Abstract
Background
Stress hyperglycemia is a relatively transient increase in blood glucose in response to inflammation of the body and neurohormonal disorders. It is still debated whether stress hyperglycemia ratio (SHR) in the acute phase, a new indicator of stress hyperglycemia, is related to poor prognosis in acute ischemic stroke (AIS) patients. This meta-analysis provides insight into the connection between SHR and prognosis in AIS patients.
Methods
We screened all potentially relevant studies using a comprehensive database search. The standardized mean difference (SMD) and 95% confidence interval (CI) were utilized to investigate the relationship between SHR in the acute phase and the prognosis of AIS.
Results
The pooled results revealed that AIS patients with poor prognoses had significantly higher SHR values than those with good prognoses (SMD = 0.56, 95%CI: 0.37–0.75, p<0.001). Subgroup analysis indicated that study design and differences in post-stroke treatment might be the sources of heterogeneity in this meta-analysis.
Conclusions
High SHR in the acute period is related to poor prognosis after AIS. SHR may be a new predictor of poor outcomes in AIS patients.
Similar content being viewed by others
Introduction
Globally, stroke is a significant cause of death and disability [1, 2]. The Global Burden of Diseases, injury, and Risk Factors Study (GBD) showed a substantial increase in stroke events attributable to exposure to risk factors from 1999 to 2019. In 2019, the number of people who died from stroke accounted for 11.6% of all deaths, and stroke remains the second leading cause of death and the third leading cause of death and disability combined [3]. In addition, a large sample survey showed that in 2020, the estimated prevalence of stroke in people aged ≥ 40 years in China was 2.6%, the incidence rate was 505.2/100 000 person-years, and the mortality rate was 343.4/100 000 person-years [4].
Hyperglycemia is a risk factor for stroke [5]. Stress hyperglycemia is a relatively transient increase in blood glucose in response to inflammation of the body and neurohormonal disorder [6, 7]. As a result of the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis being engaged during a stroke, catecholamines and cortisol are released [7, 8]. Stress hormone disorders inhibit insulin secretion and promote glycogenolysis and hepatic gluconeogenesis, resulting in a relatively transient increase in blood glucose level [6, 9]. Absolute hyperglycemia does not distinguish between chronic poor glycemic management and stress response in acute ischemic stroke(AIS). Roberts et al. proposed the concept of relative hyperglycemia - stress hyperglycemia ratio (SHR) [10]. Compared to absolute hyperglycemia, stress hyperglycemia ratio is a more predictive prognostic marker. Studies have demonstrated a significant correlation between elevated stress hyperglycemia ratio, measured as fasting blood glucose/glycosylated hemoglobin (FBG/HbA1c), and the poor outcomes of AIS patients [11, 12], including an increased risk of functional impairments, stroke recurrence, and death [13,14,15]. However, Nathan et al. suggested using fasting blood glucose/estimated average glucose (FBG/EAG) to define the stress hyperglycemia ratio, where EAG= (1.59 ×HbA1c) -2.59 [16]. Two previous studies have shown that SHR calculated as FBG/EAG predicts poor outcomes in patients with AIS or critical illness [17, 18], suggesting that elevated SHR may predict poor clinical prognosis in AIS patients.
However, there are few studies on the association between SHR and prognosis in patients with AIS, and the underlying pathological mechanisms have not been thoroughly investigated. Still, there are several possible explanations: (1) Hyperglycemia during cerebral ischemia affects the energy metabolism of brain cells and aggravates anaerobic glycolysis, which can lead to intracellular acidosis [6]. Intracellular acidosis can further exacerbate ischemic brain injury [7]; (2) At the time of ischemic stroke, glutamate accumulates extracellular and activates postsynaptic glutamate receptors in a hyperglycemic state [10]. The excessive opening of Ca2 + channels leads to intracellular Ca2 + overload and impaired mitochondrial function, which eventually leads to neuronal death [6]; (3) After ischemic stroke, hyperglycemia promotes inflammatory response and oxidative stress and activates matrix metalloproteinase-9 activity [9]. This series of reactions further disrupts the blood-brain barrier, exacerbates cerebral edema, and causes hemorrhagic transition [19, 20]. (4) Elevated blood glucose leads to vascular endothelial dysfunction in patients with AIS [11], and the decrease of cerebral blood flow further aggravates ischemic injury [19]. (5) Hyperglycemia may directly damage the ischemic penumbra by transferring intracellular electrolytes [21, 22]. This cascade of reactions can form a vicious cycle affecting stroke patients’ short-term and long-term neurological recovery. Indeed, many factors complicate the prognosis of ischemic stroke, and their relevance requires further investigation. In light of this, we performed the meta-analysis to investigate the significance of acute phase SHR in predicting clinical outcomes in AIS patients.
