Abstract
Objectives
The effects of different concentrations of titanium dioxide (TiO2) into 40% hydrogen peroxide (HP) were evaluated as regards the effectiveness of dental color change either associated with activation by polywave LED light or not.
Materials and methods
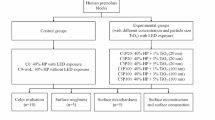
TiO2 (0, 1, 5, or 10%) was incorporated into HP to be applied during in-office bleaching (3 sessions/40 min each). Polywave LED light (Valo Corded/Ultradent) was applied or not in activation cycles of 15 s (total time of 2 min). The color of 80 third molars separated into groups according to TiO2 concentration and light activation (n = 10) was evaluated at baseline and at time intervals after the 1st, 2nd, and 3rd bleaching sessions.
Results
WID value was significantly higher when using HP with 5% TiO2 in the 2nd session than the values in the other groups (p < 0.05). After the 2nd and 3rd sessions, the ΔEab value was significantly higher when activated with light (p < 0.05) for all agents containing TiO2 or not. Zeta potential and pH of the agents were not modified by incorporating TiO2 at the different concentrations.
Conclusions
The 5% TiO2 in the bleaching agent could enhance tooth bleaching, even without light application. Association with polywave LED light potentiated the color change, irrespective of the presence of TiO2 in the bleaching gel.
Clinical significance
HP with 5% TiO2 could lead to a greater tooth bleaching response in the 2nd clinical session, as well as the polywave light can enhance color change.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Smile esthetics has been desired by patients, and dental procedures need to provide the natural characteristics of the teeth, reproducing the shape and esthetics in a harmonious and beautiful way [1, 2]. From this aspect, the color of the teeth can be changed with the use of dental bleaching techniques, which are performed under the supervision of the dentist, using trays (used at home by the patient with the low concentrations of hydrogen peroxide or carbamide peroxide), in-office (applied by the dentist with high concentrations of hydrogen peroxide), or the combined technique (consisting of in-office bleaching associated with the at-home technique) [1, 3]. However, painful symptoms have been reported during the bleaching procedure, in which this sensitivity can be influenced by the time of use, concentration of the bleaching agent, and technique applied [3,4,5,6].
With the purpose of accelerating the degradation reaction of the bleaching agent and minimizing the time of exposure of dental tissues to the bleaching agent, titanium dioxide (TiO2) can be incorporated into bleaching agents to promote electron excitation and generation of oxygen ions, thus generating superoxide that contributes to the breakdown of chromophores present in the dental structure [4, 7, 8]. By incorporating TiO2 into the composition of bleaching agents, the concentration of hydrogen peroxide can even be reduced (between 3.5 and 23% in different studies [9, 10]), with similar bleaching effectiveness when compared with the use of high concentration agents, and the time of application of the bleaching gel may even be reduced [8,9,10]. Addition of this catalyst agent to the in-office hydrogen peroxide-based bleaching gel has been used at concentrations of approximately 1% [10,11,12] and has demonstrated a positive bleaching effect. However, different concentrations of TiO2 in the bleaching agent have not yet been evaluated, and it is necessary to assess their physicochemical properties on being incorporated into the gel and their influence on the degree of bleaching in the periods between the different clinical sessions.
TiO2 is considered a photocatalytic agent, as it allows acceleration of the reaction rate when excited by light [13]. It also acts as a catalyst and photocatalyst semiconductor that reacts to blue and violet light [10, 13, 14]. When using a visible light source in the blue–violet range with a wavelength of 405 to 480 nm, it was shown that TiO2 showed reactivity, suggesting that violet light (in the range of 405 nm) would increase the catalytic reaction of TiO2 [13].
However, professionals do not always have a specific violet LED light device in their offices to perform bleaching procedures, while the presence of photoactivating devices for polymerization of polywave-type composite resins is more common. Polywave light activation devices have LEDs (light emitting diodes) that emit blue light between 445 and 460 nm, as well as violet light close to 407 nm, and the totality of these emissions leads to the possibility of improved excitation of photoinitiators present in part of the contemporary composite resins [15]. Although this is a light-curing device used by professionals when they perform restorative procedures [16, 17], there are no studies that have evaluated polywave LED light as an activating agent for TiO2 when it is incorporated into the bleaching agent. Therefore, the association between the bleaching agent, incorporation of TiO2, and the use of polywave LED light could result in requiring a shorter time for performing the in-office technique or even in fewer sessions, which could reduce dentin sensitivity.
Thus, the aim of this study was to evaluate the influence of different concentrations of TiO2 incorporated into 40% hydrogen peroxide on the physicochemical properties of the bleaching agent and on the color change of dental enamel, either associated with the polywave LED light or not. The following null hypotheses were studied: (H01) the incorporation of TiO2 at different concentrations would not influence the physicochemical properties of pH, particle size, polydispersity, and zeta potential of 40% hydrogen peroxide; (H02) the incorporation of TiO2 at different concentrations into 40% hydrogen peroxide would not influence the change in dental color; (H03) activation of 40% hydrogen peroxide with or without polywave LED light would not influence the dental color change.
