Abstract
Objective
The study aims to assess the effects of a 10% H2O2 bleaching gel with different MnO2 concentrations on the bleaching efficacy (BE), degradation kinetics (DK) of H2O2, and trans-amelodentinal cytotoxicity (TC).
Materials and methods
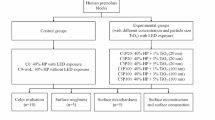
Standardized bovine enamel/dentin disks (n = 96) were placed in artificial pulp chambers, and the bleaching gels were applied for 45 min. Thus, the following groups were established: (G1) no treatment (negative control/NC); (G2) 35% H2O2 (positive control/PC); (G3) 10% H2O2; (G4) 10% H2O2 + 2 mg/mL MnO2; (G5) 10% H2O2 + 6 mg/mL MnO2; and (G6) 10% H2O2 + 10 mg/mL MnO2. After analyzing bleaching efficacy (ΔE00 and ΔWI), the degradation kinetics of H2O2 and trans-amelodentinal cytotoxicity were determined (n = 8, ANOVA/Tukey; p < 0.05).
Results
G6 presented BE (ΔE00 and ΔWI) statistically similar to G2, which represented conventional in-office bleaching (p = 0.6795; p > 0.9999). A significant reduction in the diffusion of H2O2 occurred in G3, G4, G5, and G6 compared to G2 (p < 0.0001). The highest DK of H2O2 occurred in G6 (p < 0.0001), which had the lowest TC in comparison with all other bleached groups (p ≤ 0.0186).
Conclusion
The addition of 10 mg/mL of MnO2 in a 10% H2O2 bleaching gel potentiates the degradation of this reactive molecule, which increases the BE of the product and decreases TC.
Clinical significance
Replacing a 35% H2O2 gel commonly used for conventional in-office dental bleaching by a 10% H2O2 gel containing 10 mg/mL of MnO2 reduces the cytotoxicity of this professional therapy, maintaining its excellent esthetic efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The main questions regarding in-office tooth bleaching are associated with the fact that it may cause bleaching sensitivity (BS) of different intensities [1,2,3]. Studies show that 50 to 93% of patients subjected to in-office bleaching complain of BS [2, 4]. Rezende et al. [5] showed that the risk of patients experiencing this adverse effect at least once during the bleaching protocol is 61%, and on a subjective pain intensity scale from 0 to 4, the mean was 2.8. Bleaching sensitivity relates to the ability of hydrogen peroxide (H2O2) to diffuse in high concentrations through the enamel and dentin, damaging pulp tissue cells [6]. Several researchers showed in vitro that the higher the amount of H2O2 in contact with pulp cells, the more intense the cellular oxidative stress produced [7,8,9]. In vivo, this may cause areas of coronal pulp necrosis and inflammation of different intensities [10, 11].
A number of clinical protocols have been proposed to minimize the adverse effects of in-office tooth bleaching. Among them are the use of desensitizing agents applied topically or incorporated into the bleaching gels [12], the prescription of analgesics or anti-inflammatories [13], and the application of bleaching gels with low H2O2 concentrations [14, 15]. The first two strategies do not prevent H2O2 diffusion through the enamel/dentin and consequent pulp damages. Using gels with low H2O2 concentrations limits the chromatic change of dental tissues [6], which makes this treatment unfeasible because it requires several clinical sessions to promote a satisfactory whitening result. Recently, some studies have shown that the association between the application of ozone (O3) and bleaching gels can enhance the esthetic efficacy and reduce BS in vivo [16, 17]. Another option of strategy widely investigated is adding catalyzing agents in bleaching gels of several H2O2 concentrations may result in a similar esthetic efficacy to that obtained with conventional in-office bleaching [18, 19]. This strategy aims to accelerate the process of H2O2 decomposition by the catalyzing agent, which potentiates the production of other highly reactive oxygen species (ROS) with an extremely short half-life [20]. Thus, after efficiently and rapidly interacting with the chromophores in dental tissues, these new ROS are eliminated, decreasing the possibility of damage to pulp cells [7, 8, 21].
Among the several known catalyzing agents are manganese oxides (MnO2) [20, 22], which are abundantly found on the planet [23] and inexpensive [24]. The catalyst potential of this transition metal is associated with the ability of the mineral phase of Mn particles to react with the H2O2 molecule, stimulating its degradation and consequently producing other ROS in a process similar to the Fenton reaction [25]. Among the reactive molecules produced with H2O2 degradation is the hydroxyl radical (OH·), which may accelerate the chromatic change of darkened dental tissues [8]. Although this important scientific knowledge is available in the literature, the adequate MnO2 concentration added to a 10% H2O2 bleaching gel to obtain a satisfactory result after 45 min of product application on the enamel (one in-office bleaching session) remains unknown.
