Abstract
No study investigated the possible detrimental effect of stress hyperglycemia on patients affected acute ischemic stroke (AIS) undergoing intravenous thrombolysis (IVT). A new index, the glucose-to-glycated hemoglobin ratio (GAR), has been developed for assessing stress hyperglycemia. We retrospectively analyzed data from a prospectively collected database of consecutive patients admitted to the Udine University Hospital with AIS that were treated with IVT from January 2015 to December 2019. Four hundred and fourteen consecutive patients with AIS undergoing IVT entered the study. The patients were then stratified into four groups by quartiles of GAR (Q1–Q4). The higher GAR index was, the more severe stress hyperglycemia was considered. Prevalence of 3 months poor outcome (37.7% for Q1, 34% for Q2, 46.9% for Q3, and 66.7% for Q4, p for trend = 0.001), 3 months mortality (10.5% for Q1, 7.5% for Q2, 11.2% for Q3, and 27.1% for Q4, p for trend = 0.001), and symptomatic intracranial hemorrhage (0.9% for Q1, 0.9% for Q2, 5.1% for Q3, and 17.7% for Q4, p for trend = 0.001) was significant different among the four groups. AIS patients with severe stress hyperglycemia had a significantly increased risk of 3 months poor outcome (OR 2.43, 95% CI 1.14–5.22, p = 0.02), 3 months mortality (OR 2.38, 95% CI 1.01–5.60, p = 0.04), and symptomatic intracranial hemorrhage (OR 16.76, 95% CI 2.09–134.58, p = 0.008) after IVT. In conclusion, we demonstrated that stress hyperglycemia, as measured by the GAR index, is associated to worse outcome in AIS patients undergoing IVT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
No study investigated the possible detrimental effect of stress hyperglycemia on patients affected acute ischemic stroke undergoing intravenous thrombolysis.
-
The glucose-to-glycated hemoglobin ratio was used for assessing stress hyperglycemia in our patients.
-
Patients with severe stress hyperglycemia had a significantly increased risk of 3 months poor outcome, 3 months mortality, and symptomatic intracranial hemorrhage.
Introduction
Before reperfusion therapy was available, elevated serum glucose has been associated with poor outcome in patients affected by acute ischemic stroke (AIS) [1]. More recently, AIS patients undergoing intravenous thrombolysis (IVT) with hyperglycemia at admission had a worse outcome and a more frequent occurrence of symptomatic intracranial hemorrhage (SICH) than those with lower glucose levels [2]. However, a proper interpretation of these results is complicated because underlying diabetes may act as potential confounder.
Diabetes mellitus is a carbohydrate metabolism disorder characterized by chronic hyperglycemia. Diabetes represents a well-established risk factor for stroke and it leads to cerebrovascular disease by several different mechanisms: vascular endothelial dysfunction, increased early-age arterial stiffness, systemic inflammation, and thickening of the capillary basal membrane [3].
Hyperglycemia may, also, occur de novo in a high proportion of patients suffering an acute stress, such as stroke [4]. Stress hyperglycemia has been associated with a high risk of mortality both in human and animal studies [5]. Recently, a new index, the glucose-to-glycated hemoglobin (HbA1c) ratio (GAR), has been developed for assessing stress hyperglycemia [6]. While HbA1c reflects the baseline average glucose status over the past 3 months, the GAR index quantifies the extent of acute elevation in plasma glucose, i.e. stress hyperglycemia, compared to background plasma glucose levels.
To date, only one study used the GAR index in AIS patients and it showed that stress hyperglycemia predicted stroke recurrence and mortality in non-diabetic patients. However, only 3% of these AIS patients were treated with IVT [7].
The aim of the present study was to investigate the possible detrimental effect of stress hyperglycemia, as measured by the GAR index, in AIS patients undergoing IVT.
Methods
Study participants
We retrospectively analyzed data from a prospectively collected database of consecutive patients admitted to the Udine University Hospital with AIS that were treated with IVT from January 2015 to December 2019. In accordance with the indication and contraindications of international guidelines, we treated patients showing symptoms onset within 4.5 h with alteplase at dosage of 0.9 mg/kg over 1 h [8, 9]. Based on the recent results of the EXTEND trial, three patients with wake-up stroke and salvageable brain tissue at the computed tomography perfusion were treated beyond 4.5 h [10]. We excluded AIS patients undergoing endovascular thrombectomy in addition to IVT.
