Abstract
Aims
Hyperglycemia on admission is associated with poor prognosis in ischemic stroke (IS) patients. We aimed to investigate the relationship between stress hyperglycemia ratio (SHR) and short-term or long-term mortality in IS patients in the ICU and to explore whether this relationship is influenced by diabetes status.
Materials and methods
We collected patients with severe IS requiring ICU admission in the Medical Information Mart for Intensive Care (MIMIC-IV) database and calculated SHR. Outcomes included 30-day, 90-day, and 1-year mortality. The association between SHR and mortality in patients with critical IS was elucidated using Multivariate Cox regression and subgroup analysis for diabetes.
Results
A total of 1376 patients were recruited. After adjusting for potential confounders, patients in the third and fourth quartiles had a significantly increased risk of death at 30 days, 90 days, and 1 year compared to the first quartile of SHR (Q3 vs. Q1: HR 1.56–1.80, all p < 0.02; Q4 vs. Q1: HR 1.75–2.15, all p < 0.001; all p for trend < 0.001). In addition, the highest quartile of SHR was significantly associated with short-term or long-term mortality compared with the first quartile, regardless of diabetes status.
Conclusions
Our results suggest that stress hyperglycemia, defined by the glucose/HbA1c ratio, is associated with increased short-term and long-term mortality in patients with ischemic stroke, independent of the patient's diabetes status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic stroke (IS) is one of the most common types of cerebrovascular disease, accounting for about 70% of all cerebrovascular diseases and 85% of strokes [1,2,3], with high rates of disability and death [4], especially in critically ill patients [5]. Studies showed that up to 60% of critical stroke patients were severely disabled or died within 90 days [6]. Therefore, early identification of risk factors associated with progressive IS is particularly important for clinical management.
As one of the manifestations of stress, hyperglycemia has been proven to be associated with poor prognosis in a lot of diseases [7,8,9,10]. Hyperglycemia is seen in more than 40% of patients with acute IS [11] and is associated with poorer functional outcomes and higher mortality rates [9, 12]. In previous studies, stress hyperglycemia defined by fasting or admission blood glucose (ABG) was often confounded by diabetes-related background hyperglycemia [13, 14]. However, unlike absolute hyperglycemia levels, stress hyperglycemia assessed by glucose/glycated hemoglobin (HbA1c) ratio provides a more objective representation of the relative elevation of glucose levels [15] and may be useful in predicting short-term and long-term mortality in patients with IS in the intensive care unit (ICU).
In this study, we aimed to investigate the relationship between stress hyperglycemia ratio (SHR) and short-term and long-term all-cause mortality in IS patients in the ICU and to explore whether this relationship is influenced by diabetes status.
Materials and methods
Data sources and study population
Data were collected from the Medical Information Mart for Intensive Care IV (MIMIC-IV) 2.0 database, which is a large, freely available database with information from patients who had critical care unit stays at the Beth Israel Deaconess Medical Center between 2008 and 2019 [16]. One of our authors was given access to the database after completing the training (authorization code: 46032459).
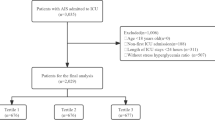
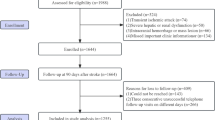
In the MIMIC-IV2.0 database, 1796 patients admitted to the ICU for IS between 2008 and 2019 were included. The criteria for exclusion were as follows: (1) not the first ICU admission for each patient; (2) age < 18 years when entering ICU; (3) length of stay in ICU < 6 h; (4) Survival time after ICU admission < 48 h; (5) Missing HbA1c or glucose records. The flow diagram of our study patient selection is shown in Supplementary Fig. 1.
Data collection and definitions
We took the first day of admission to the ICU as the baseline and extracted the following patient data: (1) demographics, including age, race, gender and body mass index (BMI); (2) comorbidities defined by the International Classification of Diseases (ICD-9-CM and ICD-10-CM) codes, including diabetes mellitus, chronic kidney disease (CKD), arterial fibrillation, myocardial infarction, peripheral vascular disease, congestive heart failure, malignant cancer, chronic pulmonary disease, and hypertension; (3) severity of illness scores at admission, including the simplified Acute Physiology Score II (SAPS-II), the Sepsis-related Organ Failure Assessment score (SOFA) and the Glasgow Coma Scale (GCS); (4) vital signs; (5) treatments, including antiplatelet (aspirin and clopidogrel) and anticoagulant (warfarin) drug use, thrombolytic therapy; (6) laboratory parameters, including HbA1c, glucose, white blood cells (WBC), platelets, red blood cells (RBC), red blood cell distribution width (RDW), hemoglobin, creatinine, serum sodium and serum potassium. Data extraction code is publicly available on GitHub (https://github.com/MIT-LCP/mimic-iv).