The present study aimed to assess the effects of a 10% H2O2 bleaching gel with different MnO2 concentrations on the bleaching efficacy, degradation kinetics of H2O2/(OH·) production, and viability of odontoblast-like cells. The null hypothesis of this study was that incorporating MnO2 into the bleaching gel does not affect esthetic efficacy, H2O2 decomposition, and trans-amelodentinal cytotoxicity of the products.
Materials and methods
Sample preparation
Ninety-six enamel/dentin disks were obtained from the buccal surface of the middle third of intact bovine incisors with a trephine diamond bur (Dinser brocas diamantadas; São Paulo, SP, Brazil) attached to a bench drill (FSB 16 Pratika; Schultz, Joinville, SC, Brazil). The disk diameter was standardized at 5.6 mm, with a total thickness (enamel/dentin) of 2.3 ± 0.2 mm; the disks have buccal enamel (0.97 ± 0.10 mm) and dentin (1.33 ± 0.10 mm), by wearing the dentin with sandpapers of granulations of 400 and 600 (T469-SF-Noton; Saint-Gobam Abrasivos, Jundiaí, SP, Brazil) [6,7,8,9].
Sample standardization
Prophylaxis of the enamel surface was performed with pumice and water to eliminate superficial pigments. The dentin was treated with EDTA (ethylenediaminetetraacetic acid; Sigma-Aldrich, St. Louis, MO, USA), 0.5 N per 30 s for smear layer removal [5,6,7,8]. Then, the disks were positioned in a standardized way in a white silicone matrix, maintaining only the enamel surface exposed. The spectrophotometer was placed on each specimen aided by support provided by the appliance, and three initial readings were performed to obtain a mean color value for each sample, determined by L*a*b* coordinates. The L*a*b* coordinate values (Commission Internationale de l'Éclairage) were measured with a UV–Vis reflection spectrophotometer (Color Guide 45/0; BYK-Gardner GmbH, Geretsried, BAV, Germany), with wavelengths ranging from 400 to 700 nm, standard D65 illumination, and 45/0° illumination/observation angle [7, 8].
Sample distributions in the experimental groups
After analyzing the initial color, the disks were subjected to a staining protocol with immersion in 2 mL of black tea solution (Matte Leão, Curitiba, PR, Brazil) for 24 h [6]. To calculate the CIEDE2000, the enamel surface of the disks was subjected to prophylaxis with pumice and water, and then a color reading of the stained disks was performed to determine the mean values of L*a*b* variables [6]. The disks with similar mean CIEDE2000 values were distributed randomly according to the groups presented in Table 1.
Gel formulations and bleaching procedure
The 10% H2O2 bleaching gel was prepared from the dilution of a stock solution of 35% H2O2 (35% hydrogen peroxide; Neon, São Paulo, SP, Brazil) in ultrapure water (ML Ultra-Pure DNase/RNase-Free Distilled Water; Thermo Fisher Scientific, Waltham, MA, USA). The thickener was prepared with 1% carbopol (polyacrylic acid Mv ~ 3,000,000; Sigma-Aldrich, St. Louis, MO, USA) diluted in ultrapure water (Thermo Fisher Scientific) with based pH (8.0).
The manganese oxide chemical activator (MnO2; Santa Cruz Biotechnology, TX, USA) was incorporated into the bleaching gel thickener to maintain the concentrations of the chemical activator at 2 mg/mL, 6 mg/mL, or 10 mg/mL, and H2O2 was added. These concentrations were preset with pilot studies. For the bleaching procedures, 20 µL of the experimental gels and the control were applied to the enamel of disks for 15 min. This procedure was repeated twice more up to 45 min of bleaching treatment (one conventional in-office bleaching session).
Assessment of bleaching efficacy (ΔE00 and ΔWI)
Forty-eight disks (n = 8) randomly distributed among the study groups were stored for 72 h in an environment of 100% humidity and 37 °C. Immediately after concluding the bleaching procedures, the disks were again stored with cotton soaked in distilled water in contact with dentin. A cotton soaked in saliva-like solution (3.9% monobasic potassium phosphate, 3.6% calcium chloride, 2% sodium chloride, 2% potassium chloride, 3.7% magnesium chloride, 0.2% Phenochem, 10% Natrosol gel, distilled water q.s.; Sigma-Aldrich) was placed on the enamel for additional 72 h in an incubator [7,8,9]. Finally, a new color reading of the disks was performed to determine the potential post-treatment chromatic change with the following equations:
-
(I)
CIEDE2000 equation
-
(II)
Whitening index equation
Experimental protocol
Cell cultivation
Immortalized odontoblast-like MDPC-23 cells were used in this study. The cells were cultivated in 96- and 24-well plates (KASVI, Curitiba, PR, Brazil) with Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA); 100 IU/mL and 100 g/mL, respectively, of penicillin and streptomycin (Gibco); and 2 mmol/L of glutamine (Gibco), in a wet atmosphere at 37 °C, 5% CO2, and 95% air.
