Abstract
Eslicarbazepine acetate (Aptiom®, Zebinix®) is approved for the adjunctive treatment of partial-onset seizures in adults aged ≥18 years. Adjunctive therapy with oral eslicarbazepine acetate 800 or 1,200 mg once daily was associated with a significantly lower standardized seizure frequency (primary endpoint) than placebo in patients aged ≥18 years with refractory partial-onset seizures in three, randomized, double-blind, multinational, phase III trials. In a fourth randomized, double-blind, multinational, phase III trial in patients aged ≥16 years with refractory partial-onset seizures, adjunctive eslicarbazepine acetate 1,200 mg once daily, but not 800 mg once daily, was associated with a significantly lower standardized seizure frequency (primary endpoint). Responder rates were significantly higher with eslicarbazepine acetate 1,200 mg once daily than with placebo in these four trials, and with eslicarbazepine acetate 800 mg once daily than with placebo in two trials. The efficacy of eslicarbazepine acetate was maintained in the longer term, according to the results of 1-year extension studies. Adjunctive therapy with oral eslicarbazepine acetate was generally well tolerated in patients with refractory partial-onset seizures, with most adverse events being of mild to moderate severity. In conclusion, eslicarbazepine acetate is a useful option for the adjunctive treatment of patients with refractory partial-onset seizures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Prodrug of eslicarbazepine |
Associated with few clinically relevant drug interactions |
Generally associated with a significantly lower standardized seizure frequency than placebo |
Efficacy maintained in the longer term |
Generally well tolerated |
1 Introduction
Epilepsy is a common neurological disorder, affecting approximately 50 million people worldwide, that is characterized by recurring seizures [1, 2]. Partial-onset (focal) seizures originate within networks limited to one hemisphere and may be discretely localized or more widely distributed [3].
Monotherapy with an antiepileptic drug (AED) is sufficient to achieve seizure control without intolerable adverse effects in ≈60 % of patients [1]. Of the remaining patients, some will initially respond to monotherapy and subsequently relapse and some will be refractory to monotherapy from the outset. A proportion of these patients will respond to combination therapy with AEDs [1].
Oral eslicarbazepine acetate (Aptiom®, Zebinix®) is approved in the US for the adjunctive treatment of adults aged ≥18 years with partial-onset seizures [4] and in the EU as adjunctive therapy in adults aged ≥18 years with partial-onset seizures with or without secondary generalization [5]. This article reviews the clinical efficacy and tolerability of eslicarbazepine acetate when used as adjunctive therapy in patients with refractory partial-onset seizures, as well as summarizing its pharmacological properties.
2 Pharmacodynamic Properties
This section provides an overview of the pharmacodynamic properties of eslicarbazepine acetate, with a summary of the findings of preclinical studies shown in Table 1. Results of pooled analyses [6–8] of phase III trials (trials 2093-301 [9], 2093-302 [10] and 2093-303 [11] or 2093-304 [12]) in patients with refractory partial-onset seizures are also discussed in this section. Patients received once-daily eslicarbazepine acetate 400, 800 or 1,200 mg or placebo in trials 2093-301 and 2093-302 and once-daily eslicarbazepine acetate 800 or 1,200 mg or placebo in trials 2093-303 and 2093-304 (see Sect. 5.1 for further information regarding these trials). Some of the studies in this section are only available as abstracts [6, 7, 13–24] and/or posters [7, 8, 25].
2.1 Mechanism of Action and Preclinical Findings
Eslicarbazepine acetate is a dibenz[b,f]azepine derivative that has a dibenazepine nucleus with a 5-carboxamide substituent, but is structurally different from carbamazepine and oxcarbazepine at the 10,11-position (Fig. 1) [26, 27]. Eslicarbazepine acetate is a prodrug that is metabolized to its major active metabolite eslicarbazepine (S-licarbazepine), and to the minor active metabolites R-licarbazepine and oxcarbazepine (see also Sect. 3).
Although the precise mechanism of the anticonvulsant activity of eslicarbazepine acetate is unknown, it is thought to involve inhibition of voltage-gated sodium channels [26]. The voltage-gated sodium channel is an α-subunit arranged in six transmembrane segments (S1–S6) and has three states: deactivated (i.e. resting state in which the channel is closed but responsive to voltage changes), activated (i.e. open) and inactivated (i.e. closed and nonresponsive to voltage changes) [28]. Activation of the voltage-gated sodium channel allows entry of Na+ ions into the neuron, permitting propagation of the action potential [28]. Eslicarbazepine acetate competitively interacted with neurotoxin receptor site 2 of the voltage-gated sodium channel in the inactivated state, stabilizing the inactive form of the sodium channel [26, 29].
Although eslicarbazepine, R-licarbazepine, oxcarbazepine and carbamazepine all had higher affinity for the inactivated versus the resting state of the voltage-gated sodium channel, the affinity of eslicarbazepine for this channel in the resting state was 5- to 15-fold lower than that of R-licarbazepine, oxcarbazepine and carbamazepine [13]. Thus, eslicarbazepine had enhanced inhibitory selectivity for rapidly-firing neurons versus those displaying normal activity [26, 29].
Eslicarbazepine also blocked high and low affinity CaV3.2 inward currents; this blockade of T-type calcium channels may contribute to the anticonvulsant activity of eslicarbazepine acetate [14, 15] (Table 1). In addition, eslicarbazepine had inhibitory effects on glycine GlyRα3 receptor-mediated inward currents [16] and on N-methyl-d-aspartate (NMDA) receptor-mediated currents [17] (Table 1).
However, eslicarbazepine did not have a marked effect on CaV2.1 calcium peak currents, voltage-gated KV7.2 potassium channels, submaximal γ-aminobutyric acid (GABA) currents or α-amino-3-hydroxy-5-methyl-isoxazole-4-propionate (AMPA) currents [14, 17–20] (Table 1).
In vitro, the release of glutamate [30, 31], aspartate [31], GABA [31] and dopamine [31] from rat hippocampal synaptosomes [30] or rat striatal slices [31] was inhibited in a concentration-dependent manner by eslicarbazepine, oxcarbazepine and carbamazepine (Table 1).
Eslicarbazepine significantly (p < 0.05) reduced maximal persistent Na+ current conductance in CA1 neurons isolated from hippocampal slices of wild-type mice, as well as reducing the firing rate [32] (Table 1). Unlike carbamazepine, eslicarbazepine was not associated with paradoxical upregulation of the persistent Na+ current in mice lacking the β1 or β2 subunit of the Na+ channel, meaning that the inhibitory effect of eslicarbazepine on repetitive firing was maintained [32].
In addition, eslicarbazepine dose-dependently inhibited neuronal firing in granule cells from the hippocampus of patients with treatment-refractory epilepsy [21] (Table 1).
Eslicarbazepine acetate and/or eslicarbazepine demonstrated anticonvulsant activity in murine models of epilepsy [22, 23, 33–36] (Table 1). In rats, eslicarbazepine acetate was associated with less neurological impairment than carbamazepine or oxcarbazepine [33].
2.2 Effects on Cognition and Psychomotor Function and Abuse Potential
Neither eslicarbazepine acetate nor oxcarbazepine was associated with clinically relevant cognitive impairment in healthy volunteers, according to the results of two nonrandomized, single-blind studies [37]. For most cognitive tests, no significant differences were seen between eslicarbazepine acetate 800 or 1,200 mg once daily or oxcarbazepine 300 or 600 mg twice daily and placebo. Parameters for which significant (p < 0.05 vs. placebo) changes were seen with eslicarbazepine acetate 1,200 mg once daily or oxcarbazepine 600 mg twice daily included increased motor reaction time and total reaction time on the Choice Reaction Time, increased reaction time on the Sternberg Short-Term Memory Test, and decreased digit discrimination ability on the Digit Vigilance Test. Beneficial treatment effects (p < 0.05 vs. placebo) seen with eslicarbazepine acetate 1,200 mg once daily included decreased mean response latency to correct responses on the Divided Attention test and an increased total number of words for category on the Controlled Oral Word Associates Test; a beneficial treatment effect was also seen with oxcarbazepine 600 mg twice daily for the latter endpoint. [37].
In patients with uncontrolled focal epilepsy who had previously been treated with oxcarbazepine (n = 10) or carbamazepine (n = 1) or were drug naïve (n = 1), treatment with eslicarbazepine acetate (mean dosage 2,083 mg/day) was not associated with any significant changes from baseline in cognitive function (attention, cognitive speed, long- and short-term memory, word fluency) [24].
Eslicarbazepine acetate had less abuse potential than alprazolam in 53 healthy recreational users of CNS depressants [25]. Volunteers received single doses of eslicarbazepine acetate 800, 1,600, 2,000 or 2,400 mg, alprazolam 1.5 or 3.0 mg or placebo. Significant (p < 0.001) differences were seen between supratherapeutic doses of eslicarbazepine acetate (i.e. 1,600, 2,000 or 2,400 mg) and placebo in terms of peak scores on a drug-liking visual analogue scale (VAS). However, peak drug-liking VAS scores were significantly (p < 0.0001) lower with all eslicarbazepine acetate doses than with alprazolam [25].
2.3 Other Effects
Administration of eslicarbazepine acetate at therapeutic (1,200 mg once daily) and supratherapeutic (2,400 mg once daily) dosages to healthy volunteers did not have a clinically relevant effect on the corrected QT (QTc) interval, according to the results of a placebo-controlled thorough QT/QTc study [38]. The mean PR interval increased by 4.6 ms with eslicarbazepine acetate 1,200 mg/day, by 8.2 ms with eslicarbazepine acetate 2,400 mg/day and by 2.2 ms with placebo; no eslicarbazepine acetate recipient had a PR interval of >200 ms [38]. The EU summary of product characteristics (SPC) recommends caution in administering eslicarbazepine acetate to patients who have medical conditions (e.g. low thyroxine levels) or who are receiving agents associated with PR prolongation [5].
Eslicarbazepine acetate was not associated with clinically relevant abnormalities in ECG recordings in patients with refractory partial-onset seizures, according to the results of pooled analyses of trials 2093-301, 2093-302 and 2093-303 [6] and trials 2093-301, 2093-302 and 2093-304 [7]; in both analyses, ECGs were over-read by cardiologists. No eslicarbazepine acetate recipients had a QTc interval exceeding 500 ms in the pooled analysis of trials 2093-301, 2093-302 and 2093-303 (856 evaluable patients) [6]. Among recipients of eslicarbazepine acetate 400, 800 or 1,200 mg once daily or placebo in the pooled analysis of trials 2093-301, 2093-302 and 2093-304, the mean change from baseline in the Bazett-corrected QT interval was −1.1, +1.6, +2.3 and +3.0 ms, respectively, and in the Fridericia-corrected QT interval was −0.9, +0.7, +2.6 and +3.4 ms, respectively [7].