All patients or his/her representatives gave informed, signed consent to use of their data for research purposes. The study was approved by the local ethics committee (Ref. No. CEUR-2020-Os-173).
Data collection
The following variables were collected: age, sex, vascular risk factors, laboratory findings, admission systolic blood pressure, and pharmacological treatment.
Previous transient ischemic attack/stroke was considered if patient had history of ischemic (transient attack or stroke) or hemorrhagic cerebrovascular disease. Presence of cardiovascular disease was based on history of previous ischemic heart disease and/or revascularization treatment using percutaneous coronary intervention/coronary artery bypass grafting. Atrial fibrillation was considered if patient had past medical history of this arrhythmia that had been confirmed in medical records. High blood pressure was defined as history of hypertension and/or use of antihypertensive medication. History of diabetes mellitus that had been confirmed in medical records and/or use of insulin/oral hypoglycemic agents were considered for defining diabetes. Presence of hypercholesterolemia was based on the use of lipid-lowering medications. Information on active tobacco use was used for defining patient as a current smoker.
Clinical assessment
The Trial of ORG 10172 in acute stroke treatment (TOAST) classification was used to determine AIS subtypes based on their etiology. In particular, cerebrovascular events were distinguished as due to large artery atherosclerosis, cardioembolism, small-vessel occlusion, other determined etiology, and undetermined etiology [11]. Stroke severity was determined with the National Institute of Health Stroke Scale (NIHSS) score at admission and at discharge. We defined patients with major neurological improvement those who had an improvement of ≥ 8 points on the NIHSS from baseline or a NIHSS score of 0 or 1 at discharge. Functional outcome was assessed by means of the modified Rankin Scale (mRS) at admission, based on pre-stroke disability, and three months after stroke. The mRS score after discharge was recorded at the patients’ routine clinical visit or through telephone interview with patients or their immediate caregivers. The mRS score was dichotomized into: favorable outcome (0–2) and poor outcome (3–6). The presence of intracranial hemorrhage (ICH) was defined as any parenchymal hematoma (PH) based on the European Cooperative Acute Stroke Study (ECASS) morphologic definitions (ECASS PH-1 or PH-2) [12], whereas presence of SICH was based on the ECASS-III protocol [13]. Finally, we collected information on time from symptom onset to IVT.
Assessment of stress hyperglycemia
The fasting venous blood samples within 24 h after hospitalization were drawn during the morning hours (range : 06.00–08:00) after an overnight fast (at least 12 h) to measure fasting plasma glucose and HbA1c. Stress hyperglycemia was estimated by the GAR index that was calculated using the following formula: fasting plasma glucose (mg/dl)/HbA1c (%). The patients were then stratified into four groups by quartiles of GAR (Q1–Q4) for further comparisons. The higher GAR index was, the more severe stress hyperglycemia was considered.
Outcome measures
The following endpoints were analyzed: 3 months poor outcome, no major neurological improvement at discharge, in-hospital mortality, 3 months mortality, presence of ICH, and presence of SICH. All the outcome measures were collected as part of our routine clinical practice in patients affected by cerebrovascular events.
Statistical analysis
Data are displayed in tables as median and interquartile range (IQR), if not otherwise specified.
Differences across the four GAR quartiles were assessed by means of the Chi square test for categorial variables. One-way analysis of variance for normally distributed continuous variables, and the Kruskal–Wallis test for non-normally distributed continuous variables and for ordinal variables were used. Post-hoc analysis was performed by means of the Bonferroni test. The Kolmogorov–Smirnov test with Lilliefors significant correction was used to assess normal distribution of data.
The impact of stress hyperglycemia, as represented by GAR quartiles, on outcome measures was evaluated by multiple logistic regression analysis with the lowest GAR quartile as reference. The potential confounding variables included in the model were: age, history of diabetes, baseline NIHSS score, pre-stroke mRS, and time from symptom onset to IVT. Systolic blood pressure > 180 mmHg was added to other confounders in the analysis that evaluated the association between stress hyperglycemia and hemorrhagic transformation (i.e. ICH, SICH).
All probability values are two-tailed. A p value < 0.05 was considered statistically significant. Statistical analysis was carried out using the SPSS Statistics, Version 22.0 (Chicago, IL, USA).
Results
Baseline characteristics
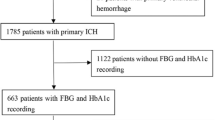
Four hundred and fourteen consecutive patients with AIS undergoing IVT entered the study (Fig. 1).