Experimental procedure
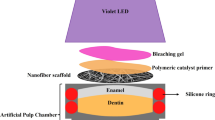
Forty-eight disks (n = 8) were adapted in artificial pulp chambers (APC) with two silicone rings. After sealing the edges of disks with utility wax (Cera 7 Rosa Wilson; Polidental, Cotia, SP, Brazil), the disk/APC sets were sterilized in ethylene oxide (Acecil; Central de Esterilização Comércio e Indústria, Campinas, SP, Brazil) (Fig. 1). Then, the disk/APC sets were placed in 24-well plates (KASVI) with 1 mL of DMEM without FBS so that the solution remained in contact with the dentin and enamel exposed to either receive the treatments or not (Table 1) [6,7,8,9].
Extracts
Immediately after finishing the bleaching procedures, the extracts (DMEM + bleaching gel components diffused through enamel/dentin disks) were collected, homogenized, and divided into aliquots of 100 mL to perform the tests described below.
Quantification of H2O2
To determine the amount of H2O2 diffused through the enamel/dentin disks, an aliquot of 100 μL of the extracts was placed in 24-well plates with 900 μL of an acetate buffer solution (2 mol/M, pH of 4.5). Next, 500 μL of this solution was transferred to tubes with 100 μL of Leuco Crystal Violet reagent (0.5 mg/mL; Sigma-Aldrich), 50 μL of a horseradish peroxidase enzyme solution (1 mg/mL; Sigma-Aldrich), and 2750 mL of distilled water. The absorbance of the solution was measured in a spectrophotometer at a wavelength of 596 nm (Synergy H1; Biotek Instruments, Winooski, VT, EUA). The optical density values were converted into μg of H2O2 per mL of extract (standard curve), and they were transformed in percentage, with the positive control group (G2) representing 100% residual H2O2 diffusion [6,7,8,9].
Degradation kinetics of H2O2
This step aimed to assess whether the presence of the catalyst in bleaching gels induced a higher OH· production in a 15-min interval. To quantify the OH· release of the bleaching gel selected, the fluorescent probe of the OxiSelect Hydroxyl Radical Antioxidant Capacity (HORAC) Activity Assay kit (Cell Biolabs, San Diego, CA, USA) was used, which principle is based on the oxidation of the probe through the OH· by transferring an oxygen atom. Thus, the reaction fluorescence decreases in the presence of OH·. For this analysis, 20 mL of the samples was incubated with 140 mL of the probe, and fluorescence was monitored at 480-nm excitation and 530-nm emission (Synergy H1; Biotek Instruments) for 15 min of incubation at room temperature [6,7,8,9].
Assessment of trans-amelodentinal cytotoxicity
Cell viability (MTT test
An aliquot of 100 mL of extracts was applied for 1 h to MDPC-23 cells (n = 8) previously cultivated in 96-well plates. Then, the extracts were aspirated, and the cells were incubated for 4 h in contact with 90 µL of DMEM + 10 µL of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma-Aldrich) solution at a concentration of 5 mg/mL of PBS and 10:1 ratio. Next, the formazan crystals were diluted in 100 μL of 0.04 N HCI-acidified isopropanol, and absorbance was measured at 570 nm (Synergy H1; Biotek Instruments). The mean absorbance value obtained in the negative control group represented 100% of cell viability, which parameter was used to calculate cell viability in the other groups [6,7,8,9].
Intracellular oxygen reactive species (oxidative stress-H2DCFDA probe
Cells cultivated in 96-well plates (KASVI) were pretreated with the carboxy-H2DCFDA fluorescent probe (Invitrogen, San Francisco, CA, USA) at a concentration of 5 mM, for 30 min at 37 °C, and exposed to 100 μL of extracts. Immediately after, fluorescence was assessed at 492-nm excitation and 518-nm emission (Synergy H1; Biotek Instruments), and the values were normalized with the mean of the negative control group [6,7,8,9].
Live/Dead analysis
This assay was performed with the Live/Dead Cell Viability/Cytotoxicity kit (Invitrogen) (n = 4), which uses the Ethyl homodimer-1 (EthD-1) fluorescence probe that connects DNA bands only to cells with membrane ruptures. The second probe was Calcein AM (CA), which is hydrolyzed with cytoplasmic esterases in viable cells [6,7,8,9].