A post hoc pooled analysis of trials 2093-301, 2093-302 and 2093-304 examined the effect of eslicarbazepine acetate on serum lipid levels in patients with refractory partial-onset seizures [8]. HMG CoA-reductase inhibitors (statins) were being administered to 46 of 1,021 eslicarbazepine acetate recipients and to 18 of 426 placebo recipients. During the study period, changes in serum lipid levels did not appear to differ between eslicarbazepine acetate and placebo recipients both in patients receiving statins and in patients not receiving statins [8].
3 Pharmacokinetic Properties
This section summarizes the pharmacokinetic properties of eslicarbazepine acetate. Some studies are only available as abstracts [39, 40].
3.1 Absorption and Distribution
Following oral administration, eslicarbazepine acetate was rapidly and extensively converted by hydrolytic first-pass metabolism to its major active metabolite eslicarbazepine [41–43]. Plasma concentrations of eslicarbazepine acetate were usually below the lower limit of quantification [42–46].
The pharmacokinetics of eslicarbazepine following administration of oral eslicarbazepine acetate 400, 800 or 1,200 mg once daily to adults with partial-onset seizures who were receiving one or two concomitant AEDs are shown in Table 2 [41]. Maximum plasma concentrations (Cmax) of eslicarbazepine were reached in a median 2.0–2.5 h, and eslicarbazepine exposure increased in a dose-proportional manner [41].
Although comparisons across studies should be made with caution [41], eslicarbazepine values for Cmax and the area under the plasma concentration–time curve (AUC) from time zero to 24 h (AUC24) appeared lower in patients with partial-onset seizures who received once-daily eslicarbazepine acetate 800 or 1,200 mg (and one or two concomitant AEDs) than in healthy volunteers receiving once-daily eslicarbazepine acetate 800 or 1,200 mg alone [47, 48] (Table 2).
Steady state was reached within 4–5 days with once-daily administration of eslicarbazepine acetate [42, 44].
Pharmacokinetic/pharmacodynamic analysis demonstrated that as the eslicarbazepine acetate dosage and eslicarbazepine concentrations increased, the seizure frequency decreased [49].
Food did not significantly affect the pharmacokinetics of eslicarbazepine [50, 51], meaning that eslicarbazepine acetate may be administered without regard to meals [4, 5].
Crushed eslicarbazepine acetate tablets were bioequivalent to intact tablets [39], and the US prescribing information states that eslicarbazepine acetate may be administered as whole or crushed tablets [4].
Plasma protein binding of eslicarbazepine was <40 % [4]. Plasma protein binding of eslicarbazepine was not affected to a clinically relevant extent by warfarin, diazepam, digoxin, phenytoin or tolbutamide, and the plasma protein binding of these agents was not affected by eslicarbazepine [4]. Eslicarbazepine accounted for 91.96 % of the total exposure of drug moieties in cerebrospinal fluid [52]. Eslicarbazepine had an apparent volume of distribution of 61 L (for a bodyweight of 70 kg) [4].
3.2 Metabolism and Elimination
Eslicarbazepine acetate was metabolized to the major active metabolite eslicarbazepine and the minor active metabolites R-licarbazepine and oxcarbazepine [42, 43, 47]. Following oral administration of eslicarbazepine acetate 1,200 mg once daily, eslicarbazepine, R-licarbazepine and oxcarbazepine accounted for 93.84, 5.20 and 0.96 % of the total exposure of drug moieties in plasma, respectively [52].
The metabolites of eslicarbazepine acetate were mainly eliminated from the systemic circulation by renal excretion, in both unchanged and glucuronide conjugate forms [4, 5]. Eslicarbazepine accounted for approximately 92 % of the total drug moieties excreted in urine, with approximately two-thirds excreted in the unchanged form and one-third excreted as the glucuronide conjugate [4, 47]. Uridine diphosphate glucuronosyltransferase (UGT) isoforms (UGT1A4, UGT1A9, UGT2B4, UGT2B7 and UGT2B17) catalysed the conjugation of eslicarbazepine with glucuronic acid [40]. The parent drug and other minor metabolites (R-licarbazepine and oxcarbazepine and their glucuronides) accounted for the remaining 8 % of total drug moieties recovered in the urine [47].
Plasma concentrations of eslicarbazepine declined in a multiphasic manner, with a terminal elimination half-life of 13–20 h in patients with partial-onset seizures who received eslicarbazepine acetate 400, 800 or 1,200 mg once daily (Table 2) [41].
3.3 Special Patient Populations
In patients with mild (creatinine clearance [CLCR] 50–80 mL/min), moderate (CLCR 30–50 mL/min) or severe (CLCR <30 mL/min) renal impairment who received a single dose of eslicarbazepine acetate 800 mg, the eslicarbazepine AUC from time zero to infinity was significantly increased by 61, 111 and 154 %, respectively, versus healthy controls [43]. The EU SPC states that no dosage adjustment is needed in patients with mild renal impairment (CLCR >60 mL/min) and recommends an eslicarbazepine acetate starting dosage of 200 mg once daily or 400 mg every other day in patients with a CLCR of 30–60 mL/min, with an increase to 400 mg once daily after 2 weeks; the dosage may be further increased based on individual response [5]. Treatment with eslicarbazepine acetate is not recommended by the EU SPC in patients with severe renal impairment (CLCR <30 mL/min) [5]. In patients with moderate or severe renal impairment (CLCR <50 mL/min), the US prescribing information recommends an eslicarbazepine acetate starting dosage of 200 mg once daily, with an increase after 2 weeks to a maximum recommended maintenance dosage of 400 mg once daily [4].
The pharmacokinetics of eslicarbazepine acetate were not significantly altered in patients with moderate hepatic impairment [47]. Thus, no dosage adjustment is needed in patients with mild or moderate hepatic impairment [4, 5]. Treatment with eslicarbazepine acetate is not recommended in patients with severe hepatic impairment, as its pharmacokinetics have not been investigated in this patient population [4, 5].
Eslicarbazepine pharmacokinetics did not significantly differ between young healthy volunteers (aged 18–40 years) and elderly healthy volunteers (aged ≥65 years) receiving single and multiple doses of eslicarbazepine acetate 600 mg [42]. The US prescribing information states that no dosage adjustment is required in elderly patients with CLCR of >50 mL/min [4], and the EU SPC states that caution should be exercised in the treatment of the elderly [5].
Gender had no significant effect on the pharmacokinetics of eslicarbazepine acetate [53].
4 Potential Drug Interactions
In vitro, eslicarbazepine weakly induced cytochrome P450 (CYP) 3A4 and UGT isoforms [5]. Eslicarbazepine had a moderate inhibitory effect on CYP2C19, but had no inhibitory effect on CYP1A2, CYP2A6, CYP2B6, CYP2D6, CYP2E1 or CYP3A4 [4, 5]. In vitro, eslicarbazepine acetate and eslicarbazepine were shown to be P-glycoprotein substrates [54]. Several studies examined potential pharmacokinetic interactions between eslicarbazepine acetate and AEDs or other drugs; most of these studies were conducted in healthy volunteers [48, 55–62]. Some of these studies are only available as abstracts [55, 57, 63] and/or posters [63, 64].
4.1 Potential Interactions with Antiepileptic Drugs
The eslicarbazepine AUC was significantly decreased by 31.9 % following coadministration of eslicarbazepine acetate 800 mg once daily and controlled-release carbamazepine 400 mg twice daily [55]. No significant changes in the AUC of carbamazepine or its carbamazepine-10,11-epoxide metabolite were observed [55]. A population pharmacokinetic analysis also found that eslicarbazepine exposure was decreased when carbamazepine was coadministered with eslicarbazepine acetate [63]. The EU SPC states that, based on individual response, the eslicarbazepine acetate dosage may need to be increased when coadministered with carbamazepine [5], and the US prescribing information states that, based on efficacy and tolerability, the eslicarbazepine acetate or carbamazepine dosage may need to be adjusted [4].
Coadministration of eslicarbazepine acetate 1,200 mg once daily with lamotrigine 150 mg once daily decreased lamotrigine exposure (which is also mainly metabolized by glucuronidation) by 14 % [5, 48], therefore no dosage adjustment is required in patients receiving concomitant eslicarbazepine acetate and lamotrigine [4, 5]. The interaction may be clinically relevant in some patients, given inter-individual variability [5].
An 18 % reduction in topiramate exposure occurred when it was administered in combination with eslicarbazepine acetate 1,200 mg once daily [56]. However, no dosage adjustment is required [4, 5].
Coadministration of eslicarbazepine acetate and phenytoin decreased the eslicarbazepine AUC by 33 % and increased the phenytoin AUC by 35 % [57]. Plasma phenytoin concentrations should be monitored and dosage adjustment may be needed based on clinical response and plasma phenytoin concentrations [4].
Population pharmacokinetic analysis indicated that coadministration of levetiracetam or valproate with eslicarbazepine acetate did not affect eslicarbazepine exposure, suggesting that no dosage adjustment is required [4, 64]. Coadministration of phenobarbital with eslicarbazepine acetate reduced eslicarbazepine exposure by 34 %, meaning that a higher dosage of eslicarbazepine acetate may be required [4, 64].
Another population pharmacokinetic analysis found that the clearance of clobazam, gabapentin, levetiracetam, phenobarbital, phenytoin or valproic acid was not affected by the coadministration of eslicarbazepine acetate [49]. The clearance of carbamazepine, lamotrigine and topiramate were increased 12–16 % by the coadministration of eslicarbazepine acetate. Eslicarbazepine exposure was not affected by coadministration of clobazam, gabapentin, levetiracetam, valproic acid, lamotrigine or topiramate [49].
4.2 Potential Interactions with Other Drugs
Coadministration of eslicarbazepine acetate 800 mg once daily and a single dose of the CYP3A4 substrate simvastatin 80 mg reduced the AUC of simvastatin and its metabolite simvastatin-β-hydroxyacid by 54 and 51 %, respectively [58]. The simvastatin dosage may need to be increased in patients receiving concomitant eslicarbazepine acetate [4, 5] if a clinically significant change in lipid levels is seen [4].