Materials and methods
Specification of the bleaching agent and the polywave LED, obtainment and incorporation of the TiO2 nanotubes and incorporation
A bleaching agent containing 40% hydrogen peroxide was used (Opalescence Boost, Ultradent, Salt Lake City, USA); composition by weight according to the Material Safety Data Sheet: Hydrogen peroxide ≤ 40%, glycerin < 12%, potassium nitrate ≤ 3%, potassium hydroxide < 3%, sodium fluoride 1.1%, and polyethylene glycol < 1%. The TiO2 nanotubes were incorporated into the different concentrations (1, 5, and 10%) of this bleaching agent.
TiO2 nanotubes formed by using a single sheet of material wound in a spiral (diameter ~ 10 nm and size ~ 200 nm) were synthesized by the alkaline method [18]. Then, they were weighed on a precision balance with readability of 0.0001 g (Engenharia BEL, Monza, Milan, Italy) and manually added to the recently manipulated bleaching agents at concentrations of 1, 5, and 10%. The incorporation procedure recommended by Monteiro et al. [12] was used and performed by using a plastic spatula for spatulating the products on a sheet of impermeable paper for 1 min. Subsequently, the content obtained was deposited in a sterile, disposable syringe. The TiO2 was always incorporated into the bleaching agents immediately before each application.
The polywave LED device (Valo, Ultradent Products INC, Utah, USA) was used in standard mode with power of 1000 mW/cm2. The device has 3 different colored chips in the single matrix set: two blue (emitting around 460 nm), one blue (emitting around 445 nm), and one violet (emitting around 400 nm) [15].
Physicochemical characterization of gels: evaluation of pH, average particle size, polydispersity, and zeta potential
The pH value of the bleaching agent incorporated into the different concentrations of TiO2 was measured in triplicate immediately after the manipulation and incorporation of TiO2, using a table pH meter (MS Tecnopon Equipamentos Especiais LTDA, RbPH-210, Piracicaba, SP, Brazil).
The mean size of the bleaching gel particles with or without the addition of TiO2 nanotubes, polydispersity (which measures the heterogeneity of the molecule or particles in the solution), and zeta potential (colloidal stability) was evaluated by dynamic light scattering (Zetasizer Nano ZS, Malvern Instruments, Malvern, UK) in triplicate at 25 °C, immediately after manipulation and incorporation of TiO2 [12]. The zeta potential was evaluated to measure the colloidal stability, using the Helmholtz–Smoluchowski [19] model, to measure the electrophoretic mobility of the dispersed particles in the electric field applied. The electrostatic potential of particles directly related to their dispersion stability by the electrostatic repulsion. Therefore, when the potential was small, attractive forces could exceed this repulsion and the dispersion could break and flocculate [20].
Zeta potential analysis was performed by laser electrophoresis with 30 runs per measurement at 25 °C. Zeta potentials were automatically calculated based on the electrophoretic mobility, using the Smoluchowski approximation: UE = 2 * ε * z * f (ka) / 3 * η → z ≈UE * η / ε, where UE was the electrophoretic mobility, ε was the dielectric constant, z was the ZP, f (ka) was the function of Henry, and η was the viscosity.
Preparation of teeth and application of bleaching treatments
The sample size calculation was dimensioned with the use of G*Power program [21] considering the experimental design (TiO2 concentration, presence of light, and time). Eighty teeth randomly distributed among 8 groups (n = 10) provided a power of 0.80 (β = 0.20) for a level of significance of 5% (α = 0.05) and large effect size for TiO2 concentration and presence of light (effect size > 0.35), and average effect size (effect size > 0.20) for time and interactions among factors under study.
After approval by the Research Ethics Committee (CAAE number 12317519.7.0000.5374), 100 freshly extracted healthy human third molars were selected. The teeth were kept frozen until the beginning of the experiment, when they were cleaned with a scalpel blade and submitted to prophylaxis with pumice stone and Robinson brush. All teeth were evaluated throughout their full length, under a stereoscopic magnifying glass (Eikonal Equipamentos Óticos e Analíticos, model EK3ST, São Paulo, SP, Brazil) with 20 × magnification. Any teeth with cracks, wear, and/or fractures were discarded.
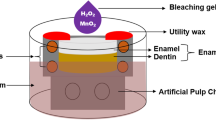
The teeth were placed in PVC molds 20 mm in diameter and 20 mm high, and the root portion, up to the cemento-enamel limit, was fixed in the mold with self-curing acrylic resin (VipiFlash, Vipi, Pirassununga, SP, Brazil). The initial color of teeth was then evaluated by means of a spectrophotometer (VITA Easyshade® Advance, Vita, Germany), using the Vita Classical scale as a criterion. Teeth with very light or very dark colors were excluded from the study, so that 80 teeth that had different colors (A3, A3.5, A4, B2, B3, and B4) were selected for homogeneous distribution among the eight groups under study (n = 10) according to the percentage of TiO2 incorporated (absent, 1, 5, and 10%) and use of polywave LED light (absence or presence of light).