Statistical analysis
The number of replicates and the sample size for each experiment were confirmed to obtain at least 80% power, using for this purpose the G*Power software (version 3.1, University Dusseldorf, Dusseldorf, Germany). The quantitative data were assessed for adherence to the normal curve, and all data presented normality (Shapiro–Wilk, p > 0.05) and homogeneity of variance (Levene, p > 0.05). Thus, these data were subjected to a one-way or two-way fixed analysis of variance, complemented by Tukey’s post-test. These analyses were performed using the SPSS (version 26.0, IBM, Chicago, IL, USA) and GraphPad Prism (version 9.0, GraphPad, San Diego, CA, USA). All statistical inferences were based on a 5% significance level. The groups were considered statistically different when p < 0.05.
Results
Assessment of bleaching efficacy (ΔE00 and ΔWI)
All bleached groups, regardless of H2O2 concentration or the presence of a catalyst (G2, G3, G4, G5, and G6), showed higher ΔE00 and ΔWI values than those of the negative control (G1) (p < 0.0001) (Fig. 2A and B). The results of the ΔE00 analysis indicated that the presence of a catalyst significantly increases the bleaching efficacy of the 10% H2O2 gel (G4, G5, and G6) compared to the same gel without the catalyst (G3) (p < 0.0001) (Fig. 2A). In G6 the color change was statistically similar to G2 (PC), which represented the conventional in-office bleaching protocol (p = 0.6795) (Fig. 2A). The ΔWI results showed that the presence of the catalyst significantly changes the bleaching potential of the 10% H2O2 gel (p < 0.0001), and G6 (10 mg/mL) presented a similar result to G2 (PC) (p > 0.9999) (Fig. 2B).
Quantification of H2O2 and analysis of its degradation kinetics
There was a significant reduction in the trans-amelodentinal diffusion of H2O2 in G3, G4, G5, and G6 (10% H2O2) compared to G2 (35% H2O2) (p < 0.0001). Regardless of the catalyst concentration added to the gel (G4, G5, and G6), the amount of H2O2 diffused was statistically lower than G3, in which the 10% H2O2 without catalyst addition was used (p ≤ 0.0359) (Fig. 3A). The quantification analysis of OH· radicals showed a higher production rate in G4, G5, and G6 than in G3 (p < 0.0001). However, G6 (10% H2O2 + 10 mg/mL MnO2) presented a statistically higher fluorescence value than the other groups (p < 0.0001), which indicates that the MnO2 concentration in the bleaching gel is directly related to the rate of H2O2 decomposition (Fig. 3B).
A Analysis of H2O2 diffusion. The bars represent mean values and standard deviations of cell viability and oxidative stress. The numbers show the mean values of each group. Different letters indicate a statistically significant difference (ANOVA/Tukey; p < 0.05). B Analysis of OH.· radical production. The data were normalized with the negative control at each analysis time interval. Uppercase and lowercase letters show a statistical difference between the analysis time intervals and among the groups, respectively. The curly brackets indicate the same behavior for the groups selected (ANOVA/Tukey, p < 0.05)
Assessment of cytotoxicity
The cells in G1 (NC) represented 100% viability. Thus, all bleached groups (G2, G3, G4, G5, and G6) showed lower cell viability than G1 (p < 0.0001) (Fig. 4A). When using 10% H2O2 bleaching gels with or without MnO2 (G3, G4, G5, and G6), the viability (p < 0.0001) and oxidative stress (p < 0.0001) values were lower than using 35% H2O2 gels (G2) (Fig. 4A and B). However, the lowest oxidative stress value (p ≤ 0.0772) and highest cell viability level (p ≤ 0.0186) occurred in G6 compared to all the other bleached groups (Fig. 4A and B). The Live/Dead assay showed that cells of all bleached groups had increased positive staining for EthD-1 (red) compared to G1 (Fig. 4C). However, there was a higher number of viable cells — stained with Calcein AM (green) — in groups that used only 10% H2O2 gels with or without MnO2 (G3, G4, G5, and G6) than G2 (Fig. 4C).
A Analysis of cell viability and B oxidative stress. The bars represent mean values and standard deviations of cell viability and oxidative stress. The numbers show the mean values of each group. Different letters indicate a statistically significant difference (ANOVA/Tukey; p < 0.05). C Fluorescence microscopy images of the Live/Dead assay (10 ×). The microscopy images show green-stained cells (Calcein AM) as viable and red-stained cells (EthD-1) in a cell death process
Discussion
The bleaching efficacy analyzed in this laboratory study allowed determining that the H2O2 concentration in the bleaching gel plays an essential role in the esthetic outcome of this therapy. There was an approximate reduction of 44% in ΔE00 and 54% in ΔWI when the 10% H2O2 bleaching gel was used (G3) compared to the 35% H2O2 commercial gel (G2), which represented the positive control group. However, adding a catalyzing agent (MnO2) to the gel, especially at a concentration of 10 mg/mL, significantly increased the bleaching potential of the product, and the esthetic outcome was comparable to that of G2. This positive effect obtained by adding MnO2 to the 10% H2O2 bleaching gel seems to be related to the catalytic ability of transition metals when in contact with oxidative agents, and this action is more intense the higher the local catalyst availability [20, 26]. In this context, researchers could show that bleaching efficacy enhances when using different transition metals such as ferrous sulfate, manganese chloride, and titanium dioxide [8, 19]. The exposure of oxidative agents such as H2O2 to transition metals produces a catalytic reaction similar to the Fenton reaction [25]. Previous studies also showed that the contact of peroxidase or catalase with H2O2 causes an enzymatic redox reaction, which catalyzes this toxic molecule and limits the indirect cell damage from bleaching gels [8, 21].