The systemic exposure of rosuvastatin was decreased by 36–39 % when it was coadministered with eslicarbazepine acetate 1,200 mg once daily [5]. Given that the mechanism of this interaction is unknown, monitoring of lipid levels in patients receiving concomitant rosuvastatin and eslicarbazepine acetate is recommended [4, 5]; the rosuvastatin dosage should be adjusted if clinically significant changes in lipid levels occur [4].
Coadministration of eslicarbazepine acetate 1,200 mg once daily and warfarin was associated with a significant 23 % reduction in the AUC of S-warfarin [59]. The pharmacokinetics of R-warfarin and the international normalized ratio (INR) were not altered to a clinically significant extent [59]. However, the INR should be monitored in patients receiving concomitant eslicarbazepine acetate and warfarin [4, 5]. Indeed, because of inter-individual variability in the interaction, the EU SPC recommends closely monitoring the INR during the first weeks after starting or stopping coadministration of warfarin and eslicarbazepine acetate [5].
The pharmacokinetics of digoxin [60] and metformin [62] were not altered to a clinically relevant extent by the coadministration of eslicarbazepine acetate.
The AUC24 values for ethinylestradiol and levonorgestrel were significantly reduced by 25 and 11 % in women receiving ethinylestradiol/levonorgestrel in combination with eslicarbazepine acetate 800 mg once daily, and by 32 and 23 % in women receiving ethinylestradiol/levonorgestrel in combination with eslicarbazepine acetate 1,200 mg once daily [61]. Women of child-bearing potential who are being treated with eslicarbazepine acetate must use additional or alternative forms of contraception [4, 5].
An interaction between monoamine oxidase inhibitors and eslicarbazepine acetate has not been studied, but is theoretically possible [5].
5 Therapeutic Efficacy
5.1 Phase III Trials
The potential of adjunctive therapy with eslicarbazepine acetate in patients with refractory partial-onset seizures was shown in a randomized, double-blind, multicentre, exploratory trial [65]. However, given the availability of four randomized, double-blind, placebo-controlled, multinational, phase III trials examining the efficacy of adjunctive therapy with eslicarbazepine acetate (2093-301 [9], 2093-302 [10], 2093-303 [11] and 2093-304 [12]), this exploratory trial is not discussed further [65].
Approval of eslicarbazepine acetate in the EU was based on trials 2093-301 [9], 2093-302 [10] and 2093-303 [11], whereas approval in the US was based on trials 2093-301 [9] and 2093-302 [10] and the more recent trial 2093-304 [12]; trial 2093-303 [11] was only used as supporting data in the US submission because of compliance issues [66].
Patients in these phase III trials were aged ≥16 [12] or ≥18 years [9–11] and had been diagnosed with simple or complex partial-onset seizures (with or without secondary generalization) [9–11] or epilepsy [12] for ≥12 months. Patients in these trials generally had a mean duration of epilepsy of >20 years (Table 3) and were experiencing partial-onset seizures despite receiving one to two [9, 11, 12] or one to three [10] concomitant AEDs. Patients were not permitted to receive concomitant oxcarbazepine in these trials [9–12]. Additional information regarding trial inclusion and exclusion criteria and baseline patient characteristics is shown in Table 3. One of these trials is available as a poster [12].
Eslicarbazepine acetate was administered once daily in all four trials. The trials comprised an observational 8-week baseline period, after which patients were randomized to the double-blind phase, which comprised a 2-week titration period followed by a 12-week maintenance period in trials 2093-301, 2093-303 and 2093-304 [9, 11, 12], or was of 14 weeks duration in trial 2093-302, with only recipients of eslicarbazepine acetate 1,200 mg/day undergoing dose titration during the first 2 weeks [10]. Patients were randomized to receive eslicarbazepine acetate 400 mg/day [9, 10], 800 mg/day [9–12] or 1,200 mg/day [9–12] or placebo [9–12]. Although results for all treatment arms are shown in Table 4, the text discussion focuses on results pertaining to the recommended maintenance dosage of eslicarbazepine acetate 800 mg/day, and the maximum recommended dosage of 1,200 mg/day [4, 5] (see also Sect. 7). Where specified, 330 of 402 patients (82 %) completed trial 2093-301 [9], 194 of 252 patients (77 %) completed trial 2093-303 [11] and 504 of 653 patients (77 %) completed trial 2093-304 [12].
The primary endpoint was the standardized seizure frequency [9–12]. Efficacy was assessed in the modified intent-to-treat population [9–12] (defined as the number of randomized patients who received at least one dose of study drug and had at least one postbaseline seizure frequency assessment) [9–11].
Adjunctive therapy with eslicarbazepine acetate reduced the seizure frequency in patients with refractory partial-onset seizures. The standardized seizure frequency was significantly lower in patients receiving eslicarbazepine acetate 800 or 1,200 mg/day than in patients receiving placebo after 12 weeks’ maintenance therapy [9, 11] or 14 weeks’ double-blind treatment [10] in trials 2093-301 [9], 2093-302 [10] and 2093-303 [11] (Table 4). In trial 2093-304, the standardized seizure frequency was significantly lower with eslicarbazepine acetate 1,200 mg/day than with placebo after 12 weeks’ maintenance therapy, with no significant difference seen between eslicarbazepine acetate 800 mg/day and placebo recipients for this endpoint [12] (Table 4).
The responder rate (defined as the proportion of patients with a ≥50 % reduction in standardized seizure frequency) was significantly higher in eslicarbazepine acetate 1,200 mg/day recipients than in placebo recipients in all four trials [9–12], with a significantly higher responder rate also seen with eslicarbazepine acetate 800 mg/day than with placebo in trials 2093-301 [9] and 2093-302 [10] (Table 4).
The median relative reduction in standardized seizure frequency is shown in Table 4. In the two studies (2093-302 and 2093-304) in which statistical analysis was reported for this endpoint, the median relative reduction in standardized seizure frequency was significantly greater with eslicarbazepine acetate 1,200 mg/day than with placebo in both studies [10, 12], and with eslicarbazepine acetate 800 mg/day than with placebo in one of the studies [10].
Among recipients of eslicarbazepine acetate 400, 800 and 1,200 mg/day and placebo, the proportion of seizure-free patients was 1–2, 4–8, 4–8 and 1–2 %, respectively [9–11].
5.1.1 Pooled Analyses
Several pooled analyses [67–72] of trials 2093-301 [9], 2093-302 [10], 2093-303 [11] and/or 2093-304 [12] have been conducted, some of which are only available as abstracts [69–72] and/or posters [68, 71, 72]. Where specified, one analysis was prespecified [67] and others were post hoc [68–71]. A pooled analysis of trials 2093-301, 2093-302 and 2093-303 assessed health-related quality of life (HR-QOL), using the Quality of Life in Epilepsy Inventory-31 (QOLIE-31) questionnaire [72].
In the prespecified pooled analysis of trials 2093-301, 2093-302 and 2093-303 (n = 1,049), the least squares mean seizure frequency per 4 weeks was significantly (p < 0.0001) lower in patients receiving eslicarbazepine acetate 800 or 1,200 mg/day than in patients receiving placebo (6.24 and 5.95 vs. 8.17) [67]. In addition, patients receiving eslicarbazepine acetate 800 or 1,200 mg/day had significantly greater (p < 0.001) least squares mean relative reductions in seizure frequency, significantly (p ≤ 0.0001) higher responder rates and a significantly (p < 0.01) lower mean number of days with seizures per 4 weeks, compared with patients receiving placebo [67].
Subgroup analyses showed that eslicarbazepine acetate 800 or 1,200 mg/day was more effective than placebo regardless of the duration of epilepsy, age at diagnosis, seizure type, number of concomitant AEDs, study location, gender, and type of concomitant AEDs, including whether or not patients were receiving concomitant carbamazepine, lamotrigine or valproic acid [67].
Similarly, in a pooled analysis of trials 2093-301, 2093-302 and 2093-304 (n = 1,450), the least squares mean standardized seizure frequency was significantly (p < 0.001) lower in patients receiving eslicarbazepine acetate 800 or 1,200 mg/day than in patients receiving placebo [68]. In patients receiving eslicarbazepine acetate 800 or 1,200 mg/day or placebo, the median percentage reduction in standardized seizure frequency was 31, 33 and 17 %, respectively, and the responder rate was 32, 41 and 21 %, respectively; statistical analysis was not conducted in the pooled population for these endpoints [68].
Another pooled analysis of trials 2093-301, 2093-302 and 2093-304 (n = 1,410) revealed that the least squares mean standardized seizure frequency was significantly (p < 0.05) lower in recipients of eslicarbazepine acetate 800 or 1,200 mg/day than in placebo recipients both in patients who did (6.5 and 6.6 vs. 8.0) and did not (5.7 and 4.9 vs. 7.5) receive concomitant carbamazepine [71]. The median percentage reduction in standardized seizure frequency was significantly (p < 0.05) greater in eslicarbazepine acetate 800 or 1,200 mg/day recipients than in placebo recipients in patients not receiving concomitant carbamazepine (−32.6 and −38.9 vs. −16.7 %), with no significant between-group difference in patients receiving concomitant carbamazepine (−30.1 and −29.8 vs. −16.7 %). The responder rate was significantly (p < 0.05) higher in recipients of eslicarbazepine acetate 800 or 1,200 mg/day than in placebo recipients both in patients who did (32.4 and 38.0 vs. 19.4 %) and did not (32.1 and 43.6 vs. 22.3 %) receive concomitant carbamazepine [71].
Pooled analyses of trials 2093-301 and 2093-302 (n = 752 [69] and 751 [70]) demonstrated that a clinically relevant effect on seizure risk was seen within 49 and 28 days in patients receiving eslicarbazepine acetate 800 and 1,200 mg/day, respectively (the analysis included the titration period of up to 2 weeks) [69] and that eslicarbazepine acetate 800 and 1,200 mg/day was significantly (p < 0.05) more effective than placebo at reducing seizure frequency in patients with co-morbid clinically relevant depressive symptoms [i.e. a Montgomergy-Asberg Depression Rating Scale (MADRS) score of ≥10] [70].