Subsequently, the teeth were kept individually immersed in Eppendorf flasks containing artificial saliva for 7 days, as recommended by Featherstone et al. [22] and modified by Serra & Cury [23] composed of (1.5 mMol/L Ca; 50 mMol/L KCl; 0.9 mMol/L PO4; 20 mMol/L Tris Buffer; pH = 7). For the application of the treatments, the teeth were removed from the saliva and dried with air jets for 5 s. An amount of 0.03 mL of the bleaching gel corresponding to each group was applied on the buccal surface of the teeth by means of a syringe. The bleaching treatment was performed in three clinical sessions (40 min each) with an interval of 7 days between sessions.
In the groups in which treatment was associated with the polywave LED light, this light was turned on immediately after application of the gel, then applied in light-activation cycles of 15 s, waiting for 5 min, new light-activation for 15 s and so on until 40 min of bleaching treatment were concluded. Therefore, when adding up the light-activation times, each tooth received a total activation time of 2 min distributed in the different time intervals in each session. This protocol was based on the study by Kishi et al. [13] which proved the effect of TiO2 photocatalysis on enamel color change when associated with blue LED light devices (7 blue LEDs — 470 nm) and 1 violet LED (405 nm) with a power of 650 mW/cm2 and application time of 1 min while the bleaching agent was left on the specimens for 5 min. As no other study had been employed polywave LED light to perform bleaching treatment, our protocol was also based on and adapted from other studies that used LED curing units, as those shown by Torres et al. [24] — who applied different types of LED light for a total time of 5 min with intervals of 1 min each — and by Ferreira et al. [25] who applied blue LED light (470 nm) and green LED light (430 nm) for 3 min associated with bleaching with 35% hydrogen peroxide. On conclusion of the recommended time, the gel was sucked up with an aspirator cannula, and the teeth were cleaned with gauze and washed with water for 10 s and then immersed in an artificial saliva solution for a period of 7 days, with the solution being changed every 2 days.
Color evaluation
The teeth were evaluated by means of a spectrophotometer (Easyshade® Advance, Vita, Germany) to analyze the color according to the parameters of the Vita Classical scale and CIEL*a*b* (L* = luminosity; a* = green–red axis; b* = blue-yellow axis). The color was evaluated in the following time intervals: initial (baseline); 7 days after the first bleaching session (after 1st session); 7 days after the second bleaching session (after the 2nd session); 7 days after the third bleaching session (after the 3rd session). For color evaluation, the teeth were placed in a box with a white background in order to standardize lighting. The procedure was always performed by the same operator and in duplicate, in each time interval, to ensure accuracy.
The tooth color obtained according to the Vita Classical scale was converted into numbers according to previously established numerical values [12], by organizing them from number 1 (B1 shade) to 16 (C4) in order of luminosity: B1, A1, B2, D2, A2, C1, C2, D4, A3, D3, B3, A3.5, B4, C3, A4, and C4. Thus, the lower the numerical value, the greater the luminosity and the lighter the tooth appeared to be.
Color changes were evaluated according to the CIEL*a*b* parameter, and the values of ΔL*, Δa*, and Δb* were obtained for each group and study period, in which the color change (ΔEab) was evaluated and estimated by formula [26]: ΔEab = √ ((ΔL*)2 + (Δa*)2 + (Δb*)2). The perceptibility and acceptability limits considered for ΔEab were 1.2 and 2.7, respectively [26, 27]. The color change was also evaluated by CIEDE2000 (ΔE00), which uses the h (hue) and C (chroma) values [28]. ΔE00 values of 0.8 and 1.8 were adopted as the perceptibility and acceptability limits [26]. Monitoring of the dental bleaching was evaluated by the Whiteness Index for Dentistry (WID), in which the parameters L*, a*, and b* were used in the following equation [29]: WID = 0,511L*—2,324a*—1,100b*. Differences in WID between the initial and final assessments were evaluated (ΔWID), using threshold values for ΔWID of 0.72 for perceptibility and 2.6 for acceptability [30].
Statistical analysis
Descriptive and exploratory analyses of all color change variables were performed. Exploratory analyses indicated that the variables did not meet the assumptions of an analysis of variance (ANOVA). The pH and zeta potential data were analyzed by the non-parametric tests of Kruskal–Wallis and Dunn. Particle size and polydispersity data were analyzed by generalized linear models. As regards the color analysis, the a* data did not fit a known distribution and were analyzed by the Kruskal–Wallis and Dunn non-parametric tests for comparisons between TiO2 concentrations; Mann–Whitney for comparisons between groups with and without light activation; and Friedman and Nemenyi tests for comparisons among time intervals. For L*, b*, WID, ΔEab, ΔE00, and ΔWID, generalized linear models were used, considering an asymmetric distribution, considering the factors under study “TiO2 concentration” and “activation with light” with repeated measures in time, as well as the interactions between them. The analyses were performed using the R Core Team (2021) program, with a significance level of 5%.