In the present study, a lower amount of H2O2 was diffused through the enamel/dentin disks in G3 (10% H2O2) than in G2 (35% H2O2). This information confirmed that the amount of H2O2 diffused and reaching the culture medium (extract) is directly related to the concentration of this molecule in the bleaching gel. It is worth noting that the trans-amelodentinal diffusion was even lower when adding the catalyzing agent, regardless of the concentration (G4, G5, and G6), to the 10% H2O2 gel. Previous studies showed that, besides the concentration of H2O2 in the bleaching gel, the time of product application to the enamel is also directly related to the rate of trans-amelodentinal diffusion of this molecule [6, 27]. The higher the amount of H2O2 in the extract, the more intense the oxidative stress and the lower the viability of pulp cells [6, 8, 9]. Therefore, the present investigation showed that the highest H2O2 catalysis in G6 resulted in the lowest diffusion of this reactive molecule, which caused reduced toxicity in MDPC-23 cells. The increased degradation kinetics of H2O2 caused by the presence of MnO2 in the gel generates several by-products, such as the hydroxyl radical (OH·), singlet oxygen (O−2), and peri-hydroxyl (HO2) that present a half-life of 10−9 s, 10−5 s, and 7 s, respectively [20, 28]. Therefore, these free radicals produced by H2O2 catalysis tend to react fast with organic compounds and decompose, and their diffusion within the pulp chamber is unexpected. In the present study, the concentration of 10 mg/mL of MnO2 added to the 10% H2O2 gel (G6) determined the best bleaching efficacy outcome, which was comparable to that obtained with the 35% H2O2 gel (G3). This investigation did not assess concentrations of MnO2 higher than 10 mg/mL, which may characterize a limitation of the study. One may expect that increasing catalyst concentrations in the gel might further benefit the esthetic outcome of the treatment. However, this possibility may not be true, since the excess of free radicals causes these molecules to interact with each other, particularly due to their instability. Consequently, a few free radicals would be available to react with the chromophores in the dental tissues, whose degradation would be harmed [29, 30].
Several clinical studies indicate bleaching sensitivity as the main side effect of this esthetic therapy [1,2,3]. The discomfort reported by most patients subjected to conventional in-office bleaching has been related, at least partially, to the intense oxidative stress induced by H2O2, which produces irreversible pulp cell lesion [31]. In the present study, immortalized MDPC-23 cells, which present an odontoblastic phenotype, were used to assess the potential trans-amelodentinal cytotoxic effect of bleaching gels with different MnO2 concentrations. This is because odontoblasts are the first pulp cells to come into contact with components of dental materials that can diffuse through the hard tissues of the tooth to reach the pulp [32, 33]. Overall, this investigation showed that a number of MDPC-23 cells were irreversibly damaged when applying the 35% H2O2 bleaching gel (G2) for 45 min to the enamel/dentin disks. Clinical/histopathological studies with human teeth showed that cell death after conventional in-office tooth bleaching was not limited to the monolayer of odontoblasts that internally lines the dentin [10, 11]. The extensive and deep areas of cell death observed after a single 45-min session of 35% H2O2 bleaching gel application resulted in the partial necrosis of the coronal pulp, associated with inflammation of the subjacent viable pulp [10, 11, 34, 35]. In the present study, the cytotoxic effects of the bleaching protocols were evaluated using the MTT assay. In spite of this laboratory test determining the level of mitochondrial respiration of cells exposed to experimental materials or their components, it does not provide data concerning the mechanism of cell damage or death [36]. Previous studies demonstrated that mechanisms of action linked to COX-2, IL-6, IL-1β, and TNF-α can be triggered when pulp cells are exposed to H2O2 [37, 38]. The authors also showed that H2O2 induces oxidative stress and apoptosis in pulp cells. These negative side effects are widely reported in vitro and in vivo when bleaching gels with high concentrations of H2O2 are used, decreasing the amount of cells in the pulp that, in turn, may reduce the potential of regeneration of this specialized connective tissue [10, 32]. In the present investigation, the cytotoxicity data obtained show the direct relationship between the rate of cell viability and H2O2 concentration in the bleaching gel. When a pure bleaching gel with 10% H2O2 (G3) or containing 10 mg/mL of MnO2 (G6) was applied to the enamel/dentin disks, the cell viability index was around 24% and 44% higher than in G2, respectively. However, considering that gels with a reduced amount of H2O2 present low bleaching efficacy [15, 39], which determines the need for complementary sessions to reach a satisfactory result [5], using catalysts may have become an interesting strategy to obtain an excellent esthetic outcome and simultaneously reduce the common side effects of this professional therapy [7, 19].