In terms of HR-QOL, unadjusted mean changes in the QOLIE-31 total score at week 14 were significantly (p < 0.001) greater in patients with a seizure frequency reduction of ≥50 % than in those with a seizure frequency reduction of <50 % (5.2 vs. 1.4) and in patients with a seizure frequency reduction of ≥75 % than in those with a seizure frequency reduction of <75 % (7.5 vs. 1.9) (842 evaluable patients) [72]. In terms of QOLIE-31 subscale scores, unadjusted mean changes in scores for cognitive functioning, medication effects, overall quality of life and seizure worry were significantly (p < 0.01) greater in patients with a seizure frequency reduction of ≥50 % than in those with a seizure frequency reduction of <50 % and unadjusted mean changes in scores for cognitive functioning, emotional well-being, energy/fatigue, medication effects, overall quality of life and seizure worry were significantly (p < 0.05) greater in patients with a seizure frequency reduction of ≥75 % than in those with a seizure frequency reduction of <75 % [72].
5.1.2 Long-Term Extensions
Results of 1-year, open-label, extension studies [73, 74] are fully published for trials 2093-301 [9] and 2093-302 [10]. In these extension studies, all patients (n = 314 in 2093-301 [73] and n = 325 in 2093-302 [74]) received an eslicarbazepine acetate starting dosage of 800 mg/day, with subsequent titration to between 400 and 1,200 mg/day (apart from Hungarian patients in the 2093-301 extension, who received a starting dosage of 400 mg/day [73]). These extension studies also assessed HR-QOL, using the QOLIE-31 questionnaire, and depressive symptoms, using MADRS [symptoms scored from 0 (absent/least severe) to 6 (most severe)]. The proportion of patients completing 6 months and 1 year of treatment was 84.9 and 76.6 %, respectively, in 2093-301 [73] and 82.2 and 68.6 %, respectively, in 2093-302 [74].
In addition, long-term data are available from a pooled analysis [75], available as an abstract, of trials 2093-301 [9], 2093-302 [10] and 2093-303 [11].
Adjunctive therapy with eslicarbazepine acetate demonstrated sustained efficacy in patients with refractory partial-onset seizures [73, 74]. In the fully published extension studies, the median absolute seizure frequency per 4 weeks was 6.0 [74] and 5.0 [73] during the first 4 weeks of the extension phase, and ranged from 4.7 to 5.7 [74] and from 3.7 to 4.0 [73] at subsequent time points throughout the 1-year follow-up period; the median absolute seizure frequency per 4 weeks at baseline was 8.4 [74] and 7.2 [73].
The median relative reduction from baseline in seizure frequency was 32.1 [74] and 39.2 % [73] at weeks 1–4, 37.6 [74] and 47.6 % [73] at weeks 5–16, 37.2 [74] and 49.8 % [73] at weeks 17–28, 38.3 [74] and 52.1 % [73] at weeks 29–40, and 39.3 [74] and 56.3 % [73] at weeks 41–52.
The responder rate was 36.6 [74] and 41.0 % [73] at weeks 1–4 and 41.5 [74] and 53.2 % [73] at weeks 41–52, and the proportion of seizure-free patients per 12-week interval was 4.6 [74] and 8.7 % [73] at weeks 5–16 and 10.8 [74] and 12.5 % [73] at weeks 41–52.
In both studies, mean overall QOLIE-31 scores significantly (p < 0.05) increased (i.e. improved) from baseline (from 55.2 to 57.5 [74] and from 54.8 to 58.3 [73]) and overall MADRS scores significantly (p < 0.0001) decreased from baseline (from 7.9 to 6.5 [74] and from 9.5 to 7.2 [73]).
In the pooled analysis of trials 2093-301, 2093-302 and 2093-303, the responder rate was 46.1 and 50.1 % during weeks 5–16 and 41–52, and the proportion of seizure-free patients was 6.3 and 13.6 % at the corresponding time points [75].
5.2 Clinical Practice Studies
This section briefly summarizes the findings of clinical practice studies that included ≥40 evaluable patients and examined the efficacy of eslicarbazepine acetate as adjunctive therapy in patients with partial-onset seizures [76–79] or a broad spectrum of refractory epilepsies [80]. Studies were conducted in Spain (n = 105 [76] and n = 61 [80]), Portugal (n = 154 [77]) and the UK (n = 202 [78] and n = 43 [79]). Three studies are available as abstracts [77–79].
After starting treatment with eslicarbazepine acetate, 20.7 % of patients remained seizure free, 58.4 % had >50 % improvement and 11.5 % had discontinued treatment at 6 months, according to the results of one of the Spanish clinical practice studies [76].
Among the 40 evaluable patients who had been followed-up for ≥3 months in the other Spanish study, the median monthly seizure frequency had significantly (p < 0.001) decreased by 63.6 %, with 30 % of patients experiencing a reduction of ≥80 % [80]. In this study, the retention rate at 3 months was 75.4 % [80].
After 6, 12, 18 and 24 months’ follow-up in the retrospective, single-centre Portuguese study, responder rates were 31.8, 34.1, 28.8 and 25.8 %, respectively, favourable Clinical Global Impression rates were 26.4, 27.3, 25.4 and 19.4 %, respectively, and retention rates were 83, 69.3, 57.8 and 32.4 %, respectively. Eslicarbazepine acetate was discontinued in 15 patients because of lack of efficacy and in 30 patients because of tolerability issues [77].
In a retrospective, multicentre UK study in patients who had received eslicarbazepine acetate for a median duration of 12 months, a ≥50 % reduction in seizure frequency was seen in 52 % of patients, with 19.8 % becoming seizure free [78]. Reasons for discontinuing treatment with eslicarbazepine acetate included lack of efficacy (3.5 % of patients), tolerability issues (21.3 %), both lack of efficacy and tolerability issues (2.0 %) and other (7.4 %) [78].
In the other retrospective, multicentre UK study, 31 of 43 (72.1 %) patients received eslicarbazepine acetate for ≥12 months, with patients discontinuing treatment because of lack of efficacy (14 %) or tolerability issues (14 %) [79]. Of the 31 evaluable patients, 61.3 % had a reduction in seizure frequency of ≥50 % and 12.9 % were seizure free [79].
6 Tolerability
6.1 General Tolerability Profile
Data relating to the tolerability of eslicarbazepine acetate were obtained from pooled analyses of the phase III trials discussed in Sect. 5.1. One prespecified pooled analysis [67] included trials 2093-301 [9], 2093-302 [10] and 2093-303 [11] (n = 1,049) and the other post hoc pooled analyses, available as abstracts [81, 82] and/or posters [81–84], included trials 2093-301 [9], 2093-302 [10] and 2093-304 [12] (n = 1,447). Data regarding the longer-term tolerability of eslicarbazepine acetate was obtained from the 1-year extension studies [73, 74] of trials 2093-301 [9] and 2093-302 [10].
Adjunctive therapy with oral eslicarbazepine acetate was generally well tolerated in patients with refractory partial-onset seizures [67, 83]. The incidence of adverse events increased as the dosage of eslicarbazepine acetate increased [67, 83]. For example, in the pooled analysis of trials 2093-301, 2093-302 and 2093-303, the incidence of treatment-emergent adverse events in patients receiving eslicarbazepine acetate 800 or 1,200 mg/day or placebo was 62.7, 67.5 and 46.4 %, respectively, and adverse events considered to be possibly related to treatment occurred in 47.2, 55.0 and 24.9 % of patients in the corresponding treatment groups [67]. In the pooled analysis of trials 2093-301, 2093-302 and 2093-304, the incidence of treatment-emergent adverse events in patients receiving eslicarbazepine acetate 400, 800 or 1,200 mg/day or placebo was 66.3, 70.8, 78.0 and 57.3 %, respectively [83].
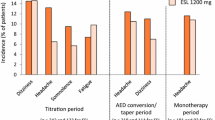
The majority of treatment-emergent adverse events were of mild to moderate severity [67, 83]. The most commonly reported treatment-emergent adverse events (occurring in ≥10 % of patients in any treatment arm) included dizziness [67, 83], somnolence [67, 83], headache [67, 83], nausea [67, 83], diplopia [83] and vomiting [83] (Fig. 2).
Tolerability of oral eslicarbazepine acetate in patients with refractory partial-onset seizures. Adverse events reported in ≥10 % of patients in any treatment arm in a a pooled analysis [67] of trials 2093-301 [9], 2093-302 [10] and 2093-303 [11], and b a pooled analysis, available as a poster [83], of trials 2093-301 [9], 2093-302 [10] and 2093-304 [12]. ESL eslicarbazepine acetate, od once daily, PL placebo
Adverse events occurred more commonly with eslicarbazepine acetate during the first few weeks of treatment [67, 84]. For example, in patients receiving eslicarbazepine acetate 400, 800 or 1,200 mg/day or placebo, treatment-emergent adverse events occurred in 28.6, 31.0, 40.4 and 15.2 % of patients, respectively, during the titration period, in 16.8, 29.6, 33.2 and 18.3 % of patients, respectively, during weeks 1–4, and in 12.8, 10.2, 6.1 and 12.5 % of patients, respectively, during weeks 9–12 [67].
Additional analysis found that the incidence of adverse events was highest during the first week at the target dosage of eslicarbazepine acetate and declined rapidly over the following 3 weeks [84]. Moreover, the incidence of adverse events during the titration period was almost twofold higher in patients who commenced treatment with eslicarbazepine acetate 800 mg without titration versus patients who were titrated to this dosage (66.0 vs. 34.5 %). In patients who initiated treatment with eslicarbazepine acetate 400 mg/day for 1 week, subsequent titration to a maintenance dosage of 800 or 1,200 mg/day had minimal impact on the adverse event profile [84].
Certain adverse events (e.g. dizziness [67, 82], diplopia [67, 82], abnormal coordination [67], vomiting [82], nausea [82]) appeared more common in eslicarbazepine acetate recipients who were receiving concomitant carbamazepine, compared with eslicarbazepine acetate recipients who were not receiving concomitant carbamazepine. By contrast, somnolence and tremor appeared less frequent in eslicarbazepine acetate 1,200 mg/day recipients who were receiving concomitant carbamazepine than in eslicarbazepine acetate 1,200 mg/day recipients who were not receiving concomitant carbamazepine [82].