Results
The pH values of the bleaching agents (Table 1) show that the incorporation of 1% TiO2 led to significantly higher pH values than when 10% was incorporated. The higher percentage of TiO2 led to a reduction in pH; however, even with the incorporation of 10%, the pH of all agents remained close to neutrality and without significant differences in comparison with the gel without TiO2. The particle size was significantly larger in the product with 10% TiO2, and only the presence of 1% TiO2 did not influence the particle size of the product in comparison with that of the bleaching agent without its incorporation. Polydispersity was significantly higher in the absence of TiO2 than in its presence. The zeta potential was significantly less negative with 10% than with 5%, with no significant differences between groups.
Irrespective of activation with or without LED light, there was no significant difference between bleaching agents with different concentrations of TiO2 for the Vita Classical, L*, and a* scale (p > 0.05) (Table 2). For b*, 5% TiO2 incorporated into the bleaching agent showed lower values than those of the other concentrations, but without significant differences in comparison with the agent without incorporation throughout the course of the bleaching sessions. For WID, after the second clinical session, 5% TiO2 led to higher values than the bleaching agent without incorporation, but there were no differences between the groups after the third bleaching session. When activated with LED light, all bleaching agents with or without incorporation of TiO2 had higher L* values than those without light-activation at all time intervals (p = 0.0172). For all bleaching agents whether they contained TiO2 or not, there was a significant decrease in the colors of the Vita Classical scale, a*, and b* throughout the clinical bleaching treatment sessions, with significant differences in comparison with baseline (p < 0.05). There was also a significant increase in L* and WID values in all groups after 3rd bleaching session compared with baseline (p < 0.05).
For b*, there was no significant difference between groups at baseline (p > 0.05). There was no significant difference between groups at baseline time intervals for WID (p < 0.05). However, after the 1st clinical bleaching session, the WID was significantly higher for bleaching agents with 1% and 5% TiO2 than those with 10% (p < 0.05). After the 2nd clinical bleaching session, the WID was significantly higher with 5% TiO2 than the other groups (p < 0.05), while after the 3rd bleaching session, the WID was significantly higher with 5% TiO2 than with 10% (p < 0.05), with no differences in comparison with the group without incorporation or with 1% TiO2.
At all assessment time intervals, there were no significant differences between groups relative to ΔEab (p > 0.05) (Table 3). However, in the assessment time intervals after the 2nd session (p = 0.0280) and 3rd bleaching session (p = 0.0272), the ΔEab value was significantly higher in groups with light activation, irrespective of the concentration of TiO2 either incorporated into the bleaching agent or not. There was no significant difference between the groups regarding ΔE00 and ΔWID (p > 0.05) irrespective of activation with or without LED light.
Discussion
Modifications in the composition of bleaching agents could favor a reduction in adverse effects on mineralized dental substrates, pulp, and periodontal tissues, as well as potentiate the beneficial effects with regard to color change. From this aspect, the incorporation of TiO2 leads to the possibility of decreasing the concentration of the bleaching agent, especially when it is associated with activation by diode laser light, with effective bleaching being obtained [7] due to the higher level of generating hydroxyl radicals [11], or even reducing the time of the bleaching treatment by a reduction of the number of the sessions. The higher generation of hydroxyl radicals may also result from the incorporation of TiO2 into the bleaching agents, especially because it affects the physicochemical properties of the bleaching agent, which led to rejection of the first null hypothesis of the present study. The presence of 1% of TiO2 in the bleaching agent was able to raise the pH from 7.38 to 8.01; however, there was no statistically significant difference, and the pH of the bleaching agent became slightly alkaline, which may have had an impact on the higher rate of release and speed of free radicals during the oxidation–reduction reaction of the bleaching agent [24, 31]. The increase in pH is capable of accelerating the rate of degradation of hydrogen peroxide, triggering the release of free radicals that promote improvement in the effectiveness of bleaching [24, 31,32,33]. When 5% TiO2 was added to the pH, the pH remained practically the same (7.27) as that of the bleaching gel without its incorporation (7.38). However, a higher concentration of TiO2 (such as the 10% used) led to a decrease in the pH of the bleaching agent (6.97), which could, to a certain extent, reduce the release of reactive free radicals and affect bleaching efficacy [24, 34].
