Abstract
Background
This study aimed to explore the effects of the titanium dioxide (TiO2) concentration and particle size in hydrogen peroxide (HP) on tooth bleaching effectiveness and enamel surface properties.
Methods
TiO2 at different concentrations and particle sizes was incorporated into 40% HP gel to form an HP/TiO2 gel. The specimens were randomly divided into 8 groups: C1P20: HP + 1% TiO2 (20 nm); C3P20: HP + 3% TiO2 (20 nm); C5P20: HP + 5% TiO2 (20 nm); C1P100: HP + 1% TiO2 (100 nm); C3P100: HP + 3% TiO2 (100 nm); C5P100: HP + 5% TiO2 (100 nm); C0: HP with LED; and C0-woL: HP without LED. Bleaching was conducted over 2 sessions, each lasting 40 min with a 7-day interval. The color differences (ΔE00), whiteness index for dentistry (WID), surface microhardness, roughness, microstructure, and composition were assessed.
Results
The concentration and particle size of TiO2 significantly affected ΔE00 and ΔWID values, with the C1P100 group showing the greatest ΔE00 values and C1P100, C3P100, and C5P100 groups showing the greatest ΔWID values (p < 0.05). No significant changes were observed in surface microhardness, roughness, microstructure or composition (p > 0.05).
Conclusions
Incorporating 1% TiO2 with a particle size of 100 nm into HP constitutes an effective bleaching strategy to achieve desirable outcomes.
Similar content being viewed by others
Background
In in-office tooth bleaching, high concentrations of hydrogen peroxide (HP) are commonly employed due to their ability to achieve similar results more quickly than lower concentrations of HP or carbamide peroxide [1]. HP penetrates the tooth structure and generates various free radicals, which interact with and decompose stain molecules [2]. Nevertheless, the high concentration of HP might induce heightened tooth sensitivity and pulp inflammation after the whitening procedure [3]. Additionally, it even has an impact on the protein matrix and mechanical properties of the enamel, leading to alterations in the surface microhardness, surface roughness, modulus of elasticity, and morphology [2, 4]. Research indicates that both the treatment effectiveness and side effects depend on the concentration of HP and the application time [5]. Consequently, accelerating the decomposition of HP or minimizing the duration of exposure to bleaching agents is proposed as a strategy to alleviate these side effects [6].
Several catalysis techniques have been reported to effectively enhance the process of dental bleaching [7, 8]. Among these, photocatalytic semiconductors, specifically titanium dioxide (TiO2), have emerged as a cost-effective and photochemically stable option with a proven safety record [9]. When incorporated into bleaching agents, TiO2 stimulates electron excitation, leading to the dissociation of the HP into ROS [10]. This process, in turn, facilitates the degradation of chromogens in the enamel and dentin [11]. Notably, superoxide is the predominant ROS generated, which mitigates the risk of hydroxyl radicals associated with tooth sensitivity [12], thereby enhancing the safety and efficacy of tooth whitening. Moreover, it has been demonstrated that the catalytic effect of TiO2 on tooth bleaching can be enhanced by the application of blue‒violet LEDs [7, 11]. In addition, the emission peak of the blue‒violet LED was well aligned with the absorption peaks of specific pigment molecules in teeth, creating an environment conducive to the disintegration of chromogens into smaller molecules [13]. However, since light of this wavelength has difficulty penetrating tooth enamel, the direct decomposition effect may be limited to a superficial level [14].
The integration of TiO2 with HP has demonstrated promise as a biocompatible approach for enhancing bleaching efficiency while maintaining the physicochemical properties of bleaching agents [7, 10, 15]. However, the results regarding the effectiveness of adding TiO2 are inconsistent. Some studies reported significant improvements, while others found comparable outcomes [16,17,18,19]. These discrepancies might stem from various factors, including the type and concentration of TiO2 [6, 20, 21], fluctuations in the HP concentration employed [20, 22], the presence of additional constituents in the bleaching agent [17, 22], the differences in bleaching procedure [17, 19, 23], or the distinct categories of dental discoloration in the experimental designs [10, 18]. The ongoing debate on this topic suggests that the concentration and particle size of TiO2 could be significant factors [10, 24]. However, no studies have investigated the optimal combination of different particle sizes and concentrations of TiO2, making this study innovative.
Therefore, this study was designed to determine the impact of varying concentrations and particle sizes of TiO2 on the process of dental bleaching. Specifically, the focus was on the effects of TiO2 on color changes and associated surface properties, including the enamel surface microhardness, surface roughness, surface morphology, and surface composition. The tested null hypotheses were as follows: (1) There would be no difference in the bleaching effectiveness between incorporating or not incorporating TiO2 in various combinations of concentrations and particle sizes into the HP gel; (2) There would be no difference in the effect on the surface microhardness, roughness, composition, or morphology of the enamel surface when using HP gel with or without TiO2.
Materials and methods
The research protocol was approved by the Research Ethics Committee at the School and Hospital of Stomatology, Fujian Medical University (approval No. 2023-34).