No dose-related trend was seen for serious adverse events, which were reported in 7.1 % [83] of eslicarbazepine acetate 400 mg/day recipients, 3.5 [67] and 7.0 % [83] of eslicarbazepine acetate 800 mg/day recipients, 3.2 [67] and 2.7 % [83] of eslicarbazepine acetate 1,200 mg/day recipients and 1.4 [67] and 2.8 % [83] of placebo recipients. Serious adverse events included nausea, vomiting, ataxia, diplopia, nystagmus and gait disturbance [83]. Death occurred in one placebo recipient in the pooled analysis of trials 2093-301, 2093-302 and 2093-303 [67], and in two placebo recipients [acute respiratory failure, possible Sudden Unexpected Death in Epilepsy (SUDEP)] and one eslicarbazepine acetate 800 mg/day recipient (status epilepticus) in the pooled analysis of trials 2093-301, 2093-302 and 2093-304 [83]. No deaths attributable to arrhythmia have occurred in the eslicarbazepine acetate development programme [7].
Treatment-emergent adverse events resulting in discontinuation occurred in 9.7 % [83] of eslicarbazepine acetate 400 mg/day recipients, 11.6 [67] and 13.5 % [83] of eslicarbazepine acetate 800 mg/day recipients, 19.3 [67] and 25.4% [83] of eslicarbazepine acetate 1,200 mg/day recipients and 4.5 [67] and 6.6 % [83] of placebo recipients. Treatment-emergent adverse events leading to study discontinuation included vertigo, diplopia, blurred vision, nausea, vomiting, fatigue, dizziness, abnormal coordination, ataxia, headache, somnolence and asthenia [67, 83]. For example, among patients receiving eslicarbazepine 400, 800 or 1,200 mg/day or placebo in the pooled analysis of trials 2093-301, 2093-302 and 2093-304, discontinuation because of dizziness occurred in 1.0, 5.5, 9.0 and 0.5 % of patients, respectively, discontinuation because of nausea occurred in 0.5, 2.2, 6.1 and 0.2 %, respectively, discontinuation because of vomiting occurred in 1.5, 1.9, 4.6 and 0.5 %, respectively, discontinuation because of ataxia occurred in 2.0, 1.9, 3.9 and 0 %, respectively, discontinuation because of diplopia occurred in 1.5, 1.9, 2.9 and 0 %, respectively, and discontinuation because of somnolence occurred in 0.5, 1.4, 2.9 and 0.5 %, respectively [83].
Treatment with eslicarbazepine acetate was not associated with clinically relevant ECG abnormalities [67, 83] (see also Sect. 2.3). In addition, clinically relevant changes in laboratory parameters, vital signs or bodyweight were generally not seen in eslicarbazepine acetate recipients [67, 83]. However, the US prescribing information states that increased transaminase levels have been reported with eslicarbazepine acetate [4].
Eslicarbazepine was generally well tolerated in the longer term [73, 74]. In the 1-year extensions of trials 2093-301 and 2093-302, treatment-emergent adverse events occurring in >10 % of patients included headache (10.2 [73] and 15.7 % [74] of patients), dizziness (10.2 [73] and 26.5 % [74]) and somnolence (12.0 % [74]). The vast majority of treatment-emergent adverse events were of mild to moderate severity [73, 74], and the incidence of treatment-emergent adverse events decreased during the 1-year treatment period [73].
6.2 Specific Adverse Events of Interest
In the pooled analyses, hyponatraemia (serum sodium level of ≤125 mEq/L) occurred in 0.5 % of eslicarbazepine acetate 400 mg/day recipients [67, 83], 0.7 [67] and 1.7 % [83] of eslicarbazepine acetate 800 mg/day recipients, 0.4 [67] and 2.2 % [83] of eslicarbazepine acetate 1,200 mg/day recipients and 0 [67] and 0.2 % [83] of placebo recipients.
In patients receiving eslicarbazepine acetate 400, 800 or 1,200 mg/day or placebo in the pooled analysis of trials 2093-301, 2093-302 and 2093-304, alterations in cognitive function (e.g. irritability, memory impairment, disturbance in attention) occurred in 7.1, 6.0, 8.3 and 4.0 % of patients, respectively [83].
Allergic reactions of any kind occurred in 9.2 % of patients receiving eslicarbazepine acetate 400 mg/day, 9.2 % of patients receiving eslicarbazepine acetate 800 mg/day, 10.5 % of patients receiving eslicarbazepine acetate 1,200 mg/day and 8.5 % of placebo recipients [81]. The most common allergic reactions were rash and pruritus, with rash occurring in 0.5, 1.2 and 3.2 % of eslicarbazepine acetate 400, 800 and 1,200 mg/day recipients, respectively, and in 1.9 % of placebo recipients. Criteria for Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) syndrome were potentially met in one eslicarbazepine acetate 400 mg/day recipient and in one eslicarbazepine acetate 1,200 mg/day recipient [81]. Across the entire eslicarbazepine acetate development programme, three potential cases of DRESS syndrome were identified in patients receiving eslicarbazepine acetate; however, none of these cases were identified as DRESS by the treating physician [83].
The risk of rash did not appear to be related to the starting dosage of eslicarbazepine acetate or to the rate of dose escalation, although the incidence of rash was more than twofold higher in patients maintained at an eslicarbazepine dosage of 1,200 versus 800 mg/day (2.6–4.9 % vs. 0–1.0 %) [84]. Serious skin reactions were reported in one patient receiving eslicarbazepine acetate 800 mg/day (leukocytoclastic vasculitis) and one patient receiving eslicarbazepine acetate 1,200 mg/day (rash) [83]. There were no reports of eslicarbazepine acetate recipients developing Stevens-Johnson syndrome in the placebo-controlled trials [5]. However, the US prescribing information states that have been reports of serious dermatological reactions, including Stevens-Johnson syndrome, associated with the use of eslicarbazepine acetate [4].
7 Dosage and Administration
Eslicarbazepine acetate is indicated in the US for the adjunctive treatment of adults aged ≥18 years with partial-onset seizures [4] and in the EU as adjunctive therapy in adults aged ≥18 years with partial-onset seizures with or without secondary generalization [5].
Eslicarbazepine acetate should be initiated at a dosage of 400 mg once daily [4, 5]. After 1 [4, 5] or 2 [5] weeks, the dosage may be increased to 800 mg once daily. Based on patient response, the eslicarbazepine acetate dosage may be further increased to the maximum recommended dosage of 1,200 mg once daily [4, 5].
It is recommended that eslicarbazepine acetate should be discontinued gradually, to minimize the potential of increased seizure frequency [4, 5].
Local prescribing information should be consulted for contraindications, warning and precautions related to eslicarbazepine acetate.
8 Place of Eslicarbazepine Acetate as Adjunctive Therapy in the Management of Refractory Partial-Onset Seizures
Pharmacological treatment of epilepsy is generally considered necessary after patients have experienced at least two unprovoked seizures [85]. The ultimate goal of epilepsy treatment is to achieve lasting seizure freedom without adverse effects [85]. Satisfactory seizure control can be achieved in approximately two-thirds of patients with epilepsy with the use of AEDs [2]. Treatment with AEDs should be individualized based on seizure type, epilepsy syndrome, comorbidities, co-medications, lifestyle factors and patient/carer preferences [1, 2].
Adjunctive therapy with an AED is recommended when monotherapy with AEDs has not resulted in seizure freedom [2]. Initial options recommended by recent National Institute for Health and Care Excellence guidelines for the adjunctive treatment of patients with refractory partial-onset seizures include carbamazepine, clobazam, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, sodium valproate or topiramate, with alternative options including eslicarbazepine acetate, lacosamide, phenobarbital, phenytoin, pregabalin, tiagabine, vigabatrin or zonisamide [2].
Carbamazepine is a commonly used AED and is generally effective, although 30–40 % of patients may not respond very well to treatment [86]. Adverse events associated with carbamazepine include nausea, headache, dizziness, diplopia and rash [44]. As well as being metabolized to the toxic mesoepoxide metabolite carbamazepine-10,11-epoxide by CYP3A, carbamazepine induces its own hepatic metabolism and that of various other drugs, and may interact with certain other AEDs and other drugs [44, 86].
Oxcarbazepine is a 10-keto analogue of carbamazepine, but does not form a mesoepoxide metabolite [44, 87]. Oxcarbazepine is metabolized to monohydroxycarbazepine (MHD), an enantiomeric mixture comprising eslicarbazepine and R-licarbazepine in a 4:1 ratio [52, 87, 88]. The elimination half-lives of oxcarbazepine and MHD were 1.3–2.3 h and 9.3 h, respectively, and it is recommended that oxcarbazepine be initiated in two divided doses [89].
Eslicarbazepine acetate was specifically designed to circumvent the production of toxic metabolites [86]. The structural differences between eslicarbazepine acetate and carbamazepine mean that eslicarbazepine acetate is not metabolized to carbamazepine-10,11-epoxide and is not susceptible to autoinduction [44, 52, 90]. Exposure to oxcarbazepine following oral administration of eslicarbazepine acetate is minimal, despite oxcarbazepine being a minor metabolite of eslicarbazepine acetate (Sect. 3.2) [27]. Eslicarbazepine acetate is associated with few clinically relevant drug interactions (Sect. 4) and has a longer elimination half-life (Sect. 3.2) than MHD, permitting a convenient once-daily administration regimen [91].
Adjunctive therapy with eslicarbazepine acetate 800 or 1,200 mg/day significantly reduced seizure frequency in patients with refractory partial-onset seizures, according to the results of phase III trials (Sect. 5.1). The patient population in these studies was considered difficult-to-treat, as they had a long history of epilepsy, were receiving at least one concomitant AED and had a high seizure frequency at baseline [9, 10]. The efficacy of eslicarbazepine acetate was maintained over time, according to the results of 1-year extension studies (Sect. 5.1.2). Additional data regarding the longer-term efficacy of eslicarbazepine acetate would be of interest [92]. Data comparing the efficacy of eslicarbazepine acetate with that of other adjunctive AEDs are also needed [92].
Results of clinical practice studies support the efficacy of eslicarbazepine acetate in reducing seizure frequency in real world settings (Sect. 5.2).
Eslicarbazepine acetate appeared effective regardless of whether or not patients were already receiving carbamazepine (Sect. 5.1). Various explanations have been proposed for the efficacy of eslicarbazepine acetate in patients refractory to current treatment with an alternative voltage-gated sodium channel blocker such as carbamazepine. For example, it has been suggested that these patients may have altered voltage-gated sodium channel protein subunits, meaning they have reduced sensitivity to some voltage-gated sodium channel blockers, whilst retaining sensitivity to others [9, 10]. Another possible explanation is that eslicarbazepine acetate has mechanisms of action besides that of voltage-gated sodium channel blockade [9]. Eslicarbazepine acetate should not be administered in combination with oxcarbazepine [4, 5], as coadministration may result in overexposure to the active metabolites [5].