In the extracted data, HbA1c was the last measurement within 1 week, glucose was the first measurement after admission to the ICU, and the rest of the laboratory parameters and vital signs were averaged over the first day of admission to the ICU.
Exposure
Stress hyperglycemia was evaluated using the following formula: Glucose (mg/dl)/HbA1c (%).
Outcomes
The primary outcome indicators in our study were short-term and long-term mortality, including 30-day, 90-day, and 1-year mortality.
Statistical analysis
Eight items had varying degrees of missing data (Supplementary Table 1), except for BMI, which had a missing proportion of < 5%. We used the random forest method to interpolate data with a missing proportion < 5%. Missing data for BMI were included in the study as categorical data.
Patient characteristics were reported as mean ± SD for normally or approximately normally distributed continuous variables, median and interquartile distance for non-normally distributed continuous variables, and frequency and proportion for categorical variables. One-way ANOVA or Kruskal–Wallis test was used to compare differences between groups for continuous variables, and Fisher’s exact test was used to compare differences between groups for categorical variables.
Multivariate Cox regression was used to assess the association between SHR and mortality, adjusting for potential confounders including age, gender, race, diabetes, CKD, arterial fibrillation, myocardial infarction, congestive heart failure, hypertension, SAPS-II, SOFA, GCS, antiplatelet and anticoagulant drug use, thrombolytic therapy, HbA1c, WBC, RDW, hemoglobin, creatinine, sodium and potassium. Results were shown as hazard ratios (HR) and 95% confidence intervals (CIs). Multicollinearity between continuous variables was assessed by the variance inflation factor. Survival curves were constructed by the Kaplan–Meier method and compared using the log-rank test. The area under the curves (AUC) of the receiver operating characteristic (ROC) curves was used to assess and compare the predictive ability of SHR, FBG, and HbA1c on mortality over time. We further explored the association of quartiles of SHR with diabetes status and risk of death and calculated P values for the interaction.
Two‐tailed values of p < 0.05 was considered statistically significant. All analyses were performed with R statistical software version 4.2.2 (The R Foundation).
Results
Baseline characteristics
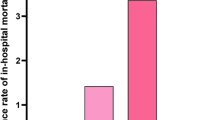
Of the 1376 patients who fulfilled the inclusion criteria, the median age was 72.9 years, of whom 47.5% were male and 63.4% were white; 210 (15.3%), 260 (18.9%), and 322 (23.4%) patients died within 30 days, 90 days, and 1 year after ICU admission, respectively. The characteristics of participants according to quartiles of SHR are shown in Table 1. The group with higher SHR had a higher proportion of patients with a history of diabetes, myocardial infarction and cancer, a higher SAPS-II, SOFA score, proportion of comatose patients, HbA1c, and glucose compared to the lower group. As the SHR increased, there was a gradual increase in the short-term and long-term mortality of the patients.
SHR and the risk of short-term or long-term mortality
The association of SHR with short-term or long-term mortality is shown in Table 2. The risk of death adjusted for different models increased significantly with increasing quartiles of SHR. Model 3 showed that after adjusting for potential confounders, patients in the third and fourth quartiles had a significantly increased risk of death at 30 days, 90 days, and 1 year compared to the first quartile of SHR (Q3 vs. Q1: HR 1.56–1.80, all p < 0.02; Q4 vs. Q1: HR 1.75–2.15, all p < 0.001; all p for trend < 0.001). Kaplan–Meier survival curves revealed that patients had a significantly higher risk of death in both the short and long terms with increasing quartiles of SHR (Fig. 1).
The predictive value of SHR, glucose, and HbA1c for mortality
The ROC analyses showed that SHR (AUC: 30-day 0.643, 90-day 0.631, 1-year 0.612) was a better predictor of mortality risk than glucose (AUC: 30-day 0.624, 90-day 0.614, 1-year 0.602) and HbA1c (AUC: 30-day 0.514, 90-day 0.513, 1-year 0.516) for both short-term and long-term mortality (Fig. 2).