When using a concentration of 1% of TiO2 incorporated into the 40% hydrogen peroxide bleaching agent, Monteiro et al. [12] observed no differences in the effectiveness of tooth enamel bleaching, suggesting that other concentrations should be evaluated. From this aspect, although the present study showed that there were no differences between the concentrations of TiO2 incorporated into the bleaching agent when evaluating the color evaluation parameters with reference to the Vita Classical scale, L*, and a*, both in the presence and absence of light, differences could be observed between the parameters b* and WID, which led to rejection of the second hypothesis of the present study. The concentration of 5% TiO2 led to lower b* values after the first clinical session compared with the other concentrations, which means that there was a higher degree of degradation of yellow pigments present in the tooth at the time of evaluation. Evaluation of the b* parameter has been pointed out as being an important factor in the evaluation of color changes during tooth bleaching because it is the parameter that has a faster and more extensive influence on color than that of the other components of the CIELab system [35], whereas it was only when evaluating the WID parameter that a significant difference in the presence of 5% TiO2 was observed in comparison with the control group (bleaching agent without TiO2) after the second clinical bleaching session. From this aspect, considering that the WID parameter was developed with the aim of establishing a strong correlation with the perception of tooth bleaching [29], in addition to demonstrating superior performance when assessing the degree of perceptible bleaching in comparison with other indices [29], the suggestion could be that among all the treatments evaluated, a higher bleaching index could be obtained in the second session when using the bleaching agent with 5% TiO2 incorporated into it. This could be an advantage in cases when the patient does not require or does not wish to perform a third tooth bleaching session. These results could suggest that 5% TiO2 might have potentiated the bleaching effect of the hydrogen peroxide used, which may have increased the degradation reaction of the bleaching agent and contributed to the breakdown of chromophores present in the tooth structure [4, 7, 8]. However, with the need for a 3rd clinical session, no significant difference was observed between the treatments evaluated, irrespective of whether or not they contained TiO2 in the composition.
Despite the bleaching agent into which a concentration of 5% TiO2 was incorporated having a pH similar to the bleaching agent without any incorporation, the former caused a significant increase in the average size of particles present in the material. This also occurred with the incorporation of a concentration of 10%. The incorporation of different concentrations of TiO2 into the bleaching agents produced gels with good viscosity and handling characteristics, but a change in the original reddish color of the gel was observed when using the concentration of 10% TiO2, as well as more thickening of the gel and significant presence of bubbles in it. The increase in the mean particle sizes in the bleaching agent may be related to the rheological characteristics of the material, considering that despite the use of nanometric particles in the tubular conformation of TiO2, their incorporation led to a change in the characteristics of polydispersity and colloidal stability of the bleaching agent. The particles used had sizes in the range from 0.1 to 100 nm. The purpose was to make the product lighter and improve its properties [36] with the use of anatase particles, especially due to their photochemical properties [18], thereby promoting an increase in surface area and quantum effects.
The polydispersity index is defined by the distribution of particles in the material, with lower values indicating uniformity of dispersion [37]. From this aspect, all TiO2 concentrations improved the polydispersity characteristics, with a lower probability of agglomeration forming in the bleaching gel. However, it was only at the 5% concentration that the zeta potential values of the bleaching agent reached “good colloidal stability” (− 44.53 mV), unlike the 10% concentration, which obtained “incipient stability” (− 18.87 mV). The zeta potential is an important indicator of the stability of colloidal dispersions. For molecules and particles that are small enough, a high zeta potential will confer stability, i.e., the solution or dispersion will resist aggregation. Therefore, solutions or colloids with high zeta potential (negative or positive) are electrically stabilized — leading to greater material stability — while colloids with low zeta potential tend to coagulate or flocculate [20, 38].
The association of the use of a light source during the bleaching treatment has been recognized as a factor that leads to an increase in temperature, which can accelerate the decomposition reaction of hydrogen peroxide [39] and could impact the reduction in bleaching treatment time. However, several systematic reviews have shown that activating the bleaching gel in in-office treatment with different types of lights and protocols does not seem to lead to greater effectiveness in color change [39,40,41,42,43], with inconclusive answers regarding tooth sensitivity, and some reviews reporting a decrease [42], while others reported that sensitivity was not affected by the use of lights [39, 43]. These differences may occur due to the sensitivity assessment criteria, protocol, and type of light used (halogen, LED or laser) [39, 41, 42]. Studies with the use of polywave LED light associated with bleaching treatment have not yet been conducted, and the present study demonstrated that the activation of the bleaching agent with this type of light promoted greater luminosity, irrespective of the presence and concentration of TiO2 in the composition of the bleaching agent, leading to the rejection of the third null hypothesis of the present study.