Eslicarbazepine acetate is not currently approved for use in paediatric patients aged <18 years [4, 5], although a phase III trial in children and adolescents with refractory partial-onset seizures examining the efficacy of adjunctive therapy with eslicarbazepine acetate is currently underway [93].
A randomized, double-blind, multicentre, historical control study has also been conducted examining the efficacy of conversion to monotherapy with eslicarbazepine acetate in patients aged ≥16 years with poorly-controlled partial-onset seizures [94], and a phase III trial is underway examining the efficacy of monotherapy with eslicarbazepine acetate in patients aged ≥18 years with newly-diagnosed partial-onset seizures [95].
Oral eslicarbazepine acetate was generally well tolerated in patients with partial-onset seizures. There was a dose-dependent increase in the incidence of adverse events in eslicarbazepine acetate recipients, with the most commonly reported adverse events including dizziness, somnolence, headache, nausea, diplopia and vomiting (Sect. 6.1). Adverse events can be minimized by appropriate titration of the eslicarbazepine acetate dosage [84]. Indeed, it is recommended that eslicarbazepine acetate be initiated at a dosage of 400 mg once daily, with an increase to 800 mg once daily after 1 [4, 5] or 2 [5] weeks (Sect. 7).
Some AEDs (e.g. carbamazepine, phenytoin) have been associated with increased serum lipid levels [96–98]. In a pooled analysis of phase III trials, eslicarbazepine acetate did not appear to affect serum lipid levels, regardless of whether or not patients were receiving concomitant statin therapy (Sect. 2.3). However, eslicarbazepine acetate has been shown to reduce the plasma exposure of simvastatin and rosuvastatin, meaning that statin dosage adjustments may be required if lipid levels are altered to a clinically significant extent (Sect. 4.2). More data in patients receiving concomitant statins would be of interest, given that the pooled analysis only included a small number of patients receiving statins [8].
CNS symptoms such as vertigo and ataxia have been reported with accidental overdose of eslicarbazepine acetate [4, 5]. Other symptoms of overdose include hyponatraemia, nausea and vomiting [4]. There is no specific antidote for eslicarbazepine acetate overdose; patients should be managed with symptomatic and supportive treatment as appropriate [4, 5]. Gastric lavage or administration of activated charcoal may be considered, as should haemodialysis, given that haemodialysis results in partial clearance of eslicarbazepine acetate [4].
Patients with a serum sodium level of <130 mEq/L were excluded from the phase III trials [9, 99]. In these trials, clinically significant hyponatraemia (i.e. a serum sodium level of <125 mEq/L) was reported infrequently in patients receiving eslicarbazepine acetate (Sect. 6.2). Hyponatraemia was dose related and usually appeared within the first 8 weeks of treatment [4]. Depending on the severity of hyponatraemia, the eslicarbazepine acetate dosage may need to be reduced or discontinued [4, 5]. The EU SPC also recommends that serum sodium levels be measured before and during eslicarbazepine acetate treatment in patients who have pre-existing renal disease leading to hyponatraemia or are receiving agents (e.g. diuretics, desmopressin) which may lead to hyponatraemia [5].
In patients of Han Chinese or Thai origin who are receiving carbamazepine, the presence of the HLA-B*1502 allele is strongly associated with the risk of developing severe cutaneous reactions such as Stevens-Johnson syndrome [5]. Given the structural similarities between eslicarbazepine acetate and carbamazepine, it is possible that patients with the HLA-B*1502 allele may be at risk for Stevens-Johnson syndrome after receiving eslicarbazepine acetate [5]. The EU SPC states that whenever possible, genetic testing for the HLA-B*1502 allele is recommended in Han Chinese and Thai patients prior to starting treatment with carbamazepine or related compounds; testing should also be considered in other at-risk Asian populations (e.g. patients from the Philippines or Malaysia) [5]. Although the HLA-A*3101 allele may also be associated with an increased risk of carbamazepine-induced cutaneous reactions, data are currently insufficient to recommend screening for this allele prior to starting treatment with carbamazepine or related compounds [5]. In patients of European or Japanese descent who are known to be positive for the HLA-A*3101 allele, treatment with carbamazepine or related compounds may be considered if the benefits are thought to exceed the risks [5].
In conclusion, adjunctive therapy with eslicarbazepine acetate 800 or 1,200 mg once daily was generally associated with a significantly lower standardized seizure frequency than placebo in patients aged ≥16 or ≥18 years with refractory partial-onset seizures in four phase III trials. In addition, responder rates were significantly higher with eslicarbazepine acetate 1,200 mg once daily than with placebo in all four studies, and with eslicarbazepine acetate 800 mg once daily than with placebo in two studies. The efficacy of eslicarbazepine acetate was maintained in the longer term, according to the results of 1-year extension studies. Adjunctive therapy with oral eslicarbazepine acetate was generally well tolerated in patients with refractory partial-onset seizures, with most adverse events being of mild to moderate severity. Thus, eslicarbazepine acetate is a useful option for the adjunctive treatment of patients with refractory partial-onset seizures.
Data selection sources:
Relevant medical literature (including published and unpublished data) on eslicarbazepine acetate was identified by searching databases including MEDLINE (from 1946) and EMBASE (from 1996) [searches last updated 9 June 2014], bibliographies from published literature, clinical trial registries/databases and websites. Additional information was also requested from the company developing the drug.
Search terms: Eslicarbazepine acetate, epilepsies, partial, partial-onset epilepsy, partial seizures, partial-onset seizures, focal epilepsy
Study selection: Studies in patients with partial-onset seizures who received eslicarbazepine acetate. When available, large, well designed, comparative trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
References
Stephen LJ, Brodie MJ. Antiepileptic drug monotherapy versus polytherapy: pursuing seizure freedom and tolerability in adults. Curr Opin Neurol. 2012;25(2):164–72.
National Institute for Health and Care Excellence. The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care (NICE clinical guideline 137). 2013. http://www.nice.org.uk/nicemedia/live/13635/57779/57779.pdf. Accessed 3 Feb 2014.
Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE commission on classification and terminology, 2005–2009. Epilepsia. 2010;51(4):676–85.
Sunovion Pharmaceuticals Inc. Aptiom® (eslicarbazepine acetate) tablets, for oral use: US prescribing information. 2013. http://www.aptiom.com. Accessed 13 Jun 2014.
European Medicines Agency. Zebinix (eslicarbazepine acetate): EU summary of product characteristics. 2014. http://www.ema.europa.eu. Accessed 13 Jun 2014.
Gil-Nagel A, Lopes-Lima J, Ben-Menachem E, et al. An integrated data analysis from three placebo-controlled clinical studies on over-read electrocardiograms of epileptic patients treated with eslicarbazepine acetate: are there any effects on cardiac impulse transmission? (abstract no. p832). Epilepsia. 2011;52(Suppl 6):252.
Vaisleib I, Penovich P, Shneker B, et al. Effects of eslicarbazepine acetate on cardiac function in patients with refractory partial-onset seizures: a pooled analysis of three phase III controlled studies (abstract no. P3.238 and poster). In: 66th American Academy of Neurology Annual Meeting, 26 April–3 May 2014, Philadelphia, PA.
Blum D, Mintzer S, Wechsler RT, et al. Effects of eslicarbazepine acetate on serum lipids in statin users and non users: pooled analysis of placebo-controlled trials (poster no. 2.139). In: 67th Annual American Epilepsy Society Meeting, 6–10 Dec 2013, Washington, DC.
Elger C, Halász P, Maia J, et al. Efficacy and safety of eslicarbazepine acetate as adjunctive treatment in adults with refractory partial-onset seizures: a randomized, double-blind, placebo-controlled, parallel-group phase III study. Epilepsia. 2009;50(3):454–63.
Ben-Menachem E, Gabbai AA, Hufnagel A, et al. Eslicarbazepine acetate as adjunctive therapy in adult patients with partial epilepsy. Epilepsy Res. 2010;89(2–3):278–85.
Gil-Nagel A, Lopes-Lima J, Almeida L, et al. Efficacy and safety of 800 and 1200 mg eslicarbazepine acetate as adjunctive treatment in adults with refractory partial-onset seizures. Acta Neurol Scand. 2009;120(5):281–7.
Sperling M, Harvey JJ, Biraben A, et al. Adjunctive eslicarbazepine acetate in patients with refractory partial-onset seizures: efficacy results of a 12 week randomized placebo-controlled study (poster no. 3.210). In: 67th Annual American Epilepsy Society Meeting; 6–10 Dec 2013, Washington, DC.
Hebeisen S, Brady K, Konrad D, et al. Inhibitory effects of eslicarbazepine acetate and its metabolites against neuronal voltage-gated sodium channels (abstract no. p851). Epilepsia. 2011;52(Suppl 6):257–8.
Bonifácio MJ, Brady K, Hebeisen S, et al. Effects of eslicarbazepine, R-licarbazepine and oxcarbazepine on ion transmission through CAV2.1 and CAV3.2 channels (abstract no. 3.211). Epilepsy Curr. 2013;13(Suppl 1):415.
Brady K, Hebeisen S, Konrad D, et al. The effects of eslicarbazepine, R-licarbazepine, oxcarbazepine and carbamazepine on ion transmission through CaV3.2 channels (abstract no. p858). Epilepsia. 2011;52(Suppl 6):260.
Wright LC, Bulling A, Hebeisen S, et al. Effects of eslicarbazepine, R-licarbazepine, oxcarbazepine and carbamazepine on glycine GLYRA3 receptor-mediated inward currents (abstract no. p375). Epilepsia. 2011;52(Suppl 6):119.
Bulling A, Hebeisen S, Konrad D, et al. Effects of eslicarbazepine, R-licarbazepine and carbamazepine on NMDA and AMPA receptor-mediated currents (abstract no. p852). Epilepsia. 2011;52(Suppl 6):258.
Soares-da-Silva P, Bulling A, Hebeisen S, et al. The effects of eslicarbazepine, R-licarbazepine and carbamazepine on ion transmission through KV7.2 channels (abstract no. p854). Epilepsia. 2011;52(Suppl 6):258–9.
