers. Dordrecht, The Netherlands 1-66
Rekharsky MV, Inoue Y (1988) Complexation thermodynamics of cyclodextrins. Chem Rev 98(5):1875–1918
Guo Y, Guo S, Li J, Wang E, Dong S (2011) Cyclodextrin–graphene hybrid nanosheets as enhanced sensing platform for ultrasensitive determination of carbendazim. Talanta 84(1):60–64. https://doi.org/10.1016/j.talanta.2010.12.007
Hapiot F, Tilloy S, Monflier E (2006) Cyclodextrins as supramolecular hosts for organometallic complexes. Chemical reviews 106(3):767–781. https://doi.org/10.1021/cr050576c
Connors KA (1997) The stability of cyclodextrin complexes in solution. Chemical reviews 97(5):1325–1358. https://doi.org/10.1021/cr960371r
Crini G, Morcellet M (2002) Synthesis and applications of adsorbents containing cyclodextrins. J Sep Sci 25(13):789–813. https://doi.org/10.1002/1615-9314(20020901)25:13<789::AID-JSSC789>3.0.CO;2-J
Gazpio C, Sánchez M, Isasi JR, Vélaz I, Martín C, Martínez-Ohárriz C, Zornoza A (2008) Sorption of pindolol and related compounds by a β-cyclodextrin polymer: isosteric heat of sorption. Carbohydr Polymers 71(1):140–146. https://doi.org/10.1016/j.carbpol.2007.06.007
Zhang J, Liu D, Meng X, Shi Y, Wang R, Xiao D, He H (2017) Solid phase extraction based on porous magnetic graphene oxide/β-cyclodextrine composite coupled with high performance liquid chromatography for determination of antiepileptic drugs in plasma samples. J Chromatogr A 1524:49–56. https://doi.org/10.1016/j.chroma.2017.09.074
Goud KY, Hayat A, Satyanarayana M, Kumar VS, Catanante G, Gobi KV, Marty JL (2017) Aptamer-based zearalenone assay based on the use of a fluorescein label and a functional graphene oxide as a quencher. Microchim Acta 184(11):4401–4408. https://doi.org/10.1007/s00604-017-2487-6
Wu J, Xiao D, Zhao H, He H, Peng J, Wang C, Zhang C, He J (2015) A nanocomposite consisting of graphene oxide and Fe3O4 magnetic nanoparticles for the extraction of flavonoids from tea, wine and urine samples. Microchim Acta 182(13-14):2299–2306. https://doi.org/10.1007/s00604-015-1575-8
Iqbal M, Ezzeldin E, Al-Rashood KA (2017) UPLC–MS/MS assay for identification and quantification of brivaracetam in plasma sample: application to pharmacokinetic study in rats. J Chromatogr B 1060:63–70. https://doi.org/10.1016/j.jchromb.2017.05.039
Bourgogne E, Culot B, Dell’Aiera S, Chanteux H, Stockis A, Nicolas JM (2018) Off-line solid phase extraction and liquid chromatography-tandem mass spectrometry method for the quantitation of brivaracetam acid metabolites: method validation and application to in vitro metabolism assays. J Chromatogr B 1086:138–145. https://doi.org/10.1016/j.jchromb.2018.04.018
Vasanth DA, Rajkamal B (2018) A validated LC-MS/MS method for pharmacokinetic study of brivaracetam in healthy rabbits. Int J Pharm Pharm Sci 10(2):24. https://doi.org/10.22159/ijpps.2018v10i2.21457
Li T, Huang B, Li D, Zhu Y, Ding L, Shu C (2019) Development and validation of a specific and sensitive LC–MS/MS method for determination of eslicarbazepine in human plasma and its clinical pharmacokinetic study. J Chromatogr B 1112:61–66. https://doi.org/10.1016/j.jchromb.2019.02.027
Loureiro AI, Fernandes-Lopes C, Wright LC, Soares-da-Silva P (2011) Development and validation of an enantioselective liquid-chromatography/tandem mass spectrometry method for the separation and quantification of eslicarbazepine acetate, eslicarbazepine, R-licarbazepine and oxcarbazepine in human plasma. J Chromatogr B Anal Technol Biomed Life Sci 879(25):2611–2618. https://doi.org/10.1016/j.jchromb.2011.07.019
Servais AC, Janicot B, Takam A, Crommen J, Fillet M (2016) Liquid chromatography separation of the chiral prodrug eslicarbazepine acetate and its main metabolites in polar organic mode. Application to their analysis after in vitro metabolism. J Chromatogr A 1467:306–311. https://doi.org/10.1016/j.chroma.2016.07.022
Mowafy HA, Alanazi FK, El Maghraby GM (2012) Development and validation of an HPLC-UV method for the quantification of carbamazepine in rabbit plasma. Saudi Pharm J 20(1):29–34. https://doi.org/10.1016/j.jsps.2011.04.003
Serralheiro A, Alves G, Fortuna A, Rocha M, Falcão A (2013) First HPLC-UV method for rapid and simultaneous quantification of phenobarbital, primidone, phenytoin, carbamazepine, carbamazepine-10,11-epoxide, 10,11-trans-dihydroxy-10,11-dihydrocarbamazepine, lamotrigine, oxcarbazepine and licarbazepine in human plasma. J Chromatogr B Anal Technol Biomed Life Sci 925:1–9. https://doi.org/10.1016/j.jchromb.2013.02.026
Acknowledgements
The authors would like to thank the National Institute of Technology-Warangal for providing essential support to carry out this research work. Thanks to Mylan and Hetero pharma laboratory managements for helping this work.
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
All the authors have read and approved the manuscript. BP has framed the methodology of the work and investigated and validated by performing the formal analysis. BP and JC have drafted the original paper. KV has also assisted in the formal analysis. KG and JC have visualized and supervised the overall experimental work. PN and JC have helped in the conceptualization of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research was approved by the ethical committee of Hindu College of Pharmacy, Guntur, Andhra Pradesh, with an approval number 1263/PO/Re/S/09/CPCSEA, and written consent was obtained from each participant for the current study.
Consent for publication
All the participant has given their written consent for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
FT-IR spectra of GO, MGO and MGO-CD.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Palakeeti, B., Reddy, K.V., Gobi, K.V. et al. Simple and efficient method for the quantification of antiepileptic drugs in human plasma by using magnetic graphene oxide-β-cyclodextrin composite as a sorbent. Futur J Pharm Sci 7, 93 (2021). https://doi.org/10.1186/s43094-021-00239-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-021-00239-x