Although the safety of the pulp temperature that can be reached in the tooth when using the light-application protocol has not yet been recognized, the suggestion could be that the increase in the temperature of the bleaching gel may have led to greater degradation of hydrogen peroxide, causing a higher level of bleaching effectiveness, which impacted the color change values obtained by ΔEab after the second and third tooth bleaching sessions. In fact, Rotstein et al. [44] showed that when increasing the temperature from 24 to 37 °C, this doubles the amount of hydrogen peroxide free radicals that reach the pulp, thereby even further increasing the penetration into the dental substrate when a temperature of 47 °C is reached. It is possible that the protocol used did not lead to reaching this high temperature, even more so considering the fact that 15 s of activation were applied at 5-min intervals, which is shorter than the time used to clinically light-activate an increment of composite resin [24, 28]. Other protocols use continuous application for 1 min of blue/violet LED light at 650 mW/cm2 [13] or halogen light at 700 mW/cm2 [24], violet LED at 140.2 mW/cm2 [45] or up to 3 min with green LED at 180 mW/cm2 [25]. However, the use of polywave LED light was shown to be safe in vivo relative to intrapulpal temperature during the light-activation of composite resin in class V cavities for the time of 60 min since the temperature obtained was close to 5.5 °C (with power of 1244 mW/cm2) [46]. Nevertheless, studies are being conducted for better evaluation, since light can potentiate the heating of the gel, which can perpetuate the increase in the temperature of the dental substrate due to its permanence on the tooth, which could increase the temperature of the pulp and, clinically, result in higher degrees of sensitivity.
However, the significantly greater effectiveness of color change promoted by activation with LED light after the second and third session in the ΔEab parameter had no impact when the threshold values of acceptability and perceptibility were observed. Even after the first tooth bleaching session, the values obtained for the parameters of color change for ΔEab [26, 27], ΔE00 [27], and ΔWID [30] were found to be much higher than the limits of perceptibility and acceptability, denoting that, irrespective of the association with light, presence and concentration of TiO2 in the composition, the bleaching obtained was clinically perceptible and acceptable.
According to Joiner & Leo [47] and Lilaj et al. [48], the WID parameter showed better correlation with visual perception data than all other parameters tested, both in studies under laboratory and clinical conditions, allowing changes to be observed in the initial and final time intervals of bleaching, a fact also observed in the present study, which may demonstrate that the WID value was significantly higher when using a bleaching agent containing TiO2, especially at a concentration of 5% in the 2nd bleaching session, than in the other groups studied. Therefore, 5% TiO2 incorporated into the bleaching agent was capable of enhancing tooth bleaching even without light application, when using the WID parameter.
The present study allowed the conclusion that the pH and zeta potential of the bleaching agent were not modified when TiO2 at different concentrations was incorporated into the bleaching agent, but it reduced the polydispersity of the gel. There was an increase in the particle size of the bleaching agent when concentrations of 5 and 10% TiO2 were incorporated into the bleaching agent. The association of a concentration of 5% TiO2 into a bleaching agent containing 40% hydrogen peroxide could lead to a greater tooth bleaching response in the second clinical session, which could be an advantage when the patient does not require or does not wish to perform a third dental bleaching session. The association of polywave LED light potentiated the color change only for the ΔEab parameter, irrespective of the presence of TiO2 in the bleaching gel. However, the color changes were noticeable and acceptable for all evaluation parameters, irrespective of activation with polywave LED light, presence and concentration of TiO2 in the bleaching agent composition.
Data Availability
Authors can confirm that all relevant data are included in the article and other supplementary information are available from the corresponding author upon reasonable request.
References
Geus JL, Wambier LM, Kossatz S, Loguercio AD, Reis A (2016) At-home vs in-office bleaching: a systematic review and meta-analysis. Oper Dent 41(4):341–56
Pavicic DK, Kolceg M, Lajnert V, Pavlic A, Brumini M, Spalj S (2018) Changes in quality of life induced by tooth whitening are moderated by perfectionism: a randomized, double-blind, placebo-controlled trial. Int J Prosthodont 31(4):394–96