Methods
The meta-analysis was carried out using the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines (PRISMA) [23].
Search strategy
We searched eight databases, including PubMed, Cochrane Library, Embase, Wed of Science, Chinese National Knowledge Infrastructure (CNKI), China Biology Medicine Literature Database (CBM), Wan fang, and VIP Chinese Journal Database, until October 2023. Publications are limited to Chinese and English. The search strategies were as follows: (“ischemic stroke” OR “acute ischemic stroke”) AND (“stress hyperglycemia” OR “stress hyperglycemia ratio” OR “stress hyperglycaemia” OR “stress hyperglycaemia ratio” OR “hyperglycemia” OR “hyperglycaemia” OR “glycated hemoglobin A” OR “HbA1C” OR “glycated hemoglobin” OR “glycosylated hemoglobin”). Finally, references to selected articles were browsed for potentially relevant research. The detailed search strategy were presented in Supplementary material.
Selection criteria
Inclusion criteria: (1) Cohort study; (2) Patients in the study were diagnosed with AIS; (3) Experiments and controls were designed to compare SHR in the good and poor prognosis groups; (4) SHR was calculated as fasting blood glucose (mmol/L)/HbA1c (%) (FBG/HbA1c) or fasting blood glucose (mmol/L)/estimated average glucose (FBG/EAG); (5) Prognostic evaluation of patients with AIS used modified Rankin scale (mRS) [24, 25]; (6) Research data were available. Exclusion Criteria: (1) Duplicate studies; (2) Review, meta-analysis, animal experiment, case report, letter, and conference abstract were excluded. When the study populations overlapped in multiple publications, we included the most recent or complete one. Two researchers independently completed the literature screening and reached a consensus through discussion.
Data extraction
The following data were extracted independently by two researchers: first author, publication year, study design, country, the proportion of men, diabetes status, post-stroke treatment, the time point of outcome assessment, prognostic outcome, and definition of SHR. When SHR was expressed as the median and interquartile range (IQR), SHR was transformed to mean and standard deviation (SD) using the quantile estimation (QE) method of Sean McGrath et al. [26]. Any disagreements were resolved through group discussions.
Quality assessment
The literature’s quality was assessed using the three criteria of selection, comparability, and outcome using the Newcastle-Ottawa Quality Assessment Scale (NOS) [27]. Each star represents 1 point, and a score of 5 or more is considered high quality. We awarded up to 1 star per category for “selection” and “outcome” and up to 2 stars for “comparability”. Discussions resolved discrepancies in quality assessment among investigators.
Statistical analysis
STATA 16.0 was used to analyze the data. Forest plots of continuous data were constructed using SMD and 95% CI. Heterogeneity was examined using the I2 statistic. The heterogeneity was considered significant when I square(I2) > 50%. If significant heterogeneity is detected, random-effects models should be used. We conducted a sensitivity analysis when there was significant inter-study heterogeneity to see how each study’s results might affect the overall effect size. Subgroup analysis and meta-regression analysis were employed to investigate potential sources of heterogeneity.
Additionally, we also performed funnel plots and Egger’s test to assess publication bias.
Results
Literature research
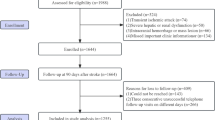
Supplementary Fig. 1 shows a flow chart of study search and screening. Eight electronic databases were searched, and 2 844 articles were obtained. First, 359 copies were eliminated. After carefully reading the titles and abstracts, 2,459 pieces were removed, including some animal studies, reviews, reviews, correspondence, case reports, meta-analyses, and articles not relevant to this study. 16 of the 26 remaining papers were removed after evaluation because they didn’t meet the criteria for inclusion. Ten articles were ultimately included.