Associations of SHR with outcomes in patients with and without diabetes
Regardless of the patient’s diabetes status, the highest quartile of the SHR was consistently and significantly associated with an increased risk of death in both the short and long term compared to the lowest quartile. The Associations of SHR with outcomes in patients with and without diabetes are shown in Table 3. In patients with diabetes, the highest quartile of SHR was significantly associated with an increased risk of short-term and long-term mortality compared with the lowest quartile (HR: 30-day 2.57, 90-day 2.33, 1-year 1.82; all p < 0.05; all p for trend ≤ 0.005), and a similar relationship was found in those without diabetes (HR: 30-day 2.04, 90-day 1.94, 1-year 1.87; all p < 0.02; all p for trend ≤ 0.001).
Discussion
This 12-year retrospective observational study demonstrated that elevated SHR is an independent predictor of higher mortality in patients with critical IS, and this result remains for both short-term and long-term mortality risk. Although SHR was a mediocre predictor of short-term and long-term mortality risk, it was consistently slightly better than blood glucose and better than Hba1c. In addition, the highest quartile of SHR was remarkably associated with short-term or long-term mortality compared with the first quartile, regardless of diabetes status. This suggests that SHR may be useful in identifying more patients in need of more aggressive treatment and patients at high risk of death during follow-up.
Previous studies have shown that stress hyperglycemia is associated with poor outcomes in patients with IS [17, 18]. However, due to the presence of high background glucose in diabetic patients, the association between glucose and adverse outcomes was often contradictory among patients with different diabetes states [13, 19]. Therefore, Roberts et al. [15] proposed a stress hyperglycemia ratio that takes into account background blood glucose levels to quantify this acute stress condition. Li and Yuan et al. [20, 21] showed that SHR was an independent risk factor for hemorrhagic transformation and neurologic deficit in IS patients and regardless of diabetes status. This is similar to our findings. In addition, in our study, the relationship between SHR and mortality at different times in critically ill IS patients was also simultaneously explored, and diabetes status was stratified to provide additional clinical support for this view.
Several plausible explanations exist for the association of stress hyperglycemia with poor prognosis in patients with IS. First, activation of the hypothalamic–pituitary–adrenal axis, the sympathetic adrenomedullary system, the glucose regulatory center, and humoral factors under stress together induce stress hyperglycemia, which accordingly represents a more severe neuroendocrine response [22, 23]. Second, acute glucose elevation and glucose fluctuations induce greater endothelial dysfunction, more endothelial apoptosis, and an oxidative stress response cascade worsening the progression of the disease [24, 25]. Moreover, stress hyperglycemia may directly damage ischemic brain tissues and accelerate the transformation of the ischemic penumbra into infarct regions by increasing lactate accumulation and intracellular acidosis through anaerobic glucose metabolism [26, 27]. In addition, hyperglycemia may provoke chronic cardiac metabolic changes, which may enlarge the sympathetic tone dysfunction and cardiac denervation, leading to cardiovascular damage [28]; interfere with vascular remodeling and apoptosis via multiple pathways, promoting atherosclerosis and leading to plaque instability and rupture [29]; and induce oxidative stress and increase intestinal barrier permeability to promote thrombosis. These may lead to increased all-cause mortality in patients [30,31,32].
In the context of IS, stress hyperglycemia has been measured in a variety of ways, including admission random glucose, admission fasting glucose, glucose gap, stress hyperglycemia index, and glucose variability [33]. Nevertheless, without knowing the state of diabetes and its glycemic control, which is the best glycemic indicator and its threshold remains undetermined. Although several recent studies have shown that the stress hyperglycemia ratio provides better prognostic insights than other metrics [34, 35], more prospective studies are still needed to assess and compare the clinical feasibility of various glycemic metrics or to try to apply them in combination.
Currently, there is no evidence to support the positive impact of early glycemic control in stroke patients. This may be due to the fact that appropriately elevated glucose under stress provides sufficient substrate for energy metabolism, maximizing the guarantee of cellular metabolism [36, 37]. It also promoted the generation of cell survival proteins, such as heat shock protein 27 and phosphorylated endothelial nitric oxide synthase, which somewhat reduced the infarct area [38]. Current prospective studies of acute stroke patients receiving islets to control stress hyperglycemia were designed using admission glucose as the definition and have not yet produced the desired results [39,40,41,42,43]. However, given the differences in the timing and duration of patient treatment, glycemic control criteria, and whether or not to differentiate between stroke type and diabetes status across studies, further research is needed in the future.