Mushashe AM, Coelho BS, Garcia PP, Rechia BN, da Cunha LF, Correr GM, Gonzaga CC (2018) Effect of different bleaching protocols on whitening efficiency and enamel superficial microhardness. J Clin Exp Dent 10(8):e772–e775
Komatsu O, Hishida H, Sekino T, Yamamoto K (2014) Application of titanium dioxide nanotubes to tooth whitening. Nanobiomedicine 6(2):63–72
Mondelli R, Rizzante F, Rosa ER, Borges A, Furuse AY, Bombonatti J (2018) Effectiveness of LED/laser irradiation on in-office dental bleaching after three years. Oper Dent 43(1):31–37
Pinto MM, Gonçalves ML, Mota AC, Deana AM, Olivan SR, Bortoletto C, Godoy CH, Vergilio KL, Altavista OM, Motta LJ, Bussadori SK (2017) Controlled clinical trial addressing teeth whitening with hydrogen peroxide in adolescents: a 12-month follow-up. Clinics 72(3):161–170
Tano E, Otsuki M, Kato J, Sadr A, Ikeda M, Tagami J (2012) Effects of 405 nm diode laser on titanium oxide bleaching activation. Photomed Laser Surg 30(11):648–654
Thacker M, Chen YN, Lin CP, Lin FH (2021) Nitrogen-doped titanium dioxide mixed with calcium peroxide and methylcellulose for dental bleaching under visible light activation. Int J Mol Sci 22(7):3759
Martín J, Vildósola P, Bersezio C, Herrera A, Bortolatto J, Saad JR, Oliveira OB Jr, Fernández E (2015) Effectiveness of 6% hydrogen peroxide concentration for tooth bleaching—a double-blind, randomized clinical trial. J Dent 43(8):965–972
Cuppini M, Leitune VCB, Souza M, Alves AK, Samuel SMW, Collares FM (2019) In vitro evaluation of visible light-activated titanium dioxide photocatalysis for in-office dental bleaching. Dent Mater J 38(1):68–74
Sakai K, Kato J, Kurata H, Nakazawa T, Akashi G, Kameyama A et al (2007) The amounts of hydroxyl radicals generated by titanium dioxide and 3.5% hydrogen peroxide under 405-nm diode laser irradiation. Laser Phys 17(8):1062–6
Monteiro NR, Basting RT, Amaral FLBD, França FMG, Turssi CP, Gomes OP, Lisboa Filho PN, Kantovitz KR, Basting RT (2020) Titanium dioxide nanotubes incorporated into bleaching agents: physicochemical characterization and enamel color change. J Appl Oral Sci 24(28):e20190771
Kishi A, Otsuki M, Sadr A, Ikeda M, Tagami J (2011) Effect of light units on tooth bleaching with visible-light activating titanium dioxide photocatalyst. Dent Mater J 30(5):723–729
Lu L, Yoshikawa C, Komatsu O, Hirota Y, Hattori Y, Inoue C (2013) Evaluation of a tooth bleaching system incorporating titanium dioxide. J Osaka Dent Univ 47(2):409–414
Rueggeberg FA, Giannini M, Arrais CAG, Price RBT (2017) Light curing in dentistry and clinical implications: a literature review. Braz Oral Res 31(suppl 1):e61
Soares CJ, Rodrigues MP, Oliveira LRS, Braga SSL, Barcelos LM, Silva GRD, Giannini M, Price RB (2017) An evaluation of the light output from 22 contemporary light curing units. Braz Dent J 28(3):362–71
Soares CJ, Braga SSL, Price RB (2021) Relationship between the cost of 12 light-curing units and their radiant power, emission spectrum, radiant exitance, and beam profile. Oper Dent 46(3):283–92
Arruda LB, Santos CM, Orlandi MO, Schreiner WH, Lisboa-Filho PN (2015) Formation and evolution of TiO2 nanotubes in alkaline synthesis. Ceram Int 41(2):2884–2891
Zha L, Li L, Bao L (2007) Synthesis and colloidal stability of poly(N- isopropylacrylamide) microgels with different ionic groups on their surfaces. J Appl Polym Sci 103(6):3893–3898
Hanaor DAH, Michelazzi M, Leonelli C, Sorrell CC (2012) The effects of carboxylic acids on the aqueous dispersion andelectrophoretic deposition of ZrO2. J Eur Ceram Soc 32:235–244
Faul F, Erdfelder E, Lang AG, Buchner A (2007) GPower 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191
Featherstone JDB, Reilly MM, Shariatti M, Brugler S (1986) Enhancement of remineralization in vitro and in vivo. In: Leach SA (ed) Factors relating to demineralization and remineralization of the teeth. Oxford, IRL Press, p 23–34
Serra MC, Cury JA (1992) The in vitro effect of glass-ionomer cement restoration on enamel subjected to a demineralization and remineralization model. Quintessence Int 23(2):143–147
Torres CR, Crastechini E, Feitosa FA, Pucci CR, Borges AB (2014) Influence of pH on the effectiveness of hydrogen peroxide whitening. Oper Dent 39(6):E261–E268
Ferreira AC, Batista AL, Neto JA, Simões TM, da Silva MG, de Barros DD, Catão JS, de Oliveira TA, Catão MV (2021) Evaluation of dental enamel microproperties after bleaching with 35% hydrogen peroxide and different light sources: an in vitro study. J Clin Exp Dent 13(10):e969–e974
Commission Internationale del clairage (2004)Colorimetry. CIE Central Bureau, Vienna
Paravina RD, Ghinea R, Herrera LJ, Bona AD, Igiel C, Linninger M et al (2015) Color difference thresholds in dentistry. J Esthet Restor Dent 27(Suppl 1):S1-9