The general characteristics of the enrolled subjects, distinguished by GAR quartiles, are presented in Table 1. History of diabetes was more common among patients in the highest quartile of GAR, thus these subjects were treated more frequently with insulin than those in the other GAR quartile groups. Differently, the higher GAR index was, the smaller number of current smokers was. Patients in the fourth GAR quartile had higher fasting glucose levels and HbA1c values than those in the other three groups. At admission, systolic blood pressure values were significantly different among the four groups and subjects affected by more severe stress hyperglycemia showed the highest blood pressure. Neurological impairment, as measured by the NIHSS score both at admission and at discharge, was more severe in patients in the fourth GAR quartile than in the other groups. Moreover, prevalence of pre-stroke functional independence, defined as an mRS score ≤ 2, was more common among AIS patients with less severe stress hyperglycemia.
Association of stress hyperglycemia with clinical outcomes in univariate analysis
Rates of 3 months poor outcome, 3 months mortality, and SICH according to GAR quartiles are reported in Figs. 2, 3 and 4. Prevalence of no major neurological improvement (30.4% for Q1, 30.8% for Q2, 49.5% for Q3, and 49.4% for Q4, p for trend = 0.001), in-hospital mortality (1.8% for Q1, 1.9% for Q2, 5.1% for Q3, and 13.5% for Q4, p for trend = 0.001), and ICH (4.4% for Q1, 4.7% for Q2, 11.2% for Q3, and 22.9% for Q4, p for trend = 0.001) was statistically different among the four groups.
Association of stress hyperglycemia with clinical outcomes in multivariate analysis
As reported in Table 2, all the outcomes were significantly associated with severe stress hyperglycemia, even after controlling for confounders. Independent predictors, other than the highest GAR quartile, were the following: (1) age (OR 1.03, 95% CI 1.01–1.05, p = 0.001), NIHSS score at admission (OR 1.18, 95% CI 1.13–1.24, p = 0.001), and pre-stroke mRS (OR 3.63, 95% CI 2.34–5.64, p = 0.001) for three-month poor outcome; (2) age (OR 1.02, 95% CI 1.01–1.04, p = 0.006), NIHSS score at admission (OR 1.06, 95% CI 1.02–1.10, p = 0.002), and pre-stroke mRS (OR 1.29, 95% CI 1.05–1.59, p = 0.02) for no major neurological improvement; (3) NIHSS score at admission (OR 1.11, 95% CI 1.04–1.18, p = 0.002) for in-hospital mortality; (4) age (OR 1.04, 95% CI 1.01–1.07, p = 0.007), NIHSS score at admission (OR 1.61, 95% CI 1.11–1.22, p = 0.001), and pre-stroke mRS (OR 1.29, 95% CI 1.02–1.65, p = 0.04) for three-month mortality; and NIHSS score at admission for both (5) ICH (OR 1.11, 95% CI 1.06–1.17, p = 0.005), and (6) SICH (OR 1.07, 95% CI 1.01–1.14, p = 0.04).
Discussion
For the first time, we demonstrated that stress hyperglycemia, as measured by the GAR index, is predictive of worse outcome in AIS patients undergoing IVT treatment.
Admission glucose level has been associated with lower rate of good outcome and increased risk of SICH after IVT for AIS [2]. These detrimental effects might be due to the presence of underlying diabetes, but the role of stress hyperglycemia cannot be excluded.
Diabetes represents a recognized risk factor for cerebrovascular disease. Indeed, chronic hyperglycemia has been associated with ischemic stroke [3] and hemorrhagic transformation after IVT [14]. Differently, consequences of stress hyperglycemia are not well established. In 1878 Claude Bernard described the first case of hyperglycemia related to acute critical illness, i.e. stress hyperglycemia, in a patient with elevated glucose levels during hemorrhagic shock [15]. Stress hyperglycemia is mediated by the hypothalamic–pituitary–adrenal axis, the sympatho-adrenal system, and the proinflammatory cytokines, i.e. TNF-α, IL-1, and IL-6. These mediators are able to induce a stress response characterized by excessive gluconeogenesis, glycogenolysis, and insulin resistance [16]. The degree of activation of the stress response and the severity of hyperglycemia are related to the intensity of the stressor [16]. Experimental studies on rodents demonstrated that intense stress, imposed by four limb prone restraint, caused high and early peaks of plasma corticosterone levels. In contrast, stressors of lesser severity, i.e. 5 min exposure to a novel open field, produced lower peak hypothalamic–pituitary–adrenal activation. Similarly, high intensity and long duration challenges, e.g. inflammatory response after AIS, cause in humans prolonged stress responses, whereas shorter responses are observed following exposure to psychological stressor [17]. In accordance with this notion, our patients in the highest GAR quartile were affected by more severe ischemic stroke, as measured by the NIHSS score at admission and discharge.
Stress hyperglycemia represents an evolutionary preserved response that allow the host to survive during period of severe stress [18]. In humans, results coming from scientific literature are conflicting. In a recent review, Marik and Bellomo claimed that acute stress hyperglycemia may be protective and may result in greater plasticity and cellular resistance to ischemic and hypoxic insults [16]. However, numerous clinical studies performed in both ICU and non-ICU patients reported that stress hyperglycemia increases the odds of poor clinical outcomes [19,20,21,22].
A limited number of studies investigated the consequences of stress hyperglycemia in stroke patients. An historical overview by Capes et al. analyzed the relationship between stress hyperglycemia and prognosis in diabetic and non-diabetic stroke patients [23]. The authors identified 32 studies and observed that acute hyperglycemia predicted increased risk of in-hospital mortality after ischemic stroke in non-diabetic patients and increased risk of poor functional recovery in non-diabetic stroke survivors [23]. However, as reported by the authors, the included studies were affected by several limitations: (1) the definition of stress hyperglycemia varied largely among the studies; (2) most studies did not specified whether whole blood or plasma glucose was measured; (3) a random glucose level drawn on admission was used to define stress hyperglycemia in numerous studies [23]. In addition, since the definition of stress hyperglycemia is intrinsically problematic in diabetic patients because the unstressed baseline level of glucose is not known, the large part of investigations on stress hyperglycemia was performed enrolling non-diabetic stroke patients [23]. No study has primarily been focused on AIS patients undergoing IVT. Our investigation, that included diabetic and non-diabetic AIS patients, demonstrated that patients with a history of diabetes were affected by more severe forms of stress hyperglycemia. In fact, the number of diabetic patients in the lowest GAR quartile was small (8.8%), whereas almost one third (33.3%) of subjects in the Q4 GAR group had diabetes. A relationship between severe stress hyperglycemia and diabetes was, also, reported by MacIntyre et al. in a study of hospitalized patients with pneumonia [24].
Recently, Su et al. introduced the GAR index for quantifying stress hyperglycemia. In their retrospective study, enrolling patients with plasma glucose concentrations > 500 mg/dl, the authors observed that GAR independently predicted 90-day mortality, ICU admission and use of mechanical ventilation. In addition, this index was a better predictor of patient outcomes than plasma glucose alone [6]. To date, only Zhu et al. adopted the GAR index in cerebrovascular patients. The authors explored the relationship between stress hyperglycemia and clinical outcome of non-diabetic Chinese patients affected by AIS. Patients affected by stress hyperglycemia had an elevated risk of stroke recurrence and all-cause death. Since only 3.3% of patients were treated with alteplase, this study did not give any information regarding the consequences of stress hyperglycemia after IVT [7]. Our study demonstrated that stress hyperglycemia significantly impaired clinical outcome in AIS patients undergoing IVT. Sixty-seven percent of subjects with severe acute post-stroke hyperglycemia was functionally dependent at 3 months follow-up and almost 50% of patients in the Q4 GAR group did not shown any significant improvement at discharge despite IVT treatment. After adjusting for potential covariates, stress hyperglycemia remained an independent predictor of poor outcome and no major neurological improvement. In addition, risk of in-hospital and three-month mortality was significantly greater among subjects with severe stress hyperglycemia undergoing IVT. Although the mechanisms of the association between stress hyperglycemia and poor clinical outcome in stroke patients are not yet fully understood, several hypotheses have been proposed. Hyperglycemia might directly cause toxic damage to ischemic brain via accumulation of lactate and intracellular acidosis [25]. In fact, intracellular acidosis enhances lipid peroxidation and free radical formation accelerating ischemic injury [26]. Furthermore, stress-induced inflammatory response is able to increase circulating free fatty acids in patients with acute illness. Free fatty acids may impair endothelium-dependent vasodilation [27] and promote intracellular calcium overload [28].
Admission hyperglycemia has been linked to an increased risk of hemorrhagic complications after alteplase treatment [29]. Our study further confirms these data, showing that acute post-stroke hyperglycemia, as measured by the GAR index, was independently associated with the occurrence of ICH and SICH. Stress hyperglycemia might cause hemorrhagic infarct conversion via reperfusion injury [30, 31] and blood–brain barrier disruption [32].
Management of hyperglycemia varies across centers and clinicians. Indeed, to date, conclusive evidences supporting strict post-stroke normoglycemia are lacking. A recent systematic literature review recognized 13 RCTs investigating aggressive treatment of admission hyperglycemia in AIS patients. None of these trials demonstrated any significant benefit of tight glycemic control on functional outcome or in survival [33]. Recently, AIS patients were randomized to receive continuous intravenous insulin (intensive treatment group) or subcutaneous insulin on a sliding scale (standard treatment group) in the SHINE trial. Although blood glucose level was significantly lower in the intensive treatment group, rates of favorable outcome were similar between the two groups. In addition, severe hypoglycemic events occurred in the intensive treatment group [34].
Our study has limitations. About one fourth of patients were excluded because of missing fasting glucose or HbA1c (n = 26), or because lost to follow-up (n = 132). Patients in the four GAR quartiles showed significant differences regarding important baseline clinical characteristics, e.g. NIHSS score at admission. Although we know that these baseline differences might have partially affected our results, we would also underline that minimizing heterogeneity was arduous. In fact, since stress hyperglycemia is strictly related to the severity of the illness, it is clear that patients in the highest GAR quartile were affected by the most severe strokes with the highest NIHSS scores at admission. In addition, NIHSS score at admission and pre-stroke mRS were included among confounders in our multivariate analysis. This is a retrospective, observational study, thus the cause-effect relationship between stress hyperglycemia and outcome should be considered speculative. The retrospective nature of the study and the quartile-based analysis might have affected an adequate control for confounding variables able to impact outcomes. Even after controlling for known confounders, residual confounding from unobserved factors might have affected our results. Finally, since some of the 3-month mRS scores were indirectly recorded through telephone interview with patients or their caregivers, it might be possible that post-discharge functional status was not captured as well as in-hospital one.
In conclusion, we demonstrated that stress hyperglycemia, as measured by the GAR index, is associated to worse outcome in AIS patients undergoing IVT. In addition, we observed that severity of hyperglycemia, i.e. the highest GAR quartile, was directly related to stressor intensity, i.e. more severe ischemic stroke as measured by the NIHSS score.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Bruno A, Biller J, Adams HPJ et al (1999) Acute blood glucose level and outcome from ischemic stroke. Trial of ORG 10172 in acute stroke treatment (TOAST) investigators. Neurology 52:280–284
Desilles JP, Meseguer E, Labreuche J et al (2013) Diabetes mellitus, admission glucose, and outcomes after stroke thrombolysis: a registry and systematic review. Stroke 44:1915–1923
Chen R, Ovbiagele B, Feng W (2016) Diabetes and stroke: epidemiology, pathophysiology, pharmaceuticals and outcomes. Am J Med Sci 351:380–386
Melamed E (1976) Reactive hyperglycaemia in patients with acute stroke. J Neurol Sci 29:267–275
Mankovsky BN, Metzger BE, Molitch ME, Biller J (1996) Cerebrovascular disorders in patients with diabetes mellitus. J Diabetes Complicat 10:228–242
Su YW, Hsu CY, Guo YW, Chen HS (2017) Usefulness of the plasma glucose concentration-to-HbA1c ratio in predicting clinical outcomes during acute illness with extreme hyperglycaemia. Diabetes Metab 43:40–47
Zhu B, Pan Y, Jing J et al (2019) Stress hyperglycemia and outcome of non-diabetic patients after acute ischemic stroke. Front Neurol 10:1003
Jauch EC, Saver JL, Adams HP Jr, Council on Cardiovascular Nursing Council on Peripheral Vascular Disease Council on Clinical Cardiology et al (2013) Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 44:870–947
Powers WJ, Rabinstein AA, Ackerson T et al (2019) Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 50:e344–e418
Ma H, Campbell BCV, Parsons MW, EXTEND Investigators et al (2019) Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med 380:1795–1803
Adams HP Jr, Bendixen BH, Kappelle LJ et al (1993) Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial TOAST Trial of Org 10172 in acute stroke treatment. Stroke 24:35–41
Hacke W, Kaste M, Fieschi C et al (1995) Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 274:1017–1025
Hacke W, Kaste M, Bluhmki E et al (2008) Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 359:1317–1329
Demchuk AM, Morgenstern LB, Krieger DW et al (1999) Serum glucose level and diabetes predict tissue plasminogen activator-related intracerebral hemorrhage in acute ischemic stroke. Stroke 30:34–39
Bernard C (1878) Lecons sur les Phenomenes de la Vie Communs aux Animaux et aux Vegetaux. JB Bailliere et fi ls, Paris
Marik PE, Bellomo R (2013) Stress hyperglycemia: an essential survival response! Crit Care 17:305
Herman JP, McKlveen JM, Ghosal S et al (2016) Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol 6:603–621
Marik PE, Levitov A (2010) The “koala stress syndrome” and adrenal responsiveness in the critically ill. Intensive Care Med 36:1805–1806
Badawi O, Waite MD, Fuhrman SA, Zuckerman IH (2012) Association between intensive care unit-acquired dysglycemia and in-hospital mortality. Crit Care Med 40:3180–3188
Bruno A, Levine SR, Frankel MR et al (2002) Admission glucose level and clinical outcomes in the NINDS rt-PA stroke trial. Neurology 59:669–674
Capes SE, Hunt D, Malmberg K, Gerstein HC (2000) Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet 355:773–778
Dungan K, Braithwaite SS, Preiser JC (2009) Stress hyperglycemia. Lancet 373:1798–1807
Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC (2001) Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 32:2426–2432
MacIntyre EJ, Majumdar SR, Gamble JM, Minhas-Sandhu JK, Marrie TJ, Eurich DT (2012) Stress hyperglycemia and newly diagnosed diabetes in 2124 patients hospitalized with pneumonia. Am J Med 125:1036.e17–23
Levine SR, Welch KM, Helpern JA et al (1988) Prolonged deterioration of ischemic brain energy metabolism and acidosis associated with hyperglycemia: human cerebral infarction studied by serial 31P NMR spectroscopy. Ann Neurol 23:416–418
Siesjö BK, Bendek G, Koide T, Westerberg E, Wieloch T (1985) Influence of acidosis on lipid peroxidation in brain tissues in vitro. J Cereb Blood Flow Metab 5:253–258
Steinberg HO, Tarshoby M, Monestel R et al (1997) Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest 100:1230–1239
Oliver MF, Opie LH (1994) Effects of glucose and fatty acids on myocardial ischaemia and arrhythmias. Lancet 343:155–158
Putaala J, Sairanen T, Meretoja A et al (2011) Post-thrombolytic hyperglycemia and 3-month outcome in acute ischemic stroke. Cerebrovasc Dis 31:83–92
Quast MJ, Wei J, Huang NC et al (1997) Perfusion deficit parallels exacerbation of cerebral ischemia/reperfusion injury in hyperglycemic rats. J Cereb Blood Flow Metab 17:553–559
Kamada H, Yu F, Nito C, Chan PH (2007) Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke 38:1044–1049
Dietrich WD, Alonso O, Busto R (1993) Moderate hyperglycemia worsens acute blood-brain barrier injury after forebrain ischemia in rats. Stroke 24:111–116
Palaiodimou L, Lioutas VA, Lambadiari V, Paraskevas GP, Voumvourakis K, Tsivgoulis G (2019) Glycemia management in acute ischemic stroke: current concepts and novel therapeutic targets. Postgrad Med 131:423–437
Johnston KC, Bruno A, Pauls Q et al (2019) Intensive vs standard treatment of hyperglycemia and functional outcome in patients with acute ischemic stroke: the SHINE randomized clinical trial. JAMA 322:326–335
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization, GM and CS; methodology, GM and CS; software, CS and SL; validation, SL, GLG and MV; formal analysis, GM; investigation, CS, SL, SP, AS, and AM; resources, CS, SL, SP, AS and AM; data curation, GM; writing—original draft preparation, GM; writing—review and editing, GM; visualization, GLG; supervision, MV.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Merlino, G., Smeralda, C., Gigli, G.L. et al. Stress hyperglycemia is predictive of worse outcome in patients with acute ischemic stroke undergoing intravenous thrombolysis. J Thromb Thrombolysis 51, 789–797 (2021). https://doi.org/10.1007/s11239-020-02252-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-020-02252-y