Our study has some potential limitations. First, because this was a retrospective observational study, it is difficult to demonstrate a causal relationship between exposure and outcome, and the use of all-cause mortality as an outcome ignores effects due to other causes of death. Second, we had no access to patients' use of insulin and oral antidiabetic medications, there was less information in the database about changes in blood glucose levels in subsequent patients, and some of the laboratory indicators with a large proportion of missing data were excluded by us, which are potential confounders. Thirdly, it was difficult to differentiate between types of IS in this study and further stratification of analyses by type of IS was not possible. Finally, because only deaths of patients within 1 year were recorded in the database, we were not able to know the follow-up over a longer period of time. In the future, well-designed multicenter prospective studies should be conducted to validate our findings.
Conclusion
Our results suggest that stress hyperglycemia, defined by the Glucose/HbA1c ratio, is associated with higher short-term and long-term mortality in patients with IS, independent of the patient’s diabetes status. Thus, the calculation of SHR can contribute to early identification and intervention in treating patients at high risk of death after IS.
Data availability
MIMIC-IV database v2.0 is freely-available on PhysioNet (https://doi.org/10.13026/7vcr-e114). The code for data query and extraction is available from the MIMIC Code Repository (https://github.com/MIT-LCP/mimic-code).
Abbreviations
- IS:
-
Glycemic variability
- SHR:
-
Standard deviation
- AUC:
-
Area under the curves
- ROC:
-
Receiver operating characteristic
References
Zhou M, Wang H, Zhu J et al (2016) Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the global burden of disease study 2013. Lancet 387:251–272. https://doi.org/10.1016/S0140-6736(15)00551-6
Wen H, Lv M (2021) Correlation analysis between serum procalcitonin and infarct volume in young patients with acute cerebral infarction. Neurol Sci 42:3189–3196. https://doi.org/10.1007/s10072-020-04856-x
Lawrence ES, Coshall C, Dundas R et al (2001) Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke 32:1279–1284. https://doi.org/10.1161/01.str.32.6.1279
Li L, Pan Y, Wang M et al (2021) Trends and predictors of myocardial infarction or vascular death after ischaemic stroke or TIA in China, 2007–2018: insights from China national stroke registries. Stroke Vasc Neurol 6:214–221. https://doi.org/10.1136/svn-2020-000503
Zhao Z, Zhang J, Jiang X et al (2020) Is endovascular treatment still good for ischemic stroke in real world?: A meta-analysis of randomized control trial and observational study in the last decade. Stroke 51:3250–3263. https://doi.org/10.1161/STROKEAHA.120.029742
Broderick JP, Palesch YY, Demchuk AM et al (2013) Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med 368:893–903. https://doi.org/10.1056/NEJMoa1214300
Baker EH, Janaway CH, Philips BJ et al (2006) Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease. Thorax 61:284–289. https://doi.org/10.1136/thx.2005.051029
Zhou Y, Liu L, Huang H et al (2022) Stress hyperglycemia ratio and in-hospital prognosis in non-surgical patients with heart failure and type 2 diabetes. Cardiovasc Diabetol 21:290. https://doi.org/10.1186/s12933-022-01728-w
Mi D, Li Z, Gu H et al (2022) Stress hyperglycemia is associated with in-hospital mortality in patients with diabetes and acute ischemic stroke. CNS Neurosci Ther 28:372–381. https://doi.org/10.1111/cns.13764
Yang J, Zheng Y, Li C et al (2022) The impact of the stress hyperglycemia ratio on short-term and long-term poor prognosis in patients with acute coronary syndrome: insight from a large cohort study in Asia. Diabetes Care 45:947–956. https://doi.org/10.2337/dc21-1526
Williams LS, Rotich J, Qi R et al (2002) Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke. Neurology 59:67–71. https://doi.org/10.1212/wnl.59.1.67