Sharma G, Wu W, Dalal EN (2005) The CIEDE2000 color-difference formula: implementation notes, supplementary test data, and mathematical observations. Color Res Appl 30:21
Pérez MM, Ghinea R, Rivas MJ, Yebra A, Ionescu AM, Paravina RD et al (2016) Development of a customized whiteness index for dentistry based on CIELAB color space. Dent Mater 32(3):461–467
Pérez MM, Herrera LJ, Carrillo F, Pecho OE, Dudea D, Gasparik C et al (2019) Whiteness difference thresholds in dentistry. Dent Mater 35(2):292–297
Frysh H, Bowles WH, Baker F, Rivera-Hidalgo F, Guillen G (1995) Effect of pH on hydrogen peroxide bleaching agents. J Esthet Dent 7(3):130–133
Young N, Fairley P, Mohan V, Jumeaux C (2012) A study of hydrogen peroxide chemistry and photochemistry in tea stain solution with relevance to clinical tooth whitening. J Dent 40(Suppl 2):e11–e16
Balladares L, Alegría-Acevedo LF, Montenegro-Arana A, Arana-Gordillo LA, Pulido C, Salazar-Gracez MT, Reis A, Loguercio AD (2019) Effects of pH and application technique of in-office bleaching gels on hydrogen peroxide penetration into the pulp chamber. Oper Dent 44(6):659–67
Acuña ED, Parreiras SO, Favoreto MW, Cruz GP, Gomes A, Borges CP et al (2022) In-office bleaching with a commercial 40% hydrogen peroxide gel modified to have different pHs: color change, surface morphology, and penetration of hydrogen peroxide into the pulp chamber. J Esthet Restor Dent 34(2):322–327
Meireles SS, Heckmann SS, Leida FL, dos Santos Ida S, Della Bona A, Demarco FF (2008) Efficacy and safety of 10% and 16% carbamide peroxide tooth- whitening gels: a randomized clinical trial. Oper Dent 33(6):606–12
Zhang Wx (2003) Nanoscale iron particles for environmental remediation: an overview. J Nanopart Res 5:323–332
Wu L, Zhang J, Watanabe W (2011) Physical and chemical stability of drug nanoparticles. Adv Drug Deliv Rev 63(6):456–469
Mohanraj VJ, Chen Y (2006) Nanoparticles - a review. Trop J Pharm Res 5(1):561–573
Buchalla W, Attin T (2007) External bleaching therapy with activation by heat, light or laser–a systematic review. Dent Mater 23(5):586–596
Alshammery S (2019) Evaluation of light activation on in-office dental bleaching: a systematic review. J Contemp Dent Pract 20(11):1355–1360
Maran BM, Ziegelmann PK, Burey A, de Paris MT, Loguercio AD, Reis A (2019) Different light-activation systems associated with dental bleaching: a systematic review and a network meta-analysis. Clin Oral Investig 23(4):1499–1512
SoutoMaior JR, de Moraes S, Lemos C, Vasconcelos BDE, Montes M, Pellizzer EP (2019) Effectiveness of light sources on in-office dental bleaching: a systematic review and meta-analyses. Oper Dent 44(3):E105–E117
Casado B, Pellizzer EP, SoutoMaior JR, Lemos C, Vasconcelos B, Moraes S (2020) Laser influence on dental sensitivity compared to other light sources used during in-office dental bleaching: systematic review and meta-analysis. Oper Dent 45(6):589–597
Rotstein I, Torek Y, Lewinstein I (1991) Effect of bleaching time and temperature on the radicular penetration of hydrogen peroxide. Endod Dent Traumatol 7(5):196–198
Brugnera AP, Nammour S, Rodrigues JA, Mayer-Santos E, de Freitas PM, Brugnera A Junior, Zanin F (2020) Clinical evaluation of in-office dental bleaching using a violet light-emitted diode. Photobiomodul Photomed Laser Surg 38(2):98–104
Zarpellon DC, Runnacles P, Maucoski C, Coelho U, Rueggeberg FA, Arrais C (2019) Controlling in vivo, human pulp temperature rise caused by LED curing light exposure. Oper Dent 44(3):235–41
Joiner A, Luo W (2017) Tooth colour and whiteness: A review. J Dent 67S:S3–S10
Lilaj B, Dauti R, Agis H, Schmid-Schwap M, Franz A, Kanz F, Moritz A, Schedle A, Cvikl B (2019) Comparison of bleaching products with up to 6% and with more than 6% hydrogen peroxide: whitening efficacy using BI and WID and side effects - an in vitro study. Front Physiol 21(10):919
Author information
Authors and Affiliations
Contributions
Edina Veloso Gonçalves Antunes and Rosanna Tarkany Basting were responsible for developing the experiment, discussion of the results, and writing the paper. Flávia Lucisano Botelho do Amaral, Fabiana Mantovani Gomes França, Kamila Rosamilia Kantovitz, and Cecilia Pedroso Turssi interpreted the results and wrote the paper. Erika Soares Bronze-Uhle and Paulo Noronha Lisboa Filho developed and provided the TiO2. Roberta Tarkany Basting developed the experimental design, wrote the paper, and helped with the discussion.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Antunes, E.V.G., Basting, R.T., do Amaral, F.L.B. et al. Titanium dioxide nanotubes in a hydrogen peroxide-based bleaching agent: physicochemical properties and effectiveness of dental bleaching under the influence of a poliwave led light activation. Clin Oral Invest 27, 1745–1755 (2023). https://doi.org/10.1007/s00784-022-04802-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-022-04802-5