Bonifácio MJ, Hebeisen S, Soares-da-Silva P. Effects of eslicarbazepine and carbamazepine on ion transmission through CaV2.1 (P/Q-type) and CaV3.2 (T-type) calcium channels (abstract no. P483). Epilepsia. 2013;54(Suppl 3):153.
Bonifacio MJ, Bulling A, Hebeisen S, et al. Eslicarbazepine and R-licarbazepine do not have effects on ion transmission through alpha1, alpha2, alpha3 and alpha5 GABA channels (abstract no. p853). Epilepsia. 2011;52(Suppl 6):258.
Döser A, Dickhof G, Uebachs M, et al. The effects of eslicarbazepine on transient Na+ currents in chronically epileptic human hippocampus (abstract no. P797). Epilepsia. 2013;54(Suppl 3):251.
Pires N, Palma N, Loureiro AI, et al. Effects of eslicarbazepine acetate, eslicarbazepine, carbamazepine and oxcarbazepine in the maximal electroconvulsive shock test in the mice (abstract no. p373). Epilepsia. 2011;52(Suppl 6):118.
Torrão L, Machado R, Pires N, et al. Effects of eslicarbazepine acetate, eslicarbazepine, carbamazepine and oxcarbazepine in the 6-Hz psychomotor seizure model in the mice (abstract no. p374). Epilepsia. 2011;52(Suppl 6):118–9.
Buschmann F, Metternich B, Wagner K, et al. Effects of eslicarbazepine on cognition in patients with focal epilepsy (abstract no. p745). Epilepsia. 2011;52(Suppl 6):226–7.
Levy-Cooperman N, Blum D, Cheng H, et al. Abuse potential assessment of eslicarbazepine acetate in healthy male and female recreational sedative users (poster no. 3.209). In: 67th Annual American Epilepsy Society Meeting, 6–10 Dec 2013, Washington, DC.
Almeida L, Soares-da-Silva P. Eslicarbazepine acetate (BIA 2-093). Neurotherapeutics. 2007;4(1):88–96.
Bialer M, Soares-da-Silva P. Pharmacokinetics and drug interactions of eslicarbazepine acetate. Epilepsia. 2012;53(6):935–46.
Verrotti A, Loiacono G, Rossi A, et al. Eslicarbazepine acetate: an update on efficacy and safety in epilepsy. Epilepsy Res. 2014;108(1):1–10.
Bonifácio MJ, Sheridan RD, Parada A, et al. Interaction of the novel anticonvulsant, BIA 2-093, with voltage-gated sodium channels: comparison with carbamazepine. Epilepsia. 2001;42(5):600–8.
Ambrósio AF, Silva AP, Malva JO, et al. Inhibition of glutamate release by BIA 2-093 and BIA 2-024, two novel derivatives of carbamazepine, due to blockade of sodium but not calcium channels. Biochem Pharmacol. 2001;61(10):1271–5.
Parada A, Soares-da-Silva P. The novel anticonvulsant BIA 2-093 inhibits transmitter release during opening of voltage-gated sodium channels: a comparison with carbamazepine and oxcarbazepine. Neurochem Int. 2002;40(5):435–40.
Doeser A, Soares-da-Silva P, Beck H, et al. The effects of eslicarbazepine on persistent Na+ current and the role of the Na+ channel β subunits. Epilepsy Res. 2014;108(2):202–11.
Benes J, Parada A, Figueiredo AA, et al. Anticonvulsant and sodium channel-blocking properties of novel 10,11-dihydro-5H-dibenz[b, f]azepine-5-carboxamide derivatives. J Med Chem. 1999;42(14):2582–7.
Sierra-Paredes G, Oreiro-García MT, Vázquez-Illanes MD, et al. Effect of eslicarbazepine acetate (BIA 2-093) on latrunculin A-induced seizures and extracellular amino acid concentrations in the rat hippocampus. Epilepsy Res. 2007;77(1):36–43.
Sierra-Paredes G, Núñez-Rodriguez A, Vázquez-López A, et al. Anticonvulsant effect of eslicarbazepine acetate (BIA 2-093) on seizures induced by microperfusion of picrotoxin in the hippocampus of freely moving rats. Epilepsy Res. 2006;72(2–3):140–6.
Potschka H, Soerensen J, Pekcec A, et al. Effect of eslicarbazepine acetate in the corneal kindling progression and the amygdala kindling model of temporal lobe epilepsy. Epilepsy Res. 2014;108(2):212–22.
Milovan D, Almeida L, Romach MK, et al. Effect of eslicarbazepine acetate and oxcarbazepine on cognition and psychomotor function in healthy volunteers. Epilepsy Behav. 2010;18(4):366–73.
Vaz-da-Silva M, Nunes T, Almeida L, et al. Evaluation of eslicarbazepine acetate on cardiac repolarization in a thorough QT/QTc study. J Clin Pharmacol. 2012;52(2):222–33.
Kharidia J, Blum D, Cheng H. A pharmacokinetic study comparing eslicarbazepine acetate administered orally as crushed or intact tablet in healthy volunteers (abstract no. 1.232). Epilepsy Curr. 2013;13(Suppl 1):106.
Loureiro AI, Fernandes-Lopez C, Bonifacio MJ, et al. The metabolism and elimination pathway through glucuronidation of eslicarbazepine acetate and its metabolites (abstract no. p338). Epilepsia. 2011;52(Suppl 6):108.
Perucca E, Elger C, Halász P, et al. Pharmacokinetics of eslicarbazepine acetate at steady-state in adults with partial-onset seizures. Epilepsy Res. 2011;96(1–2):132–9.
Almeida L, Falcão A, Maia J, et al. Single-dose and steady-state pharmacokinetics of eslicarbazepine acetate (BIA 2-093) in healthy elderly and young subjects. J Clin Pharmacol. 2005;45(9):1062–6.
Maia J, Almeida L, Falcão A, et al. Effect of renal impairment on the pharmacokinetics of eslicarbazepine acetate. Int J Clin Pharmacol Ther. 2008;46(3):119–30.
Almeida L, Soares-da-Silva P. Safety, tolerability, and pharmacokinetic profile of BIA 2-093, a novel putative antiepileptic, in a rising multiple-dose study in young healthy humans. J Clin Pharmacol. 2004;44(8):906–18.
Almeida L, Soares-da-Silva P. Safety, tolerability and pharmacokinetic profile of BIA 2-093, a novel putative antiepileptic agent, during first administration to humans. Drugs R D. 2003;4(5):269–84.
Fontes-Ribeiro C, Nunes T, Falcão A, et al. Eslicarbazepine acetate (BIA 2-093): relative bioavailability and bioequivalence of 50 mg/mL oral suspension and 200 mg and 800 mg tablet formulations. Drugs R D. 2005;6(5):253–60.
Almeida L, Potgieter JH, Maia J, et al. Pharmacokinetics of eslicarbazepine acetate in patients with moderate hepatic impairment. Eur J Clin Pharmacol. 2008;64(3):267–73.
Almeida L, Nunes T, Sicard E, et al. Pharmacokinetic interaction study between eslicarbazepine acetate and lamotrigine in healthy subjects. Acta Neurol Scand. 2010;121(4):257–64.
Falcão A, Fuseau E, Nunes T, et al. Pharmacokinetics, drug interactions and exposure-response relationship of eslicarbazepine acetate in adult patients with partial-onset seizures: population pharmacokinetic and pharmacokinetic/pharmacodynamic analyses. CNS Drugs. 2012;26(1):79–91.
Maia J, Vaz-da-Silva M, Almeida L, et al. Effect of food on the pharmacokinetic profile of eslicarbazepine acetate (BIA 2-093). Drugs R D. 2005;6(4):201–6.
Fontes-Ribeiro C, Macedo T, Nunes T, et al. Dosage form proportionality and food effect of the final tablet formulation of eslicarbazepine acetate: randomized, open-label, crossover, single-centre study in healthy volunteers. Drugs R D. 2008;9(6):447–54.
Nunes T, Rocha JF, Falcão A, et al. Steady-state plasma and cerebrospinal fluid pharmacokinetics and tolerability of eslicarbazepine acetate and oxcarbazepine in healthy volunteers. Epilepsia. 2013;54(1):108–16.
Falcão A, Maia J, Almeida L, et al. Effect of gender on the pharmacokinetics of eslicarbazepine acetate (BIA 2-093), a new voltage-gated sodium channel blocker. Biopharm Drug Dispos. 2007;28(5):249–56.
Zhang C, Zuo Z, Kwan P, et al. In vitro transport profile of carbamazepine, oxcarbazepine, eslicarbazepine acetate, and their active metabolites by human P-glycoprotein. Epilepsia. 2011;52(10):1894–904.
Perucca E, Fauchoux N, Falcão A, et al. In healthy subjects, concomitant use of carbamazepine with eslicarbazepine acetate can decrease exposure to eslicarbazepine: lack of pharmacokinetic effects of eslicarbazepine acetate on carbamazepine and its 10,11-epoxide metabolite confirms findings from clinical phase III studies (abstract no. p346). Epilepsia. 2011;52(Suppl 6):110.
Nunes T, Sicard E, Almeida L, et al. Pharmacokinetic interaction study between eslicarbazepine acetate and topiramate in healthy subjects. Curr Med Res Opin. 2010;26(6):1355–62.
Falcão A, Rocha JF, Pinto R, et al. Pharmacokinetic drug interaction study between eslicarbazepine acetate 1200 mg and phenytoin 300 mg: a phase I, open-label, multiple dose administration study in healthy volunteers (abstract no. P528). Epilepsia. 2013;54(Suppl 3):166.
Falcão A, Pinto R, Nunes T, et al. Effect of repeated administration of eslicarbazepine acetate on the pharmacokinetics of simvastatin in healthy subjects. Epilepsy Res. 2013;106(1–2):244–9.
Vaz-da-Silva M, Almeida L, Falcão A, et al. Effect of eslicarbazepine acetate on the steady-state pharmacokinetics and pharmacodynamics of warfarin in healthy subjects during a three-stage, open-label, multiple-dose, single-period study. Clin Ther. 2010;32(1):179–92.
Vaz da Silva M, Costa R, Soares E, et al. Effect of eslicarbazepine acetate on the pharmacokinetics of digoxin in healthy subjects. Fundam Clin Pharmacol. 2009;23(4):509–14.
Falcão A, Vaz-da-Silva M, Gama H, et al. Effect of eslicarbazepine acetate on the pharmacokinetics of a combined ethinylestradiol/levonorgestrel oral contraceptive in healthy women. Epilepsy Res. 2013;105(3):368–76.