Study characteristics and quality assessment
Supplementary Table 3 presents the main features of this study. Shen et al. evaluated SHR using both FBG/HbA1c and FBG/EAG [28]. Thus, ten articles were included, including 11 cohort studies investigating the relationship between SHR and AIS prognosis. Among them, one study was conducted in Singapore [29], one study was conducted in Australia [30], and the remaining studies were conducted in China [17, 28, 31,32,33,34,35,36]. For the evaluation of SHR, FBG/HbA1c was used in five studies [28, 29, 31,32,33,34] while FBG/EAG was used in the remaining five [28, 30, 33, 35, 36]. Eight studies performed intravenous thrombolysis or mechanical thrombectomy after a stroke [17, 28,29,30, 33,34,35,36], while the other two did not [31, 32]. Eight studies evaluated patient outcomes three months after stroke [17, 28, 29, 32,33,34,35,36], one study evaluated patient outcomes six months after stroke [31] and one study evaluated stroke outcomes at discharge [30]. The quality scores of the selected studies were acceptable, with NOS scores of 7–9 points (Supplementary Table 2).
Relationship between SHR and the prognosis of AIS
To assess the connection between SHR and clinical prognosis in AIS patients, a total of 11 cohort studies were incorporated. The post-stroke prognosis was assessed by Modified Rankin Scale(mRS) [24, 25], including 3,390 patients with good outcomes and 2,000 patients with poor outcomes. The result showed that AIS patients with poor prognoses had significantly higher SHR values than those with good prognoses (SMD = 0.56, 95%CI: 0.37–0.75, p<0.001) (Supplementary Fig. 2). Due to significant heterogeneity among studies (I2 = 90.0%, p<0.001), SMDs were pooled based on a random effect model.
Sensitivity analysis
After a particular study was eliminated sequentially, the corresponding SMD values neither reversed the result nor changed significantly (Supplementary Fig. 3). This indicates that the result of this meta-analysis is relatively stable.
Subgroup analysis
Seven subgroup analyses (including study design, country, diabetes status, post-stroke treatment, the time point of outcome assessment, the definition of SHR, and presentation of primary data) were conducted to explore factors affecting heterogeneity according to clinical characteristics of patients and study methodology. Supplementary Table 4 presents the results of the subgroup analysis. The results of the seven subgroup analyses were statistically significant, and in the poor outcome group, compared to the good outcome group, the SHR value was considerably higher. Heterogeneity was reduced to varying degrees in most subgroup analyses, particularly in study design and poststroke treatment subgroups. Subgroup analysis showed that study design (RC: SMD = 0.50, 95%CI: 0.29–0.71, I2 = 86.8%; PC: SMD = 0.72, 95%CI: 0.53–0.92, I2 = 68.6%) and differences in post-stroke treatment (IVT or MT: SMD = 0.51, 95%CI: 0.33–0.70, I2 = 86.3%; none: SMD = 0.80, 95%CI: 0.57–1.04, I2 = 42.7%) might be the sources of heterogeneity.
Meta-regression analysis
Meta-regression analysis showed that study design, country, diabetes mellitus, post-stroke treatment, the time point of outcome assessment, the definition of SHR, and original data presentation were not the source of heterogeneity (Supplementary Table 5).
Publication bias
To evaluate publication bias, we created a funnel plot (Supplementary Fig. 4). Subjectively, the scatter in the funnel plot is less symmetric, with more scatter on the right side. The Egger’s test (Supplementary Fig. 5) confirmed no significant publication bias.
Discussion
SHR and the prognosis of AIS
Comprehensive searches of eight electronic databases led to the selection of ten articles, comprising eleven cohort studies investigating the connection between SHR and prognosis in AIS patients. Six articles have demonstrated that poor short-term outcomes following AIS were associated with higher SHR in the acute period [17, 28, 29, 32, 33, 35]. Two articles suggested that SHR could independently predict short-term poor outcomes [28, 32]. In addition, one piece suggested a correlation between SHR and poor long-term outcomes in AIS patients [31]. Our findings showed that high SHR in the acute phase might predict poor outcomes after AIS. The finding was demonstrated to be reliable and stable by sensitivity analysis and publication bias analysis.
Discussion on the sources of heterogeneity
At the same time, significant heterogeneity is worth exploring. Meta-regression analysis showed that study design, country, diabetes mellitus(DM), post-stroke treatment, the time point of outcome assessment, the definition of SHR, and original data presentation were not the source of heterogeneity.
Based on clinical characteristics and study methodology, we continued to perform subgroup analyses of the included studies into seven categories. The results were statistically significant in each subgroup, with the SHR value being considerably higher in the poor outcome group than in the good outcome group. Heterogeneity was reduced to varying degrees in most subgroup analyses, particularly in study design and poststroke treatment subgroups.
Differences in study design may be one source of heterogeneity. Of the eleven studies, eight were retrospective cohort studies, and the remaining three were prospective cohort studies. Subgroup analysis indicated that the reduction in heterogeneity was more pronounced in the prospective study compared to the retrospective study. The main reason is that retrospective studies are more biased, while prospective studies are more scientific.
Different poststroke interventions may be another source of heterogeneity. In nine of the eleven studies, intravenous thrombolysis (IVT) or mechanical thrombectomy (MT) was performed after the stroke, while the remaining two did not. The subgroup that did not use IVT or MT showed a significant reduction in heterogeneity. Currently, the main treatments for acute ischemic stroke are IVT, MT, and antiplatelet therapy. In clinical practice, however, there are still patients with varying degrees of poor prognosis, even if the corresponding treatment is given proactively. According to previous studies, elevated SHR in the acute period is linked to a poorer functional outcome in AIS patients after IVT or MT [12, 17, 29], as well as increased risk of stroke recurrence [14] and post-stroke hemorrhagic transition [10, 37]. Stress hyperglycemia promotes oxidative stress and inflammatory response, reducing collateral circulation in the ischemic penumbra surrounding the infarction and transforming the ischemic penumbra into irreversible infarction [8, 38, 39]. Even if the vessel is realized in time, it may result in a poor clinical prognosis [37]. However, the conclusions of these studies are limited. More research is still required to fully understand the connection between SHR and prognosis in patients with AIS, given that mechanical thrombectomy and intravenous thrombolysis may somewhat affect patients’ prognosis trajectories.
When stratified by the proportion of diabetes in the study population, the heterogeneity in the subgroup with < 30% diabetes was significantly reduced. This suggests that the findings of this meta-analysis may be more relevant in people without diabetes. Absolute hyperglycemia does not reflect glycemic stress changes in critical situations. The relative stress hyperglycemia index-SHR considers background glucose and is a good predictor of poor outcomes in acute patients [28]. Previous research has revealed that the likelihood of recurrence in people with mild stroke or transient ischemic attack is the same for newly diagnosed and previously diagnosed diabetes [40]. In contrast, in patients with stress hyperglycemia, the risk is significantly higher [40]. Merlino et al. showed that SHR was related to poor outcomes after IVT in AIS patients, regardless of diabetes [12]. However, Zhang et al. verified that SHR is an independent predictor of poor outcomes following AIS and that the predictive effect is particularly notable in non-diabetic individuals [35]. In addition, it has been revealed that stress hyperglycemia only leads to adverse outcomes in non-diabetic patients, as people with diabetes chronically exposed to high glucose levels may have better cellular adaptation and response to hyperglycemia [41]. Therefore, the predictive value of SHR in AIS patients with or without diabetes remains to be investigated further.
Heterogeneity was significantly reduced when FBG/HbA1c was used to assess SHR. Previous studies used receiver operating characteristic(ROC) curves to determine the predictive value of admission blood glucose, FBG, HbA1c, FBG/HbA1c, and FBG/EAG for poor clinical prognosis three months after AIS. The results showed that the stress hyperglycemia ratio as measured by FBG/HbA1c and FBG/EAG was independently related to poor outcomes in AIS patients and that FBG/HbA1c was a better predictor of poor prognosis in AIS patients than blood glucose, FBG, HbA1c and FBG/EAG at admission [28]. Therefore, FBG/HbA1c may be a more appropriate prognostic predictor of AIS than FBG/EAG.
Due to the need for meta-analysis, the median and interquartile range of the raw data had to be converted to mean and standard deviation. Therefore, this heterogeneity is inevitable.
Clinical significance
Acute ischemic stroke is a common cerebrovascular disease with high incidence, disability, and mortality. Disability due to stroke imposes a considerable burden on individuals, families, and society, and early identification and management of high-risk patients are essential. Diabetes mellitus is a recognized risk factor for cerebrovascular disease. However, the consequences of stress hyperglycemia have not been fully established, and the association of stress hyperglycemia with poor functional outcomes is controversial. The result of this meta-analysis may shed some light on glycemic management in patients with AIS. Whether SHR can be a new predictor and target for early intervention still deserves further research.
Limitations and prospects
First, the high degree of heterogeneity may reduce the reliability of the findings. Meanwhile, additional research is required to explore the association between SHR and the prognosis of AIS patients due to the dearth of included studies. Most of the studies included in this study were limited to China, and more studies from other regions are needed to verify the reliability and generalization of the conclusions of this study. Furthermore, just one of the studies we included had participants who were followed for six months, necessitating further research into how SHR affects stroke outcomes in the short and long term. Whether the association between SHR and stroke outcome is more pronounced in non-diabetic patients and whether FBG/HbA1c is a more appropriate predictor of AIS outcome compared to FBG/EAG remains to be investigated.
Conclusion
In conclusion, the findings of this meta-analysis provided new insight into the connection between SHR and clinical outcomes in AIS patients. We found that elevated SHR in the acute phase was associated with a worse outcome, indicating that elevated SHR may predict poor clinical outcomes after AIS. Nonetheless, given the low number of included studies, more studies may be needed to explore the predictive value of SHR in AIS.
Data availability
All data are available and taken from the previously published article.
Abbreviations
- SHR:
-
Stress hyperglycemia ratio
- AIS:
-
Acute ischemic stroke
- SMD:
-
Standardized mean difference
- CI:
-
Confidence interval
- HbA1C:
-
Glycosylated hemoglobin
- FBG:
-
Fasting blood glucose
- EAG:
-
Estimated average glucose
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- mRS:
-
Modified Rankin Scale
- IQR:
-
Interquartile range
- SD:
-
Standard deviation
- QE:
-
Quantile estimation
- NOS:
-
Newcastle-Ottawa Quality Assessment Scale
- I2 :
-
I square
- RC:
-
Retrospective cohort
- PC:
-
Prospective cohort
- IVT:
-
Intravenous thrombolysis
- MT:
-
Mechanical thrombectomy
- DM:
-
Diabetes mellitus
- ROC:
-
Receiver Operating Characteristic
- CNKI:
-
Chinese National Knowledge Infrastructure
- CBM:
-
China Biology Medicine Literature Database
References
Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, et al. Global burden of Stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the global burden of Disease Study 2013. Lancet Neurol. 2016;15(9):913–24.
Tu WJ, Qiu HC, Liu YK, Liu Q, Zeng X, Zhao J. Elevated levels of adiponectin associated with major adverse cardiovascular and cerebrovascular events and mortality risk in ischemic Stroke. Cardiovasc Diabetol. 2020;19(1):125.
Feigin VL, Stark BA, Johnson CO, Roth GA, Bisignano C, Abady GG, et al. Global, regional, and national burden of Stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795–820.
Tu WJ, Zhao Z, Yin P, Cao L, Zeng J, Chen H et al. Estimated burden of Stroke in China in 2020. JAMA Netw Open. 2023;6(3).
Ali I, Abuissa M, Alawneh A, Subeh O, Abu Sneineh A, Mousa S, et al. The prevalence of dyslipidemia and hyperglycemia among Stroke patients: preliminary findings. Stroke Res Treat. 2019;2019:1–6.
Dungan KM, Braithwaite SS, Preiser J. Stress hyperglycaemia. The Lancet. 2009;373:1798–807.
Marik PE, Bellomo R. Stress hyperglycemia: an essential survival response! Crit Care. 2013;17(2):305.
Luitse MJ, Biessels GJ, Rutten GE, Kappelle LJ. Diabetes, hyperglycaemia, and acute ischaemic Stroke. Lancet Neurol. 2012;11(3):261–71.
Farrokhi F, Smiley D, Umpierrez GE. Glycemic control in non-diabetic critically ill patients. Best Pract Res Clin Endocrinol Metab. 2011;25(5):813–24.
Roberts GW, Quinn SJ, Valentine N, Alhawassi T, O’Dea H, Stranks SN, et al. Relative hyperglycemia, a marker of critical Illness: introducing the stress hyperglycemia ratio. J Clin Endocrinol Metab. 2015;100(12):4490–7.
Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57(5):1349–54.
Merlino G, Smeralda C, Gigli GL, Lorenzut S, Pez S, Surcinelli A, et al. Stress hyperglycemia is predictive of worse outcome in patients with acute ischemic Stroke undergoing intravenous thrombolysis. J Thromb Thrombolysis. 2021;51(3):789–97.
Pan Y, Cai X, Jing J, Meng X, Li H, Wang Y, et al. Stress hyperglycemia and prognosis of minor ischemic Stroke and transient ischemic Attack: the CHANCE study (clopidogrel in high-risk patients with acute nondisabling cerebrovascular events). Stroke. 2017;48(11):3006–11.
Zhu B, Pan Y, Jing J, Meng X, Zhao X, Liu L, et al. Stress hyperglycemia and outcome of non-diabetic patients after acute ischemic Stroke. Front Neurol. 2019;10:1003.
Li J, Quan K, Wang Y, Zhao X, Li Z, Pan Y, et al. Effect of stress hyperglycemia on neurological deficit and mortality in the acute ischemic Stroke people with and without Diabetes. Front Neurol. 2020;11:576895.
Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ, et al. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31(8):1473–8.
Chen X, Liu Z, Miao J, Zheng W, Yang Q, Ye X, et al. High stress hyperglycemia ratio predicts poor outcome after mechanical thrombectomy for ischemic Stroke. J Stroke Cerebrovasc Dis. 2019;28(6):1668–73.
Lee TF, Drake SM, Roberts GW, Bersten A, Stranks SN, Heilbronn LK, et al. Relative hyperglycemia is an Independent determinant of in-hospital mortality in patients with critical Illness. Crit Care Med. 2020;48(2):e115–e22.
Allport LE, Butcher KS, Baird TA, MacGregor L, Desmond PM, Tress BM, et al. Insular cortical ischemia is independently associated with acute stress hyperglycemia. Stroke. 2004;35(8):1886–91.
Wada S, Yoshimura S, Inoue M, Matsuki T, Arihiro S, Koga M et al. Outcome prediction in acute Stroke patients by continuous glucose monitoring. J Am Heart Assoc. 2018;7(8).
Luo Y, Wang X, Matsushita K, Wang C, Zhao X, Hu B, et al. Associations between estimated glomerular filtration rate and Stroke outcomes in diabetic versus nondiabetic patients. Stroke. 2014;45(10):2887–93.
Su YW, Hsu CY, Guo YW, Chen HS. Usefulness of the plasma glucose concentration-to-HbA(1c) ratio in predicting clinical outcomes during acute Illness with extreme hyperglycaemia. Diabetes Metab. 2017;43(1):40–7.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021;134:178–89.
Van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJA, van Gijn J. Interobserver agreement for the assessment of handicap in Stroke patients. Stroke. 1988;19:604–7.
Lai SM, Duncan PW. Stroke recovery profile and the Modified Rankin Scale. Neuroepidemiology. 2001;20:26–30.
McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A, Collaboration DESD. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. 2020;29(9):2520–37.
Wells GA, Shea B, O’Connel D. The Newcastle-Ottawa Scale (NOS) for assessing the quailty of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute (ohri.ca); 2009.
Shen CL, Xia NG, Wang H, Zhang WL. Association of stress hyperglycemia ratio with acute ischemic Stroke outcomes post-thrombolysis. Front Neurol. 2021;12:785428.
Ngiam JN, Cheong CWS, Leow AST, Wei YT, Thet JKX, Lee IYS, et al. Stress hyperglycaemia is associated with poor functional outcomes in patients with acute ischaemic Stroke after intravenous thrombolysis. QJM. 2022;115(1):7–11.
Roberts G, Sires J, Chen A, Thynne T, Sullivan C, Quinn S, et al. A comparison of the stress hyperglycemia ratio, glycemic gap, and glucose to assess the impact of stress-induced hyperglycemia on ischemic Stroke outcome. J Diabetes. 2021;13(12):1034–42.
Xie SH. Effect of the stress hyperglycemia ratio in first-ever penetrating artery infarction, a comparison study between different subtypes ischemic Stroke patients. 2018.
Liu TT, He ML, Xu BC, Zhang YJ, Song YH, Sun B, et al. Stress hyperglycemia predicts the outcomes in patients with acute ischemic Stroke. Int J Cerebrovasc Dis. 2021;29(12):885–92.
Chen G, Ren J, Huang H, Shen J, Yang C, Hu J, et al. Admission random blood glucose, fasting blood glucose, stress hyperglycemia ratio, and functional outcomes in patients with acute ischemic Stroke treated with intravenous thrombolysis. Front Aging Neurosci. 2022;14:782282.
Li D, Wei XE, Duan ZW, Liu HY. Effect of blood glucose on early neurological deterioration and outcome in patients with acute ischemic Stroke after intravenous thrombolytic therapy. Int J Cerbrovasc Dis. 2022;30(9):679–83.
Zhang D, Li ZZ, Ma RN, Yue YH. Correlation between stress hyperglycemia ratio and clinical prognosis of patients with acute ischemic Stroke. Chin J Stroke. 2022;17(5):483–90.
Sun Y, Guo Y, Ji Y, Wu K, Wang H, Yuan L, et al. New stress-induced hyperglycaemia markers predict prognosis in patients after mechanical thrombectomy. BMC Neurol. 2023;23(1):132.
Yuan C, Chen S, Ruan Y, Liu Y, Cheng H, Zeng Y, et al. The stress hyperglycemia ratio is associated with hemorrhagic transformation in patients with acute ischemic Stroke. Clin Interv Aging. 2021;16:431–42.
Mazighi M, Labreuche J, Amarenco P. Glucose level and brain infarction: a prospective case-control study and prospective study. Int J Stroke. 2009;4(5):346–51.
Koracevic GP. Proposal of a new approach to study and categorize stress hyperglycemia in acute Myocardial Infarction. J Emerg Med. 2016;51(1):31–6.
Guo YZ, Wang GY, Jing J, Wang AX, Zhang XL, Meng X, et al. Stress hyperglycemia may have higher risk of Stroke recurrence than previously diagnosed Diabetes Mellitus. Aging. 2021;13(6):9108–18.
Bosarge PL, Shoultz TH, Griffin RL, Kerby JD. Stress-induced hyperglycemia is associated with higher mortality in severe traumatic brain injury. J Trauma Acute Care Surg. 2015;79(2):289–94.
Acknowledgements
Not applicable. We have no acknowledgments to disclose.
Funding
Financial support: This study was supported by the National Natural Science Foundation of China (No. 81873794).
Author information
Authors and Affiliations
Contributions
ZJ and SF contributed to the design and methodology of the study. ZJ, KW, HYD, HQD, SG, and JC participated in the literature search and data extraction. ZJ and KW performed the statistical analysis and validated the results. ZJ wrote the manuscript. SF and KW read the manuscript and made revisions.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
An ethics statement is not applicable because this study is based exclusively on published literature.
Consent for publication
The data used in the meta-analysis were all from published studies, so consent for publication did not apply.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jiang, Z., Wang, K., Duan, H. et al. Association between stress hyperglycemia ratio and prognosis in acute ischemic stroke: a systematic review and meta-analysis. BMC Neurol 24, 13 (2024). https://doi.org/10.1186/s12883-023-03519-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-023-03519-6