Besides the low cost, manganese-derived ionic compounds work as enzymatic cofactors, and this metal has the potential to work as a central element for producing several other chemical groupings [24, 40, 41]. Combining that with the interesting esthetic outcomes obtained in this study, adding MnO2 to bleaching gels with low H2O2 concentrations seems an attractive and promising strategy. The amount of 10 mg/mL of MnO2 in the bleaching gel worked decisively to maintain the viability of MDPC-23 cells. This catalyst increased the degradation kinetics of the H2O2 molecule, limiting the trans-amelodentinal diffusion of this toxic molecule and consequently reducing the cellular oxidative stress index. In this context, the null hypothesis of this study was rejected.
Besides the excellent results obtained with this investigation, it is worth noting the limitations of in vitro studies, considering that scientific data from laboratory studies should not be immediately extrapolated to clinical conditions [32, 33, 42]. As much as several contemporary laboratory protocols attempt to simulate specific clinical conditions, this objective has not been completely fulfilled [42]. The pulp of vital teeth is characterized as a specialized connective tissue with inherent regeneration potential. Besides containing cytoplasmic prolongations of odontoblasts, collagen, and other structures, the dentinal tubules have a dentinal fluid that exerts constant exudation pressure. All these factors combined, which have not been fully mimicked in the laboratory, interfere with the inward diffusion of components released from dental materials to the pulp tissue. Consequently, greater pulp cell damages are expected from in vitro tests of indirect cytotoxicity of dental materials and new treatment protocols than those of in vivo tests [32, 42]. Another limitation of this study is related to the fact that all analyses of cytotoxicity were performed immediately after the bleaching procedure. Take into consideration that pulp reaction at long term is a fundamental aspect for clinical practice, more studies are necessary to determine the cytotoxic effects of the proposed tooth bleaching protocols over time. Thus, in spite of the interesting data obtained in this investigation, further in vitro and in vivo studies are needed to assess how safe is to use catalyzing agents, such as MnO2, for in-office dental bleaching.
Conclusion
The methodology used in the present study concludes that adding MnO2 to the 10% H2O2 bleaching gel favors H2O2 degradation, significantly increasing the esthetic efficacy of the procedure. The concentration of 10 mg/mL of MnO2 determines an esthetic outcome similar to the one obtained with conventional in-office bleaching and, at the same time, reduces the cytotoxicity of this professional therapy.
References
Maran BM, de Paris Matos T, de Castro ADS et al (2020) In-Office bleaching with low/medium vs. high concentrate hydrogen peroxide: a systematic review and meta-analysis. J Dent 103499. https://doi.org/10.1016/j.jdent.2020.103499
Kielbassa AM, Maier M, Gieren AK, Eliav E (2015) Tooth sensitivity during and after vital tooth bleaching: a systematic review on an unsolved problem. Quintessence Int 46(10). https://doi.org/10.3290/j.qi.a34700
Meireles SS, Santos ME, Lustosa ÍMC et al (2021) Effects of a reduced in-office bleaching protocol with 37.5% hydrogen peroxide on effectiveness and tooth sensitivity: a double-blind randomized clinical trial. J Esthet Restor Dent 33(5):824–831. https://doi.org/10.1111/jerd.12744
Rezende M, da Silva KL, Miguel TC et al (2020) Prior application of 10% potassium nitrate to reduce postbleaching sensitivity: a randomized triple-blind clinical trial. J Evid Based Dent Pract 20(2):101406. https://doi.org/10.1016/j.jebdp.2020.101406
Rezende M, Loguercio AD, Kossatz S et al (2016) Predictive factors on the efficacy and risk/intensity of tooth sensitivity of dental bleaching: a multi regression and logistic analysis. J Dent 45:1–6. https://doi.org/10.1016/j.jdent.2015.11.003
Soares DG, Basso FG, Hebling J et al (2014) Concentrations of and application protocols for hydrogen peroxide bleaching gels: effects on pulp cell viability and whitening efficacy. J Dent 42(2):185–198. https://doi.org/10.1016/j.jdent.2013.10.021