Rocha J-F, Vaz-da-Silva M, Almeida L, et al. Effect of eslicarbazepine acetate on the pharmacokinetics of metformin in healthy subjects. Int J Clin Pharmacol Ther. 2009;47(4):255–61.
Vazquez B, Wechsler R, Rosenfeld W, et al. Co-administration of carbamazepine with eslicarbazepine acetate decreases eslicarbazepine exposure: a population pharmacokinetic analysis (abstract no. P3.243 plus poster). In: 66th American Academy of Neurology Annual Meeting, April 26–May 3 2014, Philadelphia, PA.
Jacobson M, Gidal BE, Kharidia J, et al. Effects of concomitant antiepileptic drugs on eslicarbazepine acetate: a population pharmacokinetic analysis (poster no. 3.202). In: 67th Annual American Epilepsy Society Meeting, 6–10 Dec 2013, Washington, DC.
Elger C, Bialer M, Cramer JA, et al. Eslicarbazepine acetate: a double-blind, add-on, placebo-controlled exploratory trial in adult patients with partial-onset seizures. Epilepsia. 2007;48(3):497–504.
US Food and Drug Administration. Medical review(s) for application number 022416Orig1s000. 2013. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/022416Orig1s000TOC.cfm. Accessed 17 March 2014.
Gil-Nagel A, Elger C, Ben-Menachem E, et al. Efficacy and safety of eslicarbazepine acetate as add-on treatment in patients with focal-onset seizures: integrated analysis of pooled data from double-blind phase III clinical studies. Epilepsia. 2013;54(1):98–107.
Biton V, Krauss G, Blum D, et al. Efficacy of eslicarbazepine acetate in patients with refractory partial-onset seizures: a pooled analysis of three phase III controlled studies (poster no. 2.127). In: 67th Annual American Epilepsy Society Meeting, 6–10 Dec 2013, Washington, DC.
Elger C, Steinhoff B, Serratosa J, et al. A post-hoc analysis of the time to onset of efficacy after initiation of eslicarbazepine acetate as adjunctive therapy in adult patients with refractory partial-onset seizures (abstract no. 1.230). Epilepsy Curr. 2013;13(Suppl 1):105–6.
Carreño M, Ben-Menachem E, O’Brien TJ, et al. A post-hoc exploratory analysis of the effect of eslicarbazepine acetate as adjunctive treatment in adult patients with partial onset seizures and comorbid clinically relevant depressive symptoms (abstract no. 3.229). Epilepsy Curr. 2013;13(Suppl 1):422–3.
Chung S, Krauss G, Sperling M, et al. Efficacy of eslicarbazepine acetate in patients with refractory partial-onset seizures treated with or without concomitant carbamazepine: a pooled analysis of three phase III controlled studies (abstract no. P3.239 plus poster). In: 66th American Academy of Neurology Annual Meeting, 26 April–3 May 2014, Philadelphia, PA.
Anastassopoulos KP, Velez F, Sousa R, et al. Impact of seizure frequency reduction on health-related quality of life among clinical trial subjects with refractory partial-onset seizures: a pooled analysis of phase III clinical trials of eslicarbazepine acetate (abstract no. P6.180 plus poster). In: 66th American Academy of Neurology Annual Meeting, 26 April–3 May 2014, Philadelphia, PA.
Halász P, Cramer JA, Hodoba D, et al. Long-term efficacy and safety of eslicarbazepine acetate: results of a 1-year open-label extension study in partial-onset seizures in adults with epilepsy. Epilepsia. 2010;51(10):1963–9.
Hufnagel A, Ben-Menachem E, Gabbai AA, et al. Long-term safety and efficacy of eslicarbazepine acetate as adjunctive therapy in the treatment of partial-onset seizures in adults with epilepsy: results of a 1-year open-label extension study. Epilepsy Res. 2013;103(2–3):262–9.
Gil-Nagel A, Elger C, Ben-Menachem E, et al. Efficacy of eslicarbazepine acetate as adjunctive therapy of adult patients with partial-onset seizures up to one year of follow-up (abstract no. 1277). J Neurol Sci. 2013;333:e43.
Serrano-Castro PJ, Payan-Ortiz M, Cimadevilla JM, et al. Eslicarbazepine acetate in clinical practice: efficacy and safety results (in Spanish). Rev Neurol. 2013;56(6):309–14.
Dias Correia F, Freitas J, Magalhães R, et al. Two-year clinical experience with eslicarbazepine acetate in a tertiary hospital in Porto, Portugal (abstract no. P221). Epilepsia. 2013;54 Suppl 3:75.
Keogh S, McDonald P, Lawthom C, et al. Safety and efficacy of eslicarbazepine acetate (Zebinix) in everyday clinical practice using a retrospective multicentre audit (abstract). J Neurol Sci. 2013;333(Suppl 1):e64.
Damodaran D, Rigby J, Cooper P, et al. Clinical effectiveness of eslicarbazepine acetate (Zebinex) as an add-on therapy in localization related epilepsy over 12 months (abstract no. 3.247). Epilepsy Curr. 2013;13(Suppl):430–1.
Massot A, Vivanco R, Principe A, et al. Post-authorisation study of eslicarbazepine as treatment for drug-resistant epilepsy: preliminary results (in Spanish). Neurologia. 2014;29(2):94–101.
Rogin J, Resnick T, Strom L, et al. Incidence of allergic reaction adverse events during adjunctive treatment with eslicarbazepine (abstract no. P3.241 and poster). In: 66th American Academy of Neurology Annual Meeting, 26 April–3 May 2014, Philadelphia, PA.
Benbadis S, Biton V, Jacobsen M, et al. Safety of eslicarbazepine acetate in patients with refractory partial-onset seizures treated with or without concomitant carbamazepine: a pooled analysis of three phase III controlled studies (abstract no. P3-237 plus poster). In: 66th American Academy of Neurology Annual Meeting, 26 April–3 May 2014, Philadelphia, PA.
Rogin JB, Abou.Khalil B, Blum D, et al. Eslicarbazepine acetate as adjunctive treatment for refractory partial-onset seizures: pooled analysis of safety data from three phase III controlled trials (poster no. 2.126). In: 67th Annual American Epilepsy Society Meeting, 6–10 Dec 2013, Washington, DC.
Krauss G, Biton V, Harvey JJ, et al. Adverse event profile of eslicarbazepine acetate according to dose titration in phase III controlled studies of patients with refractory partial-onset seizures (poster no. 3.208). In: 67th Annual American Epilepsy Society Meeting, 6–10 Dec 2013, Washington, DC.
Perucca E, Tomson T. The pharmacological treatment of epilepsy in adults. Lancet Neurol. 2011;10(5):446–56.
Ambrósio AF, Soares-da-Silva P, Carvalho CM, et al. Mechanisms of action of carbamazepine and its derivatives, oxcarbazepine, BIA 2-093, and BIA 2-024. Neurochem Res. 2002;27(1–2):121–30.
Volosov A, Xiaodong S, Perucca E, et al. Enantioselective pharmacokinetics of 10-hydroxycarbazepine after oral administration of oxcarbazepine to healthy Chinese subjects. Clin Pharmacol Ther. 1999;66(6):547–53.
Elger C, Bialer M, Falcão A, et al. Pharmacokinetics and tolerability of eslicarbazepine acetate and oxcarbazepine at steady state in healthy volunteers. Epilepsia. 2013;54(8):1453–61.
Novartis Pharmaceuticals UK Ltd. Trileptal (oxcarbazepine) film-coated tablets: UK summary of product characteristics. 2013. http://www.medicines.org.uk/emc/medicine/2673/SPC/Trileptal+150+mg%2c+300+mg%2c+600+mg+Film-coated+tablets/. Accessed 9 May 2014.
Stephen LJ, Brodie MJ. Pharmacotherapy of epilepsy: newly approved and developmental agents. CNS Drugs. 2011;25(2):89–107.
Fattore C, Perucca E. Novel medications for epilepsy. Drugs. 2011;71(16):2151–78.
Chang XC, Yuan H, Wang Y, et al. Eslicarbazepine acetate add-on for drug-resistant partial epilepsy. Cochrane Database Syst Rev. 2011;12:CD008907.
Bial—Portela C.S.A. Eslicarbazepine acetate (BIA 2 093) as therapy for refractory partial seizures in children (ClinicalTrials.gov identifier NCT00988156). US National Institutes of Health, ClinicalTrials.gov. 2013. http://www.clinicaltrials.gov/ct2/show/NCT00988156. Accessed 7 Feb 2014.
Sperling MR, Harvey J, Blum D, et al. Conversion to monotherapy with eslicarbazepine acetate in adults with partial-onset seizures: results of a North-American study (poster). In: 67th Annual American Epilepsy Society Meeting, 6–10 Dec 2013, Washington, DC.
Bial—Portela C.S.A. Efficacy and safety of eslicarbazepine acetate as monotherapy for patients with newly diagnosed partial-onset seizures (ClinicalTrials.gov identifier NCT01162460). US National Institutes of Health, ClinicalTrials.gov. 2013. http://www.clinicaltrials.gov/ct2/show/NCT01162460. Accessed 7 Feb 2014.
Nikolaos T, Stylianos G, Chryssoula N, et al. The effect of long-term antiepileptic treatment on serum cholesterol (TC, HDL, LDL) and triglyceride levels in adult epileptic patients on monotherapy. Med Sci Monit. 2004;10(4):MT50-2.
Brämswig S, Sudhop T, Luers C, et al. Lipoprotein(a) concentration increases during treatment with carbamazepine. Epilepsia. 2003;44(3):457–60.
Chuang YC, Chuang HY, Lin TK, et al. Effects of long-term antiepileptic drug monotherapy on vascular risk factors and atherosclerosis. Epilepsia. 2012;53(1):120–8.
European Medicines Agency. Zebinix (eslicarbazepine acetate): EU CHMP assessment report. 2009. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/000988/WC500047229.pdf. Accessed 17 Jan 2014.
Disclosure
The preparation of this review was not supported by any external funding. Gillian Keating is a salaried employee of Adis/Springer. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the author on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keating, G.M. Eslicarbazepine Acetate: A Review of Its Use as Adjunctive Therapy in Refractory Partial-Onset Seizures. CNS Drugs 28, 583–600 (2014). https://doi.org/10.1007/s40263-014-0182-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-014-0182-2