Specimen preparation
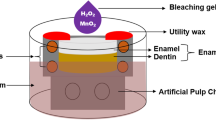
Human premolars, which were extracted for orthodontic purposes and free of evident cracks, were selected. The tooth color was evaluated with a spectrophotometer (Easyshade Advance 4.0, Vita ZahnFabrik, Germany). Only teeth that were darker than shade A3, as specified by the Vita Classical shades, were retained [25]. Blocks (7 mm ×7 mm ×3 mm) were sectioned from the buccal surfaces with a low-speed diamond saw (Isomet, Buehler, USA) and water cooling. After ultrasonic cleaning (KQ3200DE, Kunshan Ultrasonic Instrument, China) for 10 min, the blocks were embedded into cylindrical shapes with diameters of 10 mm and thicknesses of 5 mm using acrylic resin (ZiRan, Nissin, China).
The specimens for color evaluation were randomly selected without any further surface treatment. The specimens for surface microhardness, roughness, microstructure, and composition analysis were ground and polished using silicon carbide water sandpaper (600#, 800#, 1000#, 1200#, 1500#, 2000#, Starcke, Germany). All specimens were stored in artificial saliva (6.8 mM NaCl, 5.4 mM KCl, 5.4 mM CaCl2·2H2O, 0.021 mM NaS·9H2O, 5.0 mM NaH2PO4·H2O, and 16.7 mM urea, and pH = 6.8) until the bleaching procedure [26].
The sample size was calculated utilizing the G*Power program (University of Düsseldorf, Düsseldorf, Germany). For the primary outcome (color difference) measure, a sample size of 10 was determined for each group, with a power of 80%, effect size of 0.40, and α = 0.05 [27]. Based on previous studies [6], 5 specimens per group were needed for surface microhardness and roughness measurements, respectively (Fig. 1).
Characterization of TiO2 particles
The morphologies of TiO2 powder were examined using scanning electron microscopy (SEM) (Sigma 300, Zeiss, Germany). TiO2 powder was dispersed in anhydrous ethanol, followed by 10 min of ultrasonic agitation to facilitate dispersion. Droplets of the solution were placed onto a silicon wafer, air-dried, and sputter-coated with gold (SC7620, Quorum Technologies, UK). The morphology was observed under SEM with an acceleration voltage of 15 kV. The SEM images were captured at a magnification of 100,000×. The particle sizes were assessed with a software program (ImageJ; National Institutes of Health, USA) [27].
Bleaching treatment
Different experimental gel formulations were prepared by incorporating TiO2 powder (Macklin, China) at different concentrations (1%, 3%, and 5%) and with different particle sizes (20 and 100 nm). The specimens were categorized into 8 groups according to the gel formulations:
-
C1P20: 1% TiO2 with a particle size of 20 nm was integrated into a 40% HP gel, applied with LED exposure.
-
C3P20: 3% TiO2 with a particle size of 20 nm was integrated into a 40% HP gel, applied with LED exposure.
-
C5P20: 5% TiO2 with a particle size of 20 nm was integrated into a 40% HP gel, applied with LED exposure.
-
C1P100: 1% TiO2 with a particle size of 100 nm was integrated into a 40% HP gel, applied with LED exposure.
-
C3P100: 3% TiO2 with a particle size of 100 nm was integrated into a 40% HP gel, applied with LED exposure.
-
C5P100: 5% TiO2 with a particle size of 100 nm was integrated into a 40% HP gel, applied with LED exposure.
-
C0: 40% HP gel applied with LED exposure.
-
C0-woL: 40% HP gel applied without LED exposure.
The TiO2 powder was precisely weighed using an electronic analytical balance (ML204, Mettler Toledo, Switzerland) following the mass percentage method. Subsequently, the TiO2 powder was placed into an EP tube, moistened, and blended with 40% HP gel (Opalescence Boost PF 40%, Ultradent, USA). To ensure thorough and uniform mixing of the experimental gel, the EP tube was centrifuged for 1 min using a centrifuge (3–16 L, Sigma‒Aldrich Ltd., USA). This was followed by repeated agitation with a pipette gun and shaking with a mixing machine (Mix-vst, Tuohe, China) for 1 min. The pH of the experimental bleaching gel was monitored using a pH meter (S20K, Mettler Toledo, China) at 0 min, 10 min, and 20 min [28]. The average pH value was recorded after 3 measurements at each time point.
The HP/TiO2 experimental gel was freshly prepared before each application and subsequently loaded into a sterile plastic syringe for consistent application onto the enamel surface of the specimen. The bleaching procedure comprised 2 sessions with a 7-day interval between sessions based on the the manufacturer’s instruction and previous studies [25, 29]. During each bleaching session, a 1 mm thick layer of bleaching gel was applied to the enamel surface and left on the teeth for two 20-min periods, totalling 40 min. The bleaching gel was refreshed after the initial 20 min of bleaching. An LED light system (405 nm ± 5 nm; 10 W, Uvgo, China) was applied for all groups except for group C0-woL. It was used to provide 20 light cycles, each consisting of 1-min exposures interrupted by 1-min intervals of no exposure [30]. After each bleaching session, the specimen surfaces were thoroughly rinsed. Throughout the intervals between the bleaching sessions, the specimens were stored in artificial saliva.
Color evaluation
The CIEDE2000 color difference (ΔE00) was the primary metric for assessing bleaching effects, guided by the 50:50% perceptibility threshold (PT) (ΔE00 = 0.8) and 50:50% acceptability threshold (AT) (ΔE00 = 1.8) [31]. Color measurements were performed at baseline (T0), 1 day after the first session of bleaching (T1), 1 day after the second session of bleaching (T2), and 14 days after bleaching (T3). The spectrophotometer was used to measure the tooth color, specifically the L* (lightness/darkness), a* (red/green chromaticity), and b* (yellow/blue chromaticity) values. Spectrophotometer calibration was performed before each assessment to minimize measurement errors, and silicone rubber molds with positioning holes were used to maintain the repeatability of the color measurements [32]. The parameters L*, a*, and b* were used to calculate C* and H*. The formula used for calculating ΔE00 was as follows [33]:
where ΔL’, ΔC’, and ΔH’ are the differences in lightness, chroma, and hue, respectively, for a pair of specimens in CIEDE2000. The weighting functions SL, SC, and SH adjusted the total color difference for variations in the locations of the color difference pairs in the L*, a*, and b* coordinates. The parametric factors KL, KC, and KH are correction terms for the experimental conditions. In the calculation, all of the parametric factors were set to 1 (KL = KC = KH = 1). RT is a rotation function that accounts for the interaction between chroma and hue differences in the blue region [27].
The whiteness index for dentistry (WID), a reliable CIELAB-based metric developed to assess tooth whiteness, was also utilized. The WID was computed using the following formula [34]: \(\:{\varvec{W}\varvec{I}}_{\varvec{D}}=0.511{\varvec{L}}^{\ast\:}-2.324{\varvec{a}}^{\ast\:}-1.100{\varvec{b}}^{\ast\:}\). Subsequently, the changes (ΔWID) on tooth bleaching treatments can be calculated as [35]: \({\Delta \varvec{W}\varvec{I}}_{\varvec{D}}={\varvec{W}\varvec{I}}_{\varvec{D}}\left(\varvec{t}\varvec{r}\varvec{e}\varvec{a}\varvec{t}\varvec{m}\varvec{e}\varvec{n}\varvec{t}\right)-{\varvec{W}\varvec{I}}_{\varvec{D}}\left(\varvec{b}\varvec{a}\varvec{s}\varvec{e}\varvec{l}\varvec{i}\varvec{n}\varvec{e}\right)\). The PT and AT for ΔWID were 0.72 and 2.62 WID units, respectively [36].
Surface microhardness measurement
Surface microhardness values (SMHs) were assessed using a surface microhardness tester (Wilson VH1102, Buehler, USA) at T0 and T3 [37]. These assessments were performed with a test force of 500 g and a load time of 15 s [38]. Three random test points were selected for each specimen, and the average value obtained from these measurements was taken as the SMH value of each experimental stage.
Surface roughness measurement
At T0 and T3, surface roughness assessments were conducted with a surface profilometer (SEF 680, Kosaka Laboratory, Japan). The measurements were conducted with a sampling length of 2.4 mm and a driving speed of 0.1 mm/s [39]. Three random measurements (average roughness, Ra) were taken, and the average value was considered the surface roughness value of the specimen.
Surface morphology and composition examination
To determine the surface morphologies and compositions, the specimens were randomly selected from each experimental group. SEM (EM8000, KYKY, China) coupled with energy-dispersive X-ray spectroscopy (EDS) (AZtecOne with X-MaxN20, Oxford Instruments, UK) was employed for this purpose. The SEM was configured in low vacuum mode at an accelerating voltage of 5 kV, and the images were captured at a magnification of 5,000× [40].
Statistical analysis
The statistical analyses were conducted using the SPSS statistical software package (SPSS 25.0 for Windows, SPSS, Chicago, IL, USA), and p < 0.05 was considered statistically significant. To assess data normality and homoscedasticity, the Shapiro-Wilk test and Levene’s test were employed [41, 42]. Two-way ANOVA and Tukey post hoc tests were employed to assess the effects of TiO2 particle size and concentration on ΔE00 and ΔWID. One-way ANOVA was utilized to analyze the baseline WID values, surface microhardness values, surface roughness values, and the ratios of surface components.
Results
The average pH of the bleaching gel ranged from 7.29 to 7.59 across each group, indicating near neutrality. This pH stability was consistently maintained throughout the bleaching process, regardless of the concentration and particle size of the TiO2.
Characterization of TiO2 particles
The SEM micrographs of 2 types of TiO2 are shown in Fig. 2. The SEM images revealed that both sizes of TiO2 particles exhibited a similar spheroid morphology. The measured particle sizes were determined to be 28.1 ± 2.1 nm and 89.8 ± 9.6 nm, respectively.
Color evaluation
The ΔE00 values determined for all groups at different time points are presented in Table 1. The WID values at baseline and the changes in the WID (ΔWID) at different time points across all groups are shown in Tables 2 and 3, respectively. There were no significant differences in baseline WID values among the groups (p > 0.05). All groups exhibited ∆E00 values greater than 1.8 and ΔWID values greater than 2.62, both of which exceeded the respective AT. There was no significant difference in the ∆E00 and ΔWID between the control groups (C0 and C0-woL) (p > 0.05). A significant impact of TiO2 concentration (p < 0.05) and particle size (p < 0.05) on both ∆E00 and ΔWID values at T3 was observed. At T3, groups C5P20, C1P100, and C5P100 exhibited notable peaks in ∆E00 values, while groups C1P20, C5P20, C1P100, C3P100, and C5P100 showed significant peaks in ΔWID values, indicating a significant difference compared to the values at T1 and T2 (p < 0.05). In comparison to all the other groups, group C1P100 exhibited significantly higher ∆E00 values at T3 (p < 0.05), and groups C1P100, C3P100, and C5P100 had significantly higher ΔWID values than other groups at T3 (p < 0.05). Across all groups, the ∆E00 values for groups C1P20, C1P100, and C3P100 and the ΔWID values for groups C1P100, and C3P100 at T3 were significantly greater than those of group C0 (p < 0.05). There were no significant differences in ∆E00 values among the groups at T1 and T2 (p > 0.05). However, the group C3P100 exhibited significantly higher WID values than those of the groups C1P20 and C5P20 at both T1 and T2 (p < 0.05).
Surface microhardness measurement
The SMH values before and after bleaching are shown in Fig. 3. No significant changes were observed in the SMHs between T0 and T3 for any of the groups (p > 0.05).
Means and standard deviations of the SMH for each group before and after bleaching
C0: 40% HP with LED; C0-woL: 40% HP without LED; C1P20: 40% HP + 1% TiO2 (20 nm); C3P20: 40% HP + 3% TiO2 (20 nm); C5P20: 40% HP + 5% TiO2 (20 nm); C1P100: 40% HP + 1% TiO2 (100 nm); C3P100: 40% HP + 3% TiO2 (100 nm); C5P100: 40% HP + 5% TiO2 (100 nm)
SMH: surface microhardness. T0: baseline; T3: 14 days after the second session
Surface roughness measurement
The Ra values before and after bleaching are illustrated in Fig. 4. There were no significant changes in the Ra values between T0 and T3 for any of the groups (p > 0.05).
Means and standard deviations of the Ra for each group before and after bleaching
C0: 40% HP with LED; C0-woL: 40% HP without LED; C1P20: 40% HP + 1% TiO2 (20 nm); C3P20: 40% HP + 3% TiO2 (20 nm); C5P20: 40% HP + 5% TiO2 (20 nm); C1P100: 40% HP + 1% TiO2 (100 nm); C3P100: 40% HP + 3% TiO2 (100 nm); C5P100: 40% HP + 5% TiO2 (100 nm)
Ra: average roughness. T0: baseline; T3: 14 days after the second session
Surface morphology and composition examination
Figure 5 displays representative SEM micrographs for the tooth surface after bleaching. No discernible pores or demineralized regions were evident on the enamel surface in any of the groups following the bleaching process. The calcium (Ca)/phosphorus (P) ratios of the enamel, as measured by EDS, are presented in Table 4. There were no significant differences observed in Ca/P ratios among the groups (p = 0.308).
Representative SEM micrographs (×5,000) of tooth surfaces after bleaching
A: 40% HP with LED (C0); B: 40% HP without LED (C0-woL); C: 40% HP + 1% TiO2 (20 nm) (C1P20); D: 40% HP + 1% TiO2 (100 nm) (C1P100); E: 40% HP + 3% TiO2 (20 nm) (C3P20); F: 40% HP + 3% TiO2 (100 nm) (C3P100); G: 40% HP + 5% TiO2 (20 nm) (C5P20); H: 40% HP + 5% TiO2 (100 nm) (C5P100)
Discussion
This study revealed a marked enhancement in bleaching effectiveness upon the addition of TiO2. Incorporating TiO2 at different concentrations and with different particle sizes into the HP gels distinctly altered the tooth color without affecting the surface properties. Consequently, the null hypothesis that there would be no difference in the bleaching effectiveness between incorporating or not incorporating TiO2 in various combinations of concentrations and particle sizes into HP gel was rejected. However, the null hypothesis that there would be no difference in the effect on the surface microhardness, roughness, composition, or morphology of the enamel surface when using HP gel with or without TiO2 was accepted.
Since TiO2 acts as a photocatalyst that requires light activation to enhance the bleaching effect, all groups with added TiO2 underwent LED illumination [11]. Monteiro et al. [10] observed that adding 1% TiO2 to HP resulted in better bleaching results. However, Antunes et al. [21] reported that 5% TiO2 can effectively enhance the bleaching effect, and the best colloidal stability (-44.53 mV) was observed in the bleaching process with a TiO2 concentration lower than 5%. To compare the impact of concentration changes on the bleaching effect, this study selected 1%, 3%, and 5% as the concentration gradient. In addition, the TiO2 particle size significantly affects the photocatalytic performance [43]. However, no related research has focused on its influence on tooth bleaching. In this study, 2 particle sizes of TiO2 powder (20 nm and 100 nm) were selected to compare the impact of this factor on the bleaching effect [24]. Therefore, this study represents a significant advancement as it is the first to investigate the effects of TiO2 on bleaching agents while simultaneously considering different concentrations and particle sizes. Since surface flatness is essential for accurate surface measurements, the natural structure of enamel and dentin influences the color evaluations [6]. Therefore, while some specimens were ground and polished to assess the surface properties, others were left unaltered for color measurements.
In this study, the concentration and particle size of TiO2 were found to significantly influence the tooth color change, particularly with the integration of 1% and 100 nm TiO2. This finding aligns with a previous study [10], although their investigation did not further explore the influence of particle size. The present results suggest that a specific combination of TiO2 concentration and particle size is necessary to enhance the bleaching efficacy of HP gels. Therefore, discrepancies in the particle size of TiO2 used across various studies may influence the determination of the optimal concentration of TiO2 [10, 21]. Sürmelioğlu et al. [23] demonstrated that changes in TiO2 concentration and particle size may affect the persistence of its catalytic effect. Discrepancies in TiO2 concentration and particle size may lead to varying degrees of dispersion and quantum effects, which could influence the interaction with HP and its oxidation properties. In addition to the concentration and particle size, the surface area and crystallinity also play important roles in determining the photocatalytic activity of TiO2 [44]. Badovinac et al. [45] studied the effects of TiO2 crystallinity (anatase, rutile, amorphous) and particle size (ranging from 55 nm to 715 nm) and found that the TiO2 film reached a maximum photocatalytic activity at 200 nm. As the average particle size decreases, an increase in the active surface area provides enhanced photocatalytic activity, but an increase in the number of particle boundaries may lead to a decrease in photocatalytic efficacy [45]. This could explain why the addition of 100 nm TiO2 had a superior bleaching effect compared to that of 20 nm TiO2. Interestingly, the catalytic effect of TiO2 at different concentrations and particle sizes was not significant until T3 based on the ΔE00 values, consistent with previous findings [22, 45]. This phenomenon may be attributed to the residual peroxide remaining on the tooth surface or diffusing into the dental tissue [16]. With the catalytic action of the TiO2 component, the remaining small amount of HP can exert a more pronounced effect [46]. Consequently, groups supplemented with TiO2 began to exhibit a more noticeable color change after a certain period following the bleaching treatment.
Furthermore, the thresholds of perceptibility and acceptability with ΔE00 and ΔWID serve as a quality control tool to guide the evaluation of clinical performance in dentistry. Our study showed that the ΔE00 values in group C1P100 exceed those of other groups, significantly the surpassing AT (1.8 units) [31]. A similar trend was observed in ΔWID values, with groups C1P100, C3P100, and C5P100 exhibiting values greater than those of other groups, significantly exceeding the AT (2.62 units) [36]. The results suggest that the color differences are clinically unacceptable and have significant clinical implications. However, although the trends in ΔE00 and ΔWID values were similar, the 2 indices differed in assessing significant differences among groups. ΔWID values exhibited significant differences at T1 and T2, whereas ΔE00 values did not show differences until T3, consistent with previous studies [6, 21]. This indicates that WID may offer greater sensitivity to tooth color alteration and can more comprehensively assess the color alteration process in conjunction with ΔE00 and the AT.
Moreover, our research revealed that there was no significant difference in bleaching effectiveness whether the 40% HP gel was exposed to LED or not, consistent with previous findings [20, 21]. This suggests that the effect of LED light on teeth may be superficial [14].However, remarkable improvement was achieved when combined with TiO2, highlighting the potential role of TiO2 for further investigation. Current bleaching products typically require 2 to 3 bleaching sessions, each spanning more than 30 min, to achieve satisfactory results [47]. Prolonged exposure to high-concentration bleaching gel may lead to irreversible damage to teeth [48]. Shortening the bleaching duration can mitigate the risks such as free radicals harming the gum tissue or causing dentin sensitivity by reaching the pulp [49]. Maintaining therapeutic efficacy while shortening treatment duration can also enhance patient comfort [50]. Further study could explore whether the addition of TiO2 can potentially shorten the treatment duration while achieving desirable outcomes.
Regarding the surface microhardness, surface roughness, SEM micrographs, EDS spectra, and Ca/P ratios, no significant differences were observed among the groups, consistent with previous studies [6, 17]. It has been proposed that the microhardness and surface roughness of tooth enamel may be influenced by pH [51]. To mitigate potential adverse effects, an experimental agent with a neutral pH was developed in this study, which could reduce tooth sensitivity risk and intensity [52]. In this study, the bleaching gel maintained an average pH range of 7.29 to 7.59 across all groups and was considered safe for dental application.
Based on the current research results, the 40% HP bleaching scheme containing a 1% concentration of TiO2 with a 100 nm particle size exhibited superior bleaching effectiveness. However, these findings are still based on the results of in vitro experiments, and clinical research is needed to further determine the bleaching effect of HP/TiO2 gel on teeth. One limitation of this study was the exclusive use of high concentration (40%) of HP. This choice was primarily dictated by its widespread clinical application. Future research should explore how TiO2 particle size and concentration affect bleaching effectiveness under varying HP concentrations. Additionally, further investigation is warranted to determine whether incorporating different concentrations and particle sizes of TiO2 affects the overall concentration of the bleaching gel. To achieve the clinical application of HP gel doped with TiO2, it is essential to thoroughly evaluate its physicochemical properties, effects on dental pulp and cell viability, and further assess it within randomized clinical trials.
Conclusions
The incorporation of TiO2 into HP gels at varying concentrations and particle sizes had a significantly positive impact on the bleaching effect. Specifically, a 40% HP gel containing 1% TiO2 (100 nm) has demonstrated the ability to produce superior tooth bleaching.
Data availability
All data generated or analyzed during this study are included in this manuscript.
Abbreviations
- HP:
-
Hydrogen peroxide
- ROS:
-
Reactive oxygen species
- TiO2 :
-
Titanium dioxide
- N-TiO2 :
-
Nitrogen-doped titanium dioxide
- PT:
-
50:50% perceptibility threshold
- AT:
-
50:50% acceptability threshold
- SMHs:
-
Surface microhardness values
- SEM:
-
Scanning electron microscopy
- EDS:
-
Energy-dispersive X-ray spectroscopy
References
Maran BM, de Paris Matos T, de Castro ADS, Vochikovski L, Amadori AL, Loguercio AD, et al. In-office bleaching with low/medium vs. high concentrate hydrogen peroxide: a systematic review and meta-analysis. J Dent. 2020;103:103499. https://doi.org/10.1016/j.jdent.2020.103499.
Kwon SR, Wertz PW. Review of the mechanism of tooth whitening. J Esthet Restor Dent. 2015;27(5):240–57. https://doi.org/10.1111/jerd.12152.
Eimar H, Siciliano R, Abdallah MN, Abi Nader S, Amin WM, Martinez PP, et al. Hydrogen peroxide whitens teeth by oxidizing the organic structure. J Dent. 2012;40:e25–33. https://doi.org/10.1016/j.jdent.2012.08.008.
Carey CM. Tooth whitening: what we now know. J Evid Based Dent Pr. 2014;14:70–6. https://doi.org/10.1016/j.jebdp.2014.02.006.
Soares DG, Basso FG, Hebling J, de Souza Costa CA. Concentrations of and application protocols for hydrogen peroxide bleaching gels: effects on pulp cell viability and whitening efficacy. J Dent. 2014;42(2):185–98. https://doi.org/10.1016/j.jdent.2013.10.021.
Matos I, Kury M, de Melo PBG, de Souza LVS, Esteban Florez FL, Cavalli V. Effects of experimental bleaching gels containing co-doped titanium dioxide and niobium pentoxide combined with violet light. Clin Oral Investig. 2023;27(8):4827–41. https://doi.org/10.1007/s00784-023-05113-z.
Lee JY, Lee ES, Kang SM, Kim BI. Application of quantitative light-induced fluorescence technology for tooth bleaching treatment and its assessment: an in vitro study. Photodiagn Photodyn. 2019;25:208–13. https://doi.org/10.1016/j.pdpdt.2018.12.001.
Bersezio C, Pardo C, Miranda S, Medeiros Maran B, Jorquera G, Rosa da Silva A Jr., et al. Evaluation of the effectiveness in teeth whitening of a single session with 6% hydrogen peroxide Laser/LED system. Photodiagn Photodyn. 2021;36:102532. https://doi.org/10.1016/j.pdpdt.2021.102532.
Zhang H, Yu M, Qin X. Photocatalytic activity of TiO2 nanofibers: the surface crystalline phase matters. Nanomaterials (Basel). 2019;9(4):535. https://doi.org/10.3390/nano9040535.
Monteiro NR, Basting RT, Amaral FLBd, França FMG, Turssi CP, Gomes OP, et al. Titanium dioxide nanotubes incorporated into bleaching agents: physicochemical characterization and enamel color change. J Appl Oral Sci. 2020;28:e20190771. https://doi.org/10.1590/1678-7757-2019-0771.
Tano E, Otsuki M, Kato J, Sadr A, Ikeda M, Tagami J. Effects of 405 nm diode laser on titanium oxide bleaching activation. Photomed Laser Surg. 2012;30(11):648–54. https://doi.org/10.1089/pho.2012.3273.
Saita M, Kobatashi K, Yoshino F, Hase H, Nonami T, Kimoto K, et al. ESR investigation of ROS generated by H2O2 bleaching with TiO2 coated HAp. Dent Mater J. 2012;31(3):458–64. https://doi.org/10.4012/dmj.2011-192.
Kury M, Wada EE, Silva DPd, Tabchoury CPM, Giannini M, Cavalli V. Effect of violet LED light on in-office bleaching protocols: a randomized controlled clinical trial. J Appl Oral Sci. 2020;28:e20190720. https://doi.org/10.1590/1678-7757-2019-0720.
Kury M, Rueggeberg FA, Soto-Montero JR, André CB, Resende BA, Giannini M, et al. Characterization and effectiveness of a violet LED light for in-office whitening. Clin Oral Investig. 2022;26(5):3899–910. https://doi.org/10.1007/s00784-021-04357-x.
Thacker M, Chen YN, Lin CP, Lin FH. Nitrogen-doped titanium dioxide mixed with calcium peroxide and methylcellulose for dental bleaching under visible light activation. Int J Mol Sci. 2021;22(7):3759. https://doi.org/10.3390/ijms22073759.
Martín J, Vildósola P, Bersezio C, Herrera A, Bortolatto J, Saad JR, et al. Effectiveness of 6% hydrogen peroxide concentration for tooth bleaching—A double-blind, randomized clinical trial. J Dent. 2015;43(8):965–72. https://doi.org/10.1016/j.jdent.2015.05.011.
Kolsuz Ozcetin H, Surmelioglu D. Effects of bleaching gel containing TiO(2) and chitosan on tooth surface roughness, microhardness and colour. Aust Dent J. 2020;65(4):269–77. https://doi.org/10.1111/adj.12786.
Cuppini M, Leitune VCB, de SOUZA M, Alves AK, Samuel SMW, Collares FM. In vitro evaluation of visible light-activated titanium dioxide photocatalysis for in-office dental bleaching. Dent Mater J. 2019;38(1):68–74. https://doi.org/10.4012/dmj.2017-199.
Carlos NR, Basting RT, Amaral F, França FMG, Turssi CP, Kantovitz KR, et al. Physicochemical evaluation of hydrogen peroxide bleaching gels containing titanium dioxide catalytic agent, and their influence on dental color change associated with violet LED. Photodiagn Photodyn. 2023;41:103254. https://doi.org/10.1016/j.pdpdt.2022.103254.
Kury M, Hiers RD, Zhao YD, Picolo MZD, Hsieh J, Khajotia SS, et al. Novel experimental in-office bleaching gels containing co-doped titanium dioxide nanoparticles. Nanomaterials (Basel). 2022;12(17):2995. https://doi.org/10.3390/nano12172995.
Antunes EVG, Basting RT, do Amaral FLB, França FMG, Turssi CP, Kantovitz KR, et al. Titanium dioxide nanotubes in a hydrogen peroxide-based bleaching agent: physicochemical properties and effectiveness of dental bleaching under the influence of a poliwave led light activation. Clin Oral Investig. 2023;27(4):1745–55. https://doi.org/10.1007/s00784-022-04802-5.
Ozcetin HK, Surmelioglu D. Three-monthevaluation of a low concentration (6% hydrogen peroxide) experimental bleaching gel containing TiO2 and chitosan: an in vitro study. Color Res Appl. 2020;45(6):1101–8. https://doi.org/10.1002/col.22543.
Sürmelioğlu D, Özçetin HK, Özdemir ZM, Yavuz SA, Aydın U. Effectiveness and SEM–EDX analysis following bleaching with an experimental bleaching gel containing titanium dioxide and/or chitosan. Odontology. 2021;109(1):114–23. https://doi.org/10.1007/s10266-020-00526-8.
Bashir S, Wahab A, Idriss H. Synergism and photocatalytic water splitting to hydrogen over M/TiO2 catalysts: effect of initial particle size of TiO2. Catal Today. 2015;240:242–7. https://doi.org/10.1016/j.cattod.2014.05.034.
Chen Y, Yang S, Hong D, Attin T, Yu H. Short-term effects of stain-causing beverages on tooth bleaching: a randomized controlled clinical trial. J Dent. 2020;95:103318. https://doi.org/10.1016/j.jdent.2020.103318.
Chen J-M, Cheng Y-L, Yang M-H, Su C, Yu H. Enhancing the inhibition of dental erosion and abrasion with quercetin-encapsulated hollow mesoporous silica nanocomposites. Front Bioeng Biotechnol. 2024;12:1343329. https://doi.org/10.3389/fbioe.2024.1343329.
Su C, Lu Z, Ji X, Yu H. Optical properties of recycled zirconia for dental applications. J Prosthet Dent. 2024;S0022–3913(24):00106–9. https://doi.org/10.1016/j.prosdent.2024.02.001.
Soares AF, Bombonatti JFS, Alencar MS, Consolmagno EC, Honório HM, Mondelli RFL. Influence of pH, bleaching agents, and acid etching on surface wear of bovine enamel. J Appl Oral Sci. 2016;24(1):24–30. https://doi.org/10.1590/1678-775720150281.
De Geus J, Wambier L, Kossatz S, Loguercio A, Reis A. At-home vs in-office bleaching: a systematic review and meta-analysis. Oper Dent. 2016;41(4):341–56. https://doi.org/10.2341/15-287-LIT.
Costa JLSG, Besegato JF, Zaniboni JF, Galvani LD, Kuga MC. Effects of tooth bleaching protocols with violet LED and hydrogen peroxide on enamel properties. Photodiagn Photodyn. 2022;38:102733. https://doi.org/10.1016/j.pdpdt.2022.102733.
Paravina RD, Ghinea R, Herrera LJ, Bona AD, Igiel C, Linninger M, et al. Color difference thresholds in dentistry. J Esthet Restor Dent. 2015;27:S1–9. https://doi.org/10.1111/jerd.12149.
Zhong B, Yang S, Hong D, Cheng Y, Attin T, Yu H. The efficacy of at-home, in-office, and combined bleaching regimens: a randomized controlled clinical trial. Oper Dent. 2023;48(3):E71–80. https://doi.org/10.2341/22-099-C.
Luo MR, Cui G, Rigg B. The development of the CIE 2000 colour-difference formula: CIEDE2000. Color Res Appl. 2001;26(5):340–50. https://doi.org/10.1002/col.1049.
del Mar Pérez M, Ghinea R, Rivas MJ, Yebra A, Ionescu AM, Paravina RD, et al. Development of a customized whiteness index for dentistry based on CIELAB color space. Dent Mater. 2016;32(3):461–7. https://doi.org/10.1016/j.dental.2015.12.008.
Pérez MM, Pecho OE, Ghinea R, Pulgar R, Della Bona A. Recent advances in color and whiteness evaluations in dentistry. Curr Dent. 2019;1(1):23–9. https://doi.org/10.2174/2542579X01666180719125137.
Pérez MM, Herrera LJ, Carrillo F, Pecho OE, Dudea D, Gasparik C, et al. Whiteness difference thresholds in dentistry. Dent Mater. 2019;35(2):292–7. https://doi.org/10.1016/j.dental.2018.11.022.
Kutuk ZB, Ergin E, Cakir FY, Gurgan S. Effects of in-office bleaching agent combined with different desensitizing agents on enamel. J Appl Oral Sci. 2018;27:e20180233. https://doi.org/10.1590/1678-7757-2018-0233.
Singh T, Garg S, Dhindsa A, Jain N. Effect of remineralization potential of ACP–CPP with fluoride and ACP–CPP on enamel subsurface lesion in primary and young permanent teeth: in situ study. J South Asian Assoc Pediatr Dent. 2018;1(2):47–53.
Izumida FE, Ribeiro RC, Giampaolo ET, Machado AL, Pavarina AC, Vergani CE. Effect of microwave disinfection on the surface roughness of three denture base resins after tooth brushing. Gerodontology. 2011;28(4):277–82. https://doi.org/10.1111/j.1741-2358.2010.00393.x.
Wang F, Yu T, Chen J. Biaxial flexural strength and translucent characteristics of dental lithium disilicate glass ceramics with different translucencies. J Prosthodont Res. 2019;64(1):71–7. https://doi.org/10.1016/j.jpor.2019.04.007.
Meireles SS, de Oliveira RDB, Barbosa MTG, da Silva KL, Loguercio AD. Efficacy and tooth sensitivity of at-home bleaching in patients with esthetic restorations: a randomized clinical trial. Clin Oral Invest. 2022;26(1):565–73. https://doi.org/10.1007/s00784-021-04035-y.
Daltro TWS, de Almeida SAG, Dias MF, Lins Filho PC, da Silva CHV, Guimarães RP. The influence of violet LED light on tooth bleaching protocols: in vitro study of bleaching effectiveness. Photodiagn Photodyn. 2020;32:102052. https://doi.org/10.1016/j.pdpdt.2020.102052.
Li D, Song H, Meng X, Shen T, Sun J, Han W, et al. Effects of particle size on the structure and photocatalytic performance by alkali-treated TiO2. Nanomaterials (Basel). 2020;10(3):546. https://doi.org/10.3390/nano10030546.
Tian G, Fu H, Jing L, Tian C. Synthesis and photocatalytic activity of stable nanocrystalline TiO2 with high crystallinity and large surface area. J Hazard Mater. 2009;161(2–3):1122–30. https://doi.org/10.1016/j.jhazmat.2008.04.065.
Badovinac IJ, Peter R, Omerzu A, Salamon K, Šarić I, Samaržija A, et al. Grain size effect on photocatalytic activity of TiO2 thin films grown by atomic layer deposition. Thin Solid Films. 2020;709:138215. https://doi.org/10.1016/j.tsf.2020.138215.
Trevisan TC, Bortolatto JF, Rizzi G, Meloto BT, Dantas AAR, de Oliveira Junior OB. Clinical performance of 6% hydrogen peroxide containing TiO(2)N nanoparticles activated by LED in varying wavelengths-a randomized clinical trial. Laser Med Sci. 2022;37(3):2017–24. https://doi.org/10.1007/s10103-021-03464-1.
Basson R, Grobler SR, Kotze Tv, Osman Y. Guidelines for the selection of tooth whitening products amongst those available on the market: material report. SADJ. 2013;68(3):122–9.
de Souza Costa CA, Riehl H, Kina JF, Sacono NT, Hebling J. Human pulp responses to in-office tooth bleaching. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(4):e59–64. https://doi.org/10.1016/j.tripleo.2009.12.002.
Sulieman MA. An overview of tooth-bleaching techniques: chemistry, safety and efficacy. Periodontol 2000. 2008;48(1):148–69. https://doi.org/10.1111/j.1600-0757.2008.00258.x.
Kothari S, Jum’ah AA, Gray AR, Lyons KM, Yap M, Brunton PA. A randomized clinical trial investigating three vital tooth bleaching protocols and associated efficacy, effectiveness and participants’ satisfaction. J Dent. 2020;95:103322. https://doi.org/10.1016/j.jdent.2020.103322.
Sa Y, Sun L, Wang Z, Ma X, Liang S, Xing W, et al. Effects of two in-office bleaching agents with different pH on the structure of human enamel: an in situ and in vitro study. Oper Dent. 2013;38(1):100–10. https://doi.org/10.2341/11-173-L.
Loguercio A, Servat F, Stanislawczuk R, Mena Serrano A, Rezende M, Prieto M, et al. Effect of acidity of in-office bleaching gels on tooth sensitivity and whitening: a two-center double-blind randomized clinical trial. Clin Oral Investig. 2017;21(9):2811–8. https://doi.org/10.1007/s00784-017-2083-5.
Acknowledgements
The authors acknowledge Lin Ling from the Public Technology Service Center of Fujian Medical University for the SEM observation.
Funding
This research project was partially supported by the Fujian Provincial Finance Research Project (2023CZZX01).
Author information
Authors and Affiliations
Contributions
YLC: Conceptualization, Methodology, Investigation, Formal analysis, Resources, Writing–original draft. BJZ&CS: Investigation, Validation, Formal analysis. ZCL: Investigation, Validation, Formal analysis. HY: Supervision, Writing–review and editing, Funding acquisition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethical approval was taken for the study from Institutional Ethical Committee of School and Hospital of Stomatology, Fujian Medical University, China (Approval no. 2023-34). Informed consent was obtained from all subjects or legal guardians, if the subjects were under 18 years old.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cheng, Yl., Zhong, Bj., Su, C. et al. Enhanced tooth bleaching with a hydrogen peroxide/titanium dioxide gel. BMC Oral Health 24, 923 (2024). https://doi.org/10.1186/s12903-024-04691-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-024-04691-y