Peng Z, Song J, Li L et al (2023) Association between stress hyperglycemia and outcomes in patients with acute ischemic stroke due to large vessel occlusion. Cns Neurosci Ther 29:2162–2170. https://doi.org/10.1111/cns.14163
Roquer J, Giralt-Steinhauer E, Cerdà G et al (2015) Glycated hemoglobin value combined with initial glucose levels for evaluating mortality risk in patients with ischemic stroke. Cerebrovasc Dis 40:244–250. https://doi.org/10.1159/000440735
Stead LG, Gilmore RM, Bellolio MF et al (2009) Hyperglycemia as an independent predictor of worse outcome in non-diabetic patients presenting with acute ischemic stroke. Neurocrit Care 10:181–186. https://doi.org/10.1007/s12028-008-9080-0
Roberts GW, Quinn SJ, Valentine N et al (2015) Relative hyperglycemia, a marker of critical illness: introducing the stress hyperglycemia ratio. J Clin Endocrinol Metab 100(4490–4497):2019041113272363300
Johnson AEW, Bulgarelli L, Shen L et al (2023) MIMIC-IV, a freely accessible electronic health record dataset. Sci Data 10:1. https://doi.org/10.1038/s41597-022-01899-x
Desilles J-P, Meseguer E, Labreuche J et al (2013) Diabetes mellitus, admission glucose, and outcomes after stroke thrombolysis: a registry and systematic review. Stroke 44:1915–1923. https://doi.org/10.1161/STROKEAHA.111.000813
Goyal N, Tsivgoulis G, Pandhi A et al (2018) Admission hyperglycemia and outcomes in large vessel occlusion strokes treated with mechanical thrombectomy. J Neurointerv Surg 10:112–117. https://doi.org/10.1136/neurintsurg-2017-012993
Yao M, Ni J, Zhou L et al (2016) Elevated fasting blood glucose is predictive of poor outcome in non-diabetic stroke patients: a sub-group analysis of SMART. PLoS ONE 11:e0160674. https://doi.org/10.1371/journal.pone.0160674
Li J, Quan K, Wang Y et al (2020) Effect of stress hyperglycemia on neurological deficit and mortality in the acute ischemic stroke people with and without diabetes. Front Neurol 11:576895. https://doi.org/10.3389/fneur.2020.576895
Yuan C, Chen S, Ruan Y et al (2021) The stress hyperglycemia ratio is associated with hemorrhagic transformation in patients with acute ischemic stroke. Clin Interv Aging 16:431–442. https://doi.org/10.2147/CIA.S280808
Marik PE, Bellomo R (2013) Stress hyperglycemia: an essential survival response! Crit Care 17:305. https://doi.org/10.1186/cc12514
Dungan KM, Braithwaite SS, Preiser J-C (2009) Stress hyperglycaemia. Lancet 373:1798–1807. https://doi.org/10.1016/S0140-6736(09)60553-5
Quagliaro L, Piconi L, Assaloni R et al (2003) Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes 52:2795–2804. https://doi.org/10.2337/diabetes.52.11.2795
Monnier L, Mas E, Ginet C et al (2006) Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 295:1681–1687. https://doi.org/10.1001/jama.295.14.1681
Wagner KR, Kleinholz M, de Courten-Myers GM, Myers RE (1992) Hyperglycemic versus normoglycemic stroke: topography of brain metabolites, intracellular pH, and infarct size. J Cereb Blood Flow Metab 12:213–222. https://doi.org/10.1038/jcbfm.1992.31
Parsons MW, Barber PA, Desmond PM et al (2002) Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol 52:20–28. https://doi.org/10.1002/ana.10241
Paolisso P, Bergamaschi L, Rambaldi P et al (2021) Impact of admission hyperglycemia on heart failure events and mortality in patients with takotsubo syndrome at long-term follow-up: data from HIGH-GLUCOTAKO investigators. Diabetes Care 44:2158–2161. https://doi.org/10.2337/dc21-0433
Sardu C, Modugno P, Castellano G et al (2021) Atherosclerotic plaque fissuration and clinical outcomes in pre-diabetics versus normoglycemics patients affected by asymptomatic significant carotid artery stenosis at 2 years of follow-up: role of microRNAs modulation: the ATIMIR study. Biomedicines 9:401. https://doi.org/10.3390/biomedicines9040401
Sardu C, Barbieri M, Balestrieri ML et al (2018) Thrombus aspiration in hyperglycemic ST-elevation myocardial infarction (STEMI) patients: clinical outcomes at 1-year follow-up. Cardiovasc Diabetol 17:152. https://doi.org/10.1186/s12933-018-0795-8
D’Onofrio N, Sardu C, Paolisso P et al (2020) MicroRNA-33 and SIRT1 influence the coronary thrombus burden in hyperglycemic STEMI patients. J Cell Physiol 235:1438–1452. https://doi.org/10.1002/jcp.29064
Sardu C, Consiglia Trotta M, Santella B et al (2021) Microbiota thrombus colonization may influence athero-thrombosis in hyperglycemic patients with ST segment elevation myocardialinfarction (STEMI). Marianella study. Diabetes Res Clin Pract 173:108670. https://doi.org/10.1016/j.diabres.2021.108670
Yao M, Hao Y, Wang T et al (2023) A review of stress-induced hyperglycaemia in the context of acute ischaemic stroke: Definition, underlying mechanisms, and the status of insulin therapy. Front Neurol 14:1149671. https://doi.org/10.3389/fneur.2023.1149671
Chen G, Ren J, Huang H et al (2022) Admission random blood glucose, fasting blood glucose, stress hyperglycemia ratio, and functional outcomes in patients with acute ischemic stroke treated with intravenous thrombolysis. Front Aging Neurosci 14:782282. https://doi.org/10.3389/fnagi.2022.782282
Roberts G, Sires J, Chen A et al (2021) A comparison of the stress hyperglycemia ratio, glycemic gap, and glucose to assess the impact of stress-induced hyperglycemia on ischemic stroke outcome. J Diabetes 13:1034–1042. https://doi.org/10.1111/1753-0407.13223
Ibayashi S, Fujishima M, Sadoshima S et al (1986) Cerebral blood flow and tissue metabolism in experimental cerebral ischemia of spontaneously hypertensive rats with hyper-, normo-, and hypoglycemia. Stroke 17:261–266. https://doi.org/10.1161/01.str.17.2.261
Kraft SA, Larson CP, Shuer LM et al (1990) Effect of hyperglycemia on neuronal changes in a rabbit model of focal cerebral ischemia. Stroke 21:447–450. https://doi.org/10.1161/01.str.21.3.447
Chu LM, Osipov RM, Robich MP et al (2010) Is hyperglycemia bad for the heart during acute ischemia? J Thorac Cardiovasc Surg 140:1345–1352. https://doi.org/10.1016/j.jtcvs.2010.05.009
Gray CS, Hildreth AJ, Sandercock PA et al (2007) Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK glucose insulin in stroke trial (GIST-UK). Lancet Neurol 6:397–406. https://doi.org/10.1016/S1474-4422(07)70080-7
Bruno A, Kent TA, Coull BM et al (2008) Treatment of hyperglycemia in ischemic stroke (THIS): a randomized pilot trial. Stroke 39:384–389. https://doi.org/10.1161/STROKEAHA.107.493544
McCormick M, Hadley D, McLean JR et al (2010) Randomized, controlled trial of insulin for acute poststroke hyperglycemia. Ann Neurol 67:570–578. https://doi.org/10.1002/ana.21983
Rosso C, Corvol J-C, Pires C et al (2012) Intensive versus subcutaneous insulin in patients with hyperacute stroke: results from the randomized INSULINFARCT trial. Stroke 43:2343–2349. https://doi.org/10.1161/STROKEAHA.112.657122
Johnston KC, Bruno A, Pauls Q et al (2019) Intensive versus standard treatment of hyperglycemia and functional outcome in patients with acute ischemic stroke: the SHINE randomized clinical trial. JAMA 322:326–335. https://doi.org/10.1001/jama.2019.9346
Funding
No specific fund supports the current analysis.
Author information
Authors and Affiliations
Contributions
HP contributed to conceptualization, methodology, and software. HP and YX done data curation and writing—original draft preparation. HP, YX, and YH helped in visualization and investigation. YH and JZ helped in supervision. JZ and HW done software and validation. HW helped in writing—reviewing and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was performed by the principals of the Declaration of Helsinki. The use of the MIMIC-IV database was approved by the review committee of the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center. The data is publicly available (in the MIMIC-IV database) hence ethical approval statements and informed consent are not required for the study.
Additional information
Managed by Massimo Federici.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pan, H., Xiong, Y., Huang, Y. et al. Association between stress hyperglycemia ratio with short-term and long-term mortality in critically ill patients with ischemic stroke. Acta Diabetol 61, 859–868 (2024). https://doi.org/10.1007/s00592-024-02259-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-024-02259-4