Ortecho-Zuta U, de Oliveira Duque CC, de Oliveira Ribeiro RA et al (2021) Polymeric biomaterials maintained the esthetic efficacy and reduced the cytotoxicity of in-office dental bleaching. J Esthet Restor Dent 33(8):1139–1149. https://doi.org/10.1111/jerd.12805
Soares DG, Marcomini N, Duque CCO et al (2019) Increased whitening efficacy and reduced cytotoxicity are achieved by the chemical activation of a highly concentrated hydrogen peroxide bleaching gel. J Appl Oral Sci 27.https://doi.org/10.1590/1678-7757-2018-0453
Soares DG, Basso FG, Hebling J et al (2015) Effect of hydrogen-peroxide-mediated oxidative stress on human dental pulp cells. J Dent 43(6):750–756. https://doi.org/10.1016/j.jdent.2014.12.006
de Souza Costa CA, Riehl H, Kina JF et al (2010) Human pulp responses to in-office tooth bleaching. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 109(4):e59–e64. https://doi.org/10.1016/j.tripleo.2009.12.002
Kina JF, Huck C, Riehl H et al (2010) Response of human pulps after professionally applied vital tooth bleaching. Int Endod J 43(7):572–580. https://doi.org/10.1111/j.1365-2591.2010.01713.x
Donassollo SH, Donassollo TA, Coser S et al (2021) Triple-blinded randomized clinical trial comparing efficacy and tooth sensitivity of in-office and at-home bleaching techniques. J Appl Oral Sci 29.https://doi.org/10.1590/1678-7757-2020-0794
Santana MLC, Leal PC, Reis A et al (2019) Effect of anti-inflammatory and analgesic drugs for the prevention of bleaching-induced tooth sensitivity: a systematic review and meta-analysis. J Am Dent Assoc 150(10):818–829. https://doi.org/10.1016/j.adaj.2019.05.004
Ferraz NKL, Nogueira LC, Neiva IM et al (2019) Longevity, effectiveness, safety, and impact on quality of life of low-concentration hydrogen peroxides in-office bleaching: a randomized clinical trial. Clin Oral Investig 23(5):2061–2070. https://doi.org/10.1007/s00784-018-2607-7
Bersezio C, Martín J, Angel P et al (2019) Teeth whitening with 6% hydrogen peroxide and its impact on quality of life: 2 years of follow-up. Odontology 107(1):118–125. https://doi.org/10.1007/s10266-018-0372-3
Al-Omiri MK, Al Nazeh AA, Kielbassa AM et al (2018) Randomized controlled clinical trial on bleaching sensitivity and whitening efficacy of hydrogen peroxide versus combinations of hydrogen peroxide and ozone. Sci Rep 8(1):1–10. https://doi.org/10.1038/s41598-018-20878-0
Al-Omiri MK, Lamfon HA, Nazeh AAA et al (2018) Randomized clinical trial on the comparison of bleaching outcomes using either ozone or hydrogen peroxide. Quintessence Int 49(8). https://doi.org/10.3290/j.qi.a40783
Monterubbianesi R, Tosco V, Bellezze T et al (2021) A comparative evaluation of nanohydroxyapatite-enriched hydrogen peroxide home bleaching system on color, hardness and microstructure of dental enamel. Materials 14(11):3072. https://doi.org/10.3390/ma14113072
Bortolatto JF, Trevisan TC, Bernardi PSI et al (2016) A novel approach for in-office tooth bleaching with 6% H2O2/TiO_N and LED/laser system—a controlled, triple-blinded, randomized clinical trial. Lasers Med Sci 31(3):437–444. https://doi.org/10.1007/s10103-016-1866-2
Yao S, Yuan S, Xu J et al (2006) A hydrogen peroxide sensor based on colloidal MnO2/Na-montmorillonite. Appl Clay Sci 33(1):35–42. https://doi.org/10.1016/j.clay.2006.03.006
Ortecho-Zuta U, de Oliveira Duque CC, Leite ML et al (2019) Effects of enzymatic activation of bleaching gels on hydrogen peroxide degradation rates, bleaching effectiveness, and cytotoxicity. Oper Dent 44(4):414–423. https://doi.org/10.2341/17-276-L
Chen G, Zhao L, Dong YH (2011) Oxidative degradation kinetics and products of chlortetracycline by manganese dioxide. J Hazard Mater 193:128–138. https://doi.org/10.1016/j.jhazmat.2011.07.039
Kuan WH, Hu CY, Liu BS et al (2013) Degradation of antibiotic amoxicillin using 1 × 1 molecular sieve-structured manganese oxide. Environ Technol 34(16):2443–2451. https://doi.org/10.1080/09593330.2013.772658
Prasad AS (2017) Green synthesis of nanocrystalline manganese (II, III) oxide. Mater Sci Semicond Process 71:342–347. https://doi.org/10.1016/j.mssp.2017.08.020
Watts RJ, Sarasa J, Loge FJ et al (2005) Oxidative and reductive pathways in manganese-catalyzed Fenton’s reactions. J Environ Eng 131:158–164. https://doi.org/10.1061/(ASCE)0733-9372(2005)131:1(158)
