Abstract
The stress hyperglycemia ratio (SHR) is established as a reliable marker for assessing the severity of stress-induced hyperglycemia. While its effectiveness in managing patients with Acute Ischemic Stroke (AIS) remains to be fully understood. We aim to explore the relationship between SHR and clinical prognosis in AIS patients and to assess how diabetes status influences this relationship. In this study, we analyzed data from the Medical Information Mart for Intensive Care (MIMIC-IV) database, selecting patients with AIS who required ICU admission. These patients were categorized into tertiles based on their SHR levels. We applied Cox hazard regression models and used restricted cubic spline (RCS) curves to investigate relationships between outcomes and SHR. The study enrolled a total of 2029 patients. Cox regression demonstrated that a strong correlation was found between increasing SHR levels and higher all-cause mortality. Patients in the higher two tertiles of SHR experienced significantly elevated 30-day and 90-day mortality rates compared to those in the lowest tertile. This pattern remained consistent regardless of diabetes status. Further, RCS analysis confirmed a progressively increasing risk of all-cause mortality with higher SHR levels. The findings indicate that SHR is association with increased 30-day and 90-day mortality among AIS patients, underscoring its potential value in risk stratification. Although the presence of diabetes may weaken this association, significant correlations persist in diabetic patients.
Similar content being viewed by others
Introduction
Acute Ischemic Stroke (AIS) is a severe neurological condition caused by disturbances in cerebral blood circulation, with ischemic events accounting for 60–70% of all strokes1. These disturbances often lead to catastrophic outcomes, including significant neurological deficits and severe cognitive and physical impairments. Notably, up to 60% of severely affected patients either experience disability or die within 90 days of the event2,3.
Stress hyperglycemia, a metabolic response to physiological stress, exacerbates stroke complications and contributes to an overall poor prognosis, serving as a crucial predictive marker4. Stress hyperglycemia induces adverse metabolic reactions, vascular endothelial dysfunction, and immune-inflammatory responses, all of which worsen outcomes in patients with acute brain injuries5. However, assessing stress hyperglycemia has traditