Abstract
Objective
This study aimed to evaluate the effects of adding chitosan to 35% hydrogen peroxide gels (for in-office bleaching), with or without calcium gluconate, on tooth properties and bleaching efficacy.
Methods
Bovine enamel/dentin specimens (4 × 4 × 2.5 mm) were randomly allocated into groups (n = 10): negative control (unbleached), bleaching with 35% hydrogen peroxide gel (35% HP, commercial gel); 35% HP with 2% chitosan (% wt) (35% HP + chitosan), 35% HP and calcium (35% HP + Ca, commercial gel), and 35% HP + Ca + 2% chitosan. Variation of surface profile (ΔRa) and color analyses (ΔL*, Δa*, Δb*, ΔE*ab, ΔE00, and ΔWID) were performed comparing specimens at baseline (initial) and 24 h after of storage in artificial saliva (final). Surface microhardness (KHN) values and scanning electron microscopy (SEM) images were obtained on conclusion. The data were analyzed by ANOVA and Tukey’s tests (KHN), generalized linear models (ΔL*, ΔEab, ΔE00, ΔWID, ΔRa), and Kruskal–Wallis and Dunn tests (Δa*, Δb*) (α = 0.05).
Results
Considering ΔL*, Δa*, Δb*, ΔE*ab, ΔE00, and ΔWID values, the bleached groups differed from negative control. For ΔRa, chitosan-based groups showed lower variation in surface roughness compared to 35% HP, without significant difference from negative control. For KHN, chitosan groups did not differ from negative control (unbleached control = chitosan groups > 35% HP + Ca > 35% HP). For SEM, slight surface changes were observed in all bleached groups, but the intensity varied according to gel used (35% HP > gels with Ca > gels with chitosan).
Conclusion
Chitosan-enriched hydrogen peroxide gels can reduce negative impacts on tooth properties without affecting bleaching efficacy.
Clinical relevance
Although commercial gels containing remineralizing agents such as calcium reduce the negative effects on the properties of teeth, the addition of chitosan appears to be a promising approach to preservation of dental properties without interfering in bleaching efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dental bleaching is an esthetic treatment considered safe, effective, easy to perform, and has satisfactory esthetic results [1]. In spite of its undisputed efficacy as treatment for changes in tooth color, some adverse results have been reported such as tooth sensitivity [2], reduction in the bond strength [3] and microhardness values [4], slight alteration of surface roughness [5], and alterations in mineral content of enamel [6,7,8] and dentin [9]. While these changes are considered small or reversible, the treatment could reduce the amount of calcium and phosphorus in the tooth structure [6, 7]. The intensity of these adverse effects is associated with properties of the gel such as its pH [10,11,12,13] and the ability to provide availability of the bleaching agent. Other contributory factors include characteristics inherent to the patient, such as the effects of saliva [14] and availability of remineralizing agents during oral health care. As regards care determined by the technique and professional choice, attention is now being given to associating bleaching agents with remineralizing agents.
Some remineralizing compounds, such as fluoride, calcium, amorphous calcium phosphate, and synthetic hydroxyapatite, have been proposed to minimize these adverse effects [5, 15, 16]. Different active principles are being added to bleaching gel in order to prevent (or decrease) demineralization events or tooth sensitivity from arising during bleaching therapies [17]. In this sense, biopolymers have been characterized since they may have remineralizing or anti-erosive potential. Chitosan is a natural molecule derived from the deacetylation of chitin [18], which has a higher potential to bind electrostatically to surfaces with negative zeta potential, as phosphate from dental tissues as well as phosphate, fluoride, and proteins from saliva, promoting remineralization [19,20,21]. Indeed, chitosan acts as a thickener and carrier with its film-forming properties [22], acting as a stable barrier under the enamel or dentin, protecting the tooth from the process of demineralization or erosive events [23]. Chitosan has already been added to the bleaching gel [24, 25] and has shown that it does not interfere with the bleaching efficacy; however, its ability to minimize the adverse effects of bleaching is still unknown.
The risk or intensity of adverse effects arising from tooth bleaching is commonly related to increased concentration of the bleaching gel [26]. In-office bleaching gels are more prone to causing morphological and structural alterations on/in enamel [9, 27]. Thus, this in vitro study aimed to evaluate the effects of adding chitosan to commercial bleaching gels (with or without calcium) on the bleaching efficacy, surface microhardness (KHN), and surface roughness (Ra), by means of scanning electron microscopy (SEM) analyses. The null hypotheses tested in this study were the following: (1) the addition of chitosan to bleaching gels would not interfere in the bleaching efficacy; (2) the addition of chitosan to bleaching gels would not affect the properties of teeth after bleaching.
Materials and methods
Study design
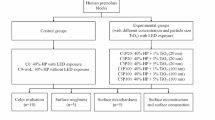
Bovine enamel/dentin blocks were randomly allocated into five groups (n = 10) according to bleaching gel used: unbleached (negative control), 35% hydrogen peroxide, 35% hydrogen peroxide with 2% chitosan, 35% hydrogen peroxide with calcium, 35% hydrogen peroxide with calcium, and 2% chitosan. The blocks were evaluated for color, roughness, microhardness, and surface images obtained by scanning electron microscopy. Information about groups and materials used are detailed in Table 1. The bovine teeth used were all donated from a slaughterhouse, where the animals are slaughtered for another reason and not for research purposes. In the present study, all applicable international guidelines for the care and use of animal portions were followed.
The sample size calculation was conducted using G*Power 3.1.5 (Heine, Universität Dusseldorf, Germany). For this, the following parameters were used: α = 0.05, 1-β = 0.8 (power), and effect size according to more relevant variables. The effect size was calculated based on means and standard deviation (n = 3). Considering differences among experimental bleached groups, the results (variable; effect size; minimum samples required) indicated for general color difference (ΔEab; 0.8873; n = 5), luminosity (ΔL*; 0.5947; n = 9), roughness (ΔRa; 0.8164; n = 6), and microhardness (KHN, 3.4471, n = 2). Thus, the study was conducted with n = 10.
Specimen preparation
Bovine teeth were stored in a 0.01% thymol solution at 4 °C for 30 days until use. A total of 50 specimens measuring 4 × 4 × 2.5 mm were obtained using a metallographic cutter (IsoMet 1000, Buehler Ltd, Lake Bluff, IL, USA) and a diamond disk (Buehler Ltd, Lake Bluff, IL, USA). Silicon carbide abrasive papers (#1200/2500/p4000, Buehler Ltd, Lake Bluff, IL, USA) were used for planing and polishing, under constant irrigation, in a polisher (Aropol E, Arotec, Cotia, SP, Brazil). The final polishing was performed with felt pads (TOP, RAM and SUPRA, Arotec, Cotia, SP, Brazil) associated with diamond pastes (1, 1/2 and 1/4 μm). Between each step and at the end of polishing, the specimens were sonicated (Marconi, Piracicaba, São Paulo, Brazil) in distilled water to remove debris, for 15 min. The specimens were inspected under an optical microscope and those without surface defects or cracks were selected.
Bleaching treatment and group division
For all bleached groups, bleaching treatments were performed in one session according to the manufacturer’s instructions. The 35% hydrogen peroxide bleaching gel (Whiteness HP, FGM, Joinville, Brazil) with or without 2% chitosan was applied three times for 15 min, each time, totaling 45 min. The 35% hydrogen peroxide bleaching gel with calcium (Whiteness HP Blue, FGM Produtos Odontológicos, Santa Catarina, Brazil) with 2% chitosan added, or not, was applied once for 45 min. For chitosan-enriched hydrogen peroxide gels, the chitosan powder (Medium molecular weight, Sigma-Aldrich Co., Madrid, Spain) was weighed using an analytical balance (AUW 220 d, Shimadzu, Kyoto, Japan) considering 2% of the weight of the manipulated gel. After this, the chitosan was incorporated into gel and immediately applied to the specimen surfaces. The chitosan concentration was determined based on previous studies [28, 29] taking account the important properties of the molecule, as molecular weight and degree of acetylation [28]. The chitosan was added to the gel and manually stirred until complete mixture. In pilot tests, no alterations on viscosity and crystallinity of the bleaching gel were verified, being it considered for use.
For the bleaching, the specimens were placed on a glass plate at room temperature. Between the applications of the 35% HP and 35% HP + chitosan, the bleaching gel was carefully removed with cotton. For all bleaching groups, the specimens were washed with distilled water, without difficulties irrespective of the bleaching gel used. When visually cleaned, they were dried with absorbent paper on conclusion of the bleaching session. The specimens were kept in remineralizing solution [30], simulating artificial saliva (1.5-mM Ca; 0.9-mM PO4; and 150-mM KCl in 20-mM Tris buffer, pH 7.0) at 37 °C, for 24 h before and 24 h after the bleaching session.
Color
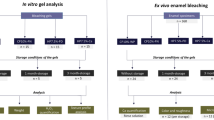
Color measurements were performed at baseline (initial) and 24 h after conclusion of the bleaching treatment. The readings were performed in a light chamber (GTI Mini Matcher MM1e, GTI Graphic Technology Inc., Newburgh, NY, USA) to obtain the standardization of the light incident on the samples and on the spectrophotometer at the time of color analysis. A reflectance spectrophotometer (CM 700D, Konica Minolta, Osaka, Japan) with an aperture size of 3 mm was used, calibrated according to the manufacturer’s recommendations prior to carrying out the readings. The readings were obtained in the coordinates of the CIE L*a*b* system. The differences in the L*, a*, and b* values between the times were expressed as ΔL*, Δa*, and Δb*.
The general color differences between the time intervals were expressed by the values of ΔEab = √ (∆L*)2 + (∆a*)2 + (∆b*)2 and CIEDE 2000 (ΔE00). The values of ∆E00 were calculated as proposed by Sharma et al. [31]. The whiteness index in dentistry (WID) was calculated in each time interval, considering the formula of WID = 0.511L* − 2.324a* − 1.100b* [32], and then its variation (ΔWID) was determined.
Surface roughness
The surface roughness analysis was performed at baseline and 24 h after bleaching procedures using a contact profilometer (Surfcorder SE1700, Kosaka Lab, Akita, Japan). Three different equidistant scans of 1.25 mm each were measured on the surface of each sample, with a cutoff of 0.25 mm and a velocity of 0.1 mm/s, and in all readings, the tip of the profilometer passed through the specimen center. The average of the three readings was considered the roughness (Ra) of each specimen. In order to assess possible changes in the surface profile, the variation in roughness values (ΔRa) were calculated between reading time intervals.
Surface microhardness
Surface microhardness analysis was performed 24 h after the bleaching treatment using a Knoop microhardness tester (HMV-2000, Shimadzu, Tokyo, Japan), with a load of 50 g for 5 s. The final microhardness values (KHN) were obtained by arithmetic mean of 5 indentations made in the central region of the specimen, with a distance of 100 µm between them. This analysis was performed at the end for all groups, so that it would not interfere with the roughness evaluation, which was performed with a contact profilometer.
Scanning electron microscopy (SEM)
For SEM analysis, representative specimens (n = 2) of each group were randomly selected and immersed in solutions with decreasing concentrations of ethanol for 20 min. After this, the specimens were mounted on an acrylic stub under an aluminum tape (3 M Adhesives Ltd., St. Paul, MN, USA) and subjected to sputter-coated under vacuum (Balzers, SCD 050 sputter coater, Germany) with a thin layer of gold. Subsequently, images (× 4000 magnification) were captured of representative areas of the specimens by scanning electron microscopy (JEOL.JSM 5600LV, Tokyo, Japan).
Statistical analysis
Initially, the data were evaluated for normality and homoscedasticity assumptions. Thus, KHN was submitted to one-way analysis of variance (ANOVA) and Tukey’s tests; ΔL*, ΔEab, ΔE00, ΔWID, and ΔRa were evaluated by generalized linear models; Δa* and Δb* results were analyzed with Kruskal–Wallis and Dunn tests. The tests were conducted using R Core Team (R Foundation for Statistical Computing, Vienna, Austria). In all evaluations, the significance level of 5% was considered.
Results
Color analyses
The color results are presented in Table 2 and Table 3. For ΔL*, the bleached groups differed statistically from the negative control (p < 0.01), irrespective of the gel composition. For Δa*, no statistical differences were found between any of the groups (p > 0.05). For Δb*, all bleached groups differed statistically from the negative control group (p < 0.0001). Considering the values found for general color change (Table 3), according to ΔEab (p < 0.05), ΔE00 (p < 0.05), or ΔWID (p < 0.05) values, the chitosan-enriched gels did not differ statistically from its respective commercial bleaching gel; the only differences found were with the negative control (p < 0.01), which was not bleached. For ΔE00 values, a statistical difference between 35% HP + 2% chitosan group and 35% HP + Ca without chitosan was found (p < 0.0001), which obtained lower values.
Surface roughness
The surface roughness results (ΔRa) are shown in Table 4. The initial Ra values were evaluated, and no statistical differences were found (general mean = 0.1113, p = 0.8495 after randomization). Considering ΔRa results, the 35% HP group showed a high positive variation in roughness values, differing from the negative control group (p < 0.01). The 35% HP + 2% chitosan, 35% HP + Ca, and 35% HP + Ca + 2% chitosan groups showed the smallest positive variation in roughness values and did not differ statistically from the negative control (p > 0.05). The 35% HP + 2% chitosan group differed from its respective commercial control (35% HP, p < 0.01); however, the 35% HP + Ca + 2% chitosan group did not differ from its respective commercial control (35% HP + Ca, p > 0.05).
Surface microhardness
The surface microhardness results (KHN) are presented in Table 4. The 35% HP group showed the lowest KHN values and differed statistically from all groups (p < 0.001). The 35% HP + Ca group differed statistically from all groups evaluated and had lower microhardness values than negative control group and higher microhardness values when compared with the 35% HP group. The 35% HP + 2% chitosan and 35% HP + Ca + 2% chitosan groups showed the highest microhardness values among all the bleached groups; chitosan-enriched hydrogen peroxide gels did not differ statistically from the negative control (p > 0.05) and differed from their respective bleaching gel without chitosan group (p < 0.01).
Scanning electron microscopy (SEM)
The SEM images according to the group are shown in Fig. 1. A smooth and uniform enamel surface was observed in the negative control (Fig. 1A), which represents an intact enamel. Slight surface changes were observed in all bleached groups, but the intensity varied according to gel used (35% HP > gel with Ca > gels with chitosan). The 35% HP group exhibited a demineralization pattern, pores, and depressions (Fig. 1B). The 35% HP + Ca showed intermediate surface changes when compared with the other bleached groups (Fig. 1D). The groups with chitosan (Fig. 1C and E) showed fewer surface changes and less evidence of dissolution among all bleached groups and exhibited surface characteristics that were more similar to those of the unbleached control group (Fig. 1A).
Discussion
This study evaluated the addition of 2% chitosan to 35% hydrogen peroxide gels with or without calcium, on the physical properties of the bleached enamel. The first null hypothesis of this study was accepted because the addition of chitosan to bleaching gels did not interfere in the bleaching efficacy; and the second null hypothesis was accepted, because the addition of chitosan to bleaching gels did not affect the properties of teeth after bleaching, whereas commercial gels without chitosan promoted changes in the properties of teeth at different intensities. The addition of chitosan to bleaching gels resulted in minor surface changes after tooth bleaching, and did not interfere in the bleaching efficacy. The bleaching gels without chitosan acted as an active control for the respective gels with the addition of chitosan, which allowed assessment of the effect of 2% chitosan (% wt).
The 35% HP group showed the lowest KHN values and the highest positive variation in roughness values (ΔRa) when compared with the other bleached groups. These changes were probably related to peroxide-based gels with non-neutral pH, capable of causing a modification/dissolution of mineralized structures such as dental enamel [5, 7]. The change in physical properties after bleaching is associated with the effects of demineralization caused by the diffusion of hydrogen peroxide and the acidic pH of the bleaching gels [33,34,35]. In addition, the 35% HP group differed statistically from all bleached groups for all analyses. This difference may have been due to the fact that the 35% HP group was the only bleached group in which the bleaching gel used did not have any remineralizing component in its formulation. The SEM images confirmed the results observed for the 35% HP group, showing greater surface demineralization in the latter, when compared with the other bleached groups.
In this study, the presence of calcium in the bleaching gel resulted in a decrease in deleterious effects after tooth bleaching as has been reported in previous studies [5, 36]. The 35% HP + Ca group showed intermediate microhardness when compared with the other bleached groups. However, in the analysis of roughness, no statistically significant differences were observed when compared with the chitosan groups. The SEM images confirmed these results because the 35% HP + Ca group exhibited intermediate changes when compared with the other bleached groups. The addition of calcium can promote saturation of the bleaching gel, allowing their incorporation into the enamel hydroxyapatite crystals [36, 37]. This would increase the resistance of these crystals to demineralization, reduce the deleterious effects caused by hydrogen peroxide [5, 36, 38, 39], and would act in favor of remineralization after the teeth were exposed to the bleaching gel. Furthermore, enamel demineralization by hydrogen peroxide gels affects the ionic balance, which allows the deposition of calcium on the enamel surface [39]. Despite the beneficial action of calcium, in the present study, its presence alone was not enough to prevent or completely reverse the changes in enamel properties after tooth bleaching, because the 35% HP + Ca group differed statistically from the unbleached group for microhardness evaluation.
The addition of 2% chitosan to the bleaching gels resulted in fewer negative effects after tooth bleaching. The 35% HP + 2% chitosan and 35% HP + Ca + 2% chitosan groups showed the highest microhardness values among the bleached groups, differing from the respective commercial bleaching gel control and being statistically similar to the unbleached group (negative control). These results could be explained by the interaction of chitosan with the dental structure. Due to its positive zetapotential, chitosan electrostatically binds to surfaces with negative ions, such as the enamel surface [40]. This confirmed the ability of chitosan to act as a film-forming agent [22]. The multiple layers of chitosan that form [22] are stable in acidic pH environments [41], such as those found in bleaching gels or on the tooth surface after gel application. In this way, the multiple layers of chitosan may have protected the enamel and influenced the demineralization process. Chitosan can inhibit mineral loss, reducing loss of phosphate ions, depending on the concentration and time of exposure to the biopolymer, in a low pH environment [28], such as the type that could occur during whitening procedures.
Chitosan’s ability to bind to ions may also have influenced the results of surface roughness. The groups with the addition of 2% chitosan showed less variation in roughness compared to 35% HP and did not differ statistically from the negative control group. This result was confirmed in the SEM images once the groups with 2% chitosan showed fewer surface changes and a surface profile similar to those found in the control group. Remineralization and mineral recovery are relevant processes in the oral environment, and the chitosan may possibly be capable of binding to some ions present on the enamel surface [40]. Lee et al. [42] have suggested that adsorption of chitosan onto the artificial saliva layer may have protected the hydroxyapatite crystals by cross-linking between the enamel surface and saliva. Thus, the chitosan layer may have favored the incorporation of minerals, such as calcium and phosphorus present in artificial saliva or the bleaching gel formulation, into the enamel. Taking into account previous studies [42,43,44], it could be assumed that its remineralizing potential in the case of tooth bleaching would be more pronounced under in situ or in vivo conditions.
Relative to the color analysis, the efficacy of bleaching was demonstrated by the increase in L* values and the decrease in b* values, which represent a lighter and less yellowish color of the tooth [45, 46]. Despite the protective effects of chitosan-enriched gels, the color results showed that the addition of 2% chitosan did not affect the result of the bleaching treatment, once all bleached groups showed an increase in L* and decrease in b* values. In addition, for the color change values (ΔE, ΔE00, ΔWID), the bleached groups were similar, with higher values than those of the unbleached group, and higher when compared with the standard values suggested for clinical acceptability of color differences [47]. The color results found in this study are in agreement with the study of Kolsuz Ozcetin and Surmelioglu [24], who showed that the presence of 0.2% of chitosan in the bleaching gel did not interfere in bleaching efficacy. Another investigation [25] showed that there were no differences in the color change of the enamel when a 6% hydrogen peroxide gel was used with or without the addition of chitosan. Thus, the color results found in this study support a safe indication for adding chitosan to bleaching gels considering the bleaching efficacy.
Although the results of this in vitro study cannot fully predict the clinical effectiveness of adding chitosan to the bleaching gel based on 35% hydrogen peroxide and 35% hydrogen peroxide with calcium, it can be suggested that chitosan is a promising agent against the deleterious effects caused by tooth bleaching. Possibly, the results testing chitosan-enriched gels in presence of human saliva would show more beneficial results. Moreover, the stability and storability of the experimental bleaching gels should also be evaluated in future researches. Beyond all of the properties of chitosan above mentioned, this biopolymer is highly biocompatible [48], making it reliable to be used and to have its potential fully explored. Thus, in vitro studies that evaluate the action of chitosan in the bleaching gel on the properties of dentin and in vivo studies are necessary in order to obtain clinical validation of the use of chitosan as an additive to bleaching gel.
Conclusion
Although commercial gels containing calcium decreased the negative impact on the evaluated properties of teeth, the addition of 2% chitosan appeared to be a promising approach to protecting the teeth from alterations in enamel roughness and microhardness without interfering in the bleaching efficacy.
References
Kwon SR, Wertz PW (2015) Review of the mechanism of tooth whitening. J Esthet Restor Dent 27:240–257. https://doi.org/10.1111/jerd.12152
Al-Omiri MK, Al Nazeh AA, Kielbassa AM, Lynch E (2018) Randomized controlled clinical trial on bleaching sensitivity and whitening efficacy of hydrogen peroxide versus combinations of hydrogen peroxide and ozone. Sci Rep 8:1–10. https://doi.org/10.1038/s41598-018-20878-0
Topcu FT, Erdemir U, Ozel E et al (2017) Influence of Bleaching Regimen and Time Elapsed on Microtensile Bond Strength of Resin Composite to Enamel. Contemp Clin Dent 8(3):451–458. https://doi.org/10.4103/ccd.ccd_234_17
Vieira-Junior WF, Lima DANL, Tabchoury CPM et al (2016) Effect of toothpaste application prior to dental bleaching on whitening effectiveness and enamel properties. Oper Dent 41:E29–E38. https://doi.org/10.2341/15-042-L
Vieira I, Vieira WF, Pauli MC et al (2020) Effect of in-office bleaching gels with calcium or fluoride on color, roughness, and enamel microhardness. J Clin Exp Dent 12:e116–e122. https://doi.org/10.4317/JCED.56006
Cavalli V, da Rosa DA, da Silva DP et al (2018) Effects of experimental bleaching agents on the mineral content of sound and demineralized enamels. J Appl Oral Sci 26:e20170589. https://doi.org/10.1590/1678-7757-2017-0589
Vieira-Junior WF, Ferraz LN, Pini NIP et al (2018) Effect of toothpaste use against mineral loss promoted by dental bleaching. Oper Dent 43:190–200. https://doi.org/10.2341/17-024-TR
Dourado Pinto AV, Carlos NR, Amaral FLBD et al (2019) At-home, in-office and combined dental bleaching techniques using hydrogen peroxide: Randomized clinical trial evaluation of effectiveness, clinical parameters and enamel mineral content. Am J Dent 32(3):124–132
Llena C, Esteve I, Forner L (2018) Effects of in-office bleaching on human enamel and dentin. Morphological and mineral changes Ann Anat 217:97–102. https://doi.org/10.1016/j.aanat.2018.01.003
Azrak B, Callaway A, Kurth P, Willershausen B (2010) Influence of bleaching agents on surface roughness of sound or eroded dental enamel specimens. J Esthet Restor Dent 22:391–399. https://doi.org/10.1111/j.1708-8240.2010.00372.x
Xu B, Li Q, Wang Y (2011) Effects of pH Values of Hydrogen Peroxide Bleaching Agents on Enamel Surface Properties. Oper Dent 36:554–562. https://doi.org/10.2341/11-045-1
Sa Y, Sun L, Wang Z et al (2013) Effects of two in-office bleaching agents with different ph on the structure of human enamel: An in situ and in vitro study. Oper Dent 38:100–110. https://doi.org/10.2341/11-173-L
Loguercio AD, Servat F, Stanislawczuk R et al (2017) Effect of acidity of in-office bleaching gels on tooth sensitivity and whitening: a two-center double-blind randomized clinical trial. Clin Oral Investig 21:2811–2818. https://doi.org/10.1007/s00784-017-2083-5
Zeczkowski M, Tenuta LMA, Ambrosano GMB et al (2015) Effect of different storage conditions on the physical properties of bleached enamel: An in vitro vs. in situ study. J Dent 43:1154–1161. https://doi.org/10.1016/j.jdent.2015.06.004
Sasaki RT, Catelan A, BertoldoEdos S et al (2009) Effect of 7.5% hydrogen peroxide containing remineralizing agents on hardness, color change, roughness and micromorphology of human enamel. Am J Dent 28(5):261–267
Ferraz LN, Vieira Júnior WF, Ambrosano GMB et al (2018) Effect of different concentrations of nanohydroxyapatite on tooth bleaching effectiveness and enamel bond strength. Brazilian Dent Sci 21:17–25. https://doi.org/10.14295/bds.2018.v21i1.1512
Chen HP, Chang CH, Liu JK et al (2008) Effect of fluoride containing bleaching agents on enamel surface properties. J Dent 36:718–725. https://doi.org/10.1016/j.jdent.2008.05.003
Muxika A, Etxabide A, Uranga J et al (2017) Chitosan as a bioactive polymer: Processing, properties and applications. Int J Biol Macromol 105:1358–1368. https://doi.org/10.1016/j.ijbiomac.2017.07.087
Svensson O, Lindh L, Cárdenas M, Arnebrant T (2006) Layer-by-layer assembly of mucin and chitosan-Influence of surface properties, concentration and type of mucin. J Colloid Interface Sci 299:608–616. https://doi.org/10.1016/j.jcis.2006.02.027
Van Der Mei HC, Engels E, De Vries J et al (2007) Chitosan adsorption to salivary pellicles. Eur J Oral Sci 115:303–307. https://doi.org/10.1111/j.1600-0722.2007.00454.x
Keegan G, Smart J, Ingram M et al (2012) Chitosan microparticles for the controlled delivery of fluoride. J Dent 40:229–240. https://doi.org/10.1016/j.jdent.2011.12.012
Guo C, Gemeinhart RA (2008) Understanding the adsorption mechanism of chitosan onto poly(lactide-co-glycolide) particles. Eur J Pharm Biopharm 70:597–604. https://doi.org/10.1016/j.ejpb.2008.06.008
Pini NIP, Lima DANL, Luka B et al (2020) Viscosity of chitosan impacts the efficacy of F/Sn containing toothpastes against erosive/abrasive wear in enamel. J Dent 92:103247. https://doi.org/10.1016/j.jdent.2019.103247
Kolsuz Ozcetin H, Surmelioglu D (2020) Effects of bleaching gel containing TiO2 and chitosan on tooth surface roughness, microhardness and colour. Aust Dent J 65:269–277. https://doi.org/10.1111/adj.12786
Sürmelioğlu D, Özçetin HK, Özdemir ZM et al (2021) Effectiveness and SEM–EDX analysis following bleaching with an experimental bleaching gel containing titanium dioxide and/or chitosan. Odontology 109:114–123. https://doi.org/10.1007/s10266-020-00526-8
Lilaj B, Dauti R, Agis H, Schmid-Schwap M et al (2019) Comparison of Bleaching Products With Up to 6% and With More Than 6% Hydrogen Peroxide: Whitening Efficacy Using BI and WI D and Side Effects – An in vitro Study. Front Physiol 10:919. https://doi.org/10.3389/fphys.2019.00919
Abouassi T, Wolkewitz M, Hahn P (2011) Effect of carbamide peroxide and hydrogen peroxide on enamel surface: An in vitro study. Clin Oral Investig 15:673–680. https://doi.org/10.1007/s00784-010-0439-1
Arnaud TMS, De Barros NB, Diniz FB (2010) Chitosan effect on dental enamel de-remineralization: An in vitro evaluation. J Dent 38:848–852. https://doi.org/10.1016/j.jdent.2010.06.004
Mhaske SP, Ambiti R, Jagga U et al (2018) Clinicomicrobiological Evaluation of 2% Chitosan Mouthwashes on Dental Plaque. J Contemp Dent Pract 19(1):94–97. https://doi.org/10.5005/jp-journals-10024-2218
Queiroz CS, Hara AT, Paes Leme AF, Cury JA (2008) pH-Cycling models to evaluate the effect of low fluoride dentifrice on enamel De- and remineralization. Braz Dent J 19:21–27. https://doi.org/10.1590/S0103-64402008000100004
Sharma G, Wu W, Dalal EN (2005) The CIEDE2000 color-difference formula: Implementation notes, supplementary test data, and mathematical observations. Color Res Appl 30:21–30. https://doi.org/10.1002/col.20070
Pérez Mdel M, Ghinea R, Rivas MJ et al (2016) Development of a customized whiteness index for dentistry based on CIELAB color space. Dent Mater 32(3):461–7. https://doi.org/10.1016/j.dental.2015.12.008
Sun L, Liang S, Sa Y et al (2011) Surface alteration of human tooth enamel subjected to acidic and neutral 30% hydrogen peroxide. J Dent 39:686–692. https://doi.org/10.1016/j.jdent.2011.07.011
Deng M, Wen HL, Dong XL et al (2013) Effects of 45S5 bioglass on surface properties of dental enamel subjected to 35% hydrogen peroxide. Int J Oral Sci 5:103–110. https://doi.org/10.1038/ijos.2013.31
Soares AF, Bombonatti JFS, Alencar MS et al (2016) Influence of pH, bleaching agents, and acid etching on surface wear of bovine enamel. J Appl Oral Sci 24:24–30. https://doi.org/10.1590/1678-775720150281
Borges AB, Guimaräes CA, Bresciani E et al (2014) Effect of incorporation of remineralizing agents into bleaching gels on the microhardness of bovine enamel in situ. J Contemp Dent Pract 15(2):195–201. https://doi.org/10.5005/jp-journals-10024-1514
Cavalli V, Rodrigues LK, Paes-Leme AF et al (2010) Effects of bleaching agents containing fluoride and calcium on human enamel. Quintessence Int 41(8):e157–e165
Borges AB, Samezima LY, Fonseca LP et al (2009) Influence of potentially remineralizing agents on bleached enamel microhardness. Oper Dent 34:593–597. https://doi.org/10.2341/08-081-L
Borges BCD, Pinheiro MHM, De Sousa Feitosa DA et al (2012) Preliminary study of a novel in-office bleaching therapy modified with a casein phosphopeptide-amorphous calcium phosphate. Microsc Res Tech 75:1571–1575. https://doi.org/10.1002/jemt.22102
Ganss C, Lussi A, Grunau O et al (2011) Conventional and anti-erosion fluoride toothpastes: Effect on enamel erosion and erosion-abrasion. Caries Res 45:581–589. https://doi.org/10.1159/000334318
Claesson PM, Ninham BW (1992) pH-Dependent Interactions between Adsorbed Chitosan Layers. Langmuir 8:1406–1412. https://doi.org/10.1021/la00041a027
Lee HS, Tsai S, Kuo CC et al (2012) Chitosan adsorption on hydroxyapatite and its role in preventing acid erosion. J Colloid Interface Sci 385:235–243. https://doi.org/10.1016/j.jcis.2012.06.074
Schlueter N, Klimek J, Ganss C (2013) Randomised in situ study on the efficacy of a tin/chitosan toothpaste on erosive-abrasive enamel loss. Caries Res 47:574–581. https://doi.org/10.1159/000351654
Pini NIP, Schlueter N, Sundfeld D et al (2018) Efficacy of Stannous Ions on Enamel Demineralization under Normal and Hyposalivatory Conditions: A Controlled Randomized in situ Pilot Trial. Caries Res 51:543–553. https://doi.org/10.1159/000479041
Cavalli V, da Silva BG, Berger SB et al (2019) Decomposition Rate, pH, and Enamel Color Alteration of At-Home and In-Office Bleaching Agents. Braz Dent J 30:385–396. https://doi.org/10.1590/0103-6440201902484
Vieira WF, Ferraz LN, Giorgi MCC et al (2019) Effect of mouth rinse treatments on bleached enamel properties, surface morphology, and tooth color. Oper Dent 44:178–187. https://doi.org/10.2341/17-250-L
Pérez MM, Pecho OE, Ghinea R et al (2018) Recent Advances in Color and Whiteness Evaluations in Dentistry. Curr Dent 1:23–29. https://doi.org/10.2174/2542579x01666180719125137
Qasim SB, Zafar MS, Najeeb S et al (2018) Electrospinning of Chitosan-Based Solutions for Tissue Engineering and Regenerative Medicine. Int J Mol Sci 19(2):407. https://doi.org/10.3390/ijms19020407
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil (CAPES), Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study does not involve humans or animals. The local ethic committee in research has confirmed that no ethical approval is required.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pini, N.I.P., Piccelli, M.R., Vieira-Junior, W.F. et al. In-office tooth bleaching with chitosan-enriched hydrogen peroxide gels: in vitro results . Clin Oral Invest 26, 471–479 (2022). https://doi.org/10.1007/s00784-021-04021-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-021-04021-4