Choudhary VR, Samanta C, Choudhary TV (2006) Factors influencing decomposition of H2O2 over supported Pd catalyst in aqueous medium. J Mol Catal A Chem 260(1–2):115–120. https://doi.org/10.1016/j.molcata.2006.07.009
Soares DG, Ribeiro APD, da Silveira VF et al (2012) Efficacy and cytotoxicity of a bleaching gel after short application times on dental enamel. Clin Oral Investig 17:1901–1909. https://doi.org/10.1007/s00784-012-0883-1
Suty H, De Traversay C, Cost M (2004) Applications of advanced oxidation processes: present and future. Water Sci Technol 49(4):227–233. https://doi.org/10.2166/wst.2004.0270
Chiam SL, Pung SY, Yeoh FY (2020) Recent developments in MnO2− based photocatalysts for organic dye removal: a review. Environ Sci Pollut Res 27(6):5759–5778. https://doi.org/10.1007/s11356-019-07568-8
Huang M, Xu C, Wu Z et al (2008) Photocatalytic discoloration of methyl orange solution by Pt modified TiO2 loaded on natural zeolite. Dyes Pigm 77(2):327–334. https://doi.org/10.1016/j.dyepig.2007.01.026
de Oliveira Duque CC, Soares DG, Basso FG et al (2017) Influence of enamel/dentin thickness on the toxic and esthetic effects of experimental in-office bleaching protocols. Clin Oral Investig 21(8):2509–2520. https://doi.org/10.1007/s00784-017-2049-7
de Souza Costa CA, Hebling J, Scheffel DL et al (2014) Methods to evaluate and strategies to improve the biocompatibility of dental materials and operative techniques. Dent Mater 30(7):769–784. https://doi.org/10.1016/j.dental.2014.04.010
de Souza Costa CA (2020) Biological aspects of dental materials. J Adhes Dent 22(5):540–544
Cintra LTA, Benetti F, da Silva Facundo AC et al (2013) The number of bleaching sessions influences pulp tissue damage in rat teeth. J Endod 39(12):1576–1580. https://doi.org/10.3290/j.jad.a45409
Sato C, Rodrigues FA, Garcia DM et al (2013) Tooth bleaching increases dentinal protease activity. J Dent Res 92(2):187–192. https://doi.org/10.1177/0022034512470831
Pagano S, Lombardo G, Costanzi E et al (2021) Morpho-functional effects of different universal dental adhesives on human gingival fibroblasts: an in vitro study. Odontology 109(2):524–539. https://doi.org/10.1007/s10266-020-00569-x
Benetti F, Gomes-Filho JE, Ferreira LL et al (2017) Hydrogen peroxide induces cell proliferation and apoptosis in pulp of rats after dental bleaching in vivo: effects of the dental bleaching in pulp. Arch Oral Biol 81:103–109. https://doi.org/10.1016/j.archoralbio.2017.04.013
Soares DG, Basso FG, Scheffel DS et al (2015) Responses of human dental pulp cells after application of a low-concentration bleaching gel to enamel. Arch Oral Biol 60(9):1428–1436. https://doi.org/10.1016/j.archoralbio.2015.06.014
Estay J, Angel P, Bersezio C et al (2020) The change of teeth color, whiteness variations and its psychosocial and self-perception effects when using low vs. high concentration bleaching gels: a one-year follow-up. BMC Oral Health 20(1):1–9. https://doi.org/10.1186/s12903-020-01244-x
Wu M, Hou P, Dong L et al (2019) Manganese dioxide nanosheets: from preparation to biomedical applications. Int J Nanomed 14:4781. https://doi.org/10.2147/IJN.S207666
Layfield RA (2008) Manganese (II): the black sheep of the organometallic family. Chem Soc Rev 37(6):1098–1107. https://doi.org/10.1039/B708850G
Rosa V, Sriram G, McDonald N et al (2022) A critical analysis of research methods and biological experimental models to study pulp regeneration. Int Endod J 00:01–10. https://doi.org/10.1111/iej.13712
Funding
The work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (grants 2020/08882–6 and 2021/01184–4) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (grants 302047/2019–0 and 408721/2018–9).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study did not need of approval by the Research Ethics Committee involving humans or animals.
Informed consent
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Oliveira Ribeiro, R.A., Zuta, U.O., Soares, I.P.M. et al. Manganese oxide increases bleaching efficacy and reduces the cytotoxicity of a 10% hydrogen peroxide bleaching gel. Clin Oral Invest 26, 7277–7286 (2022). https://doi.org/10.1007/s00784-022-04688-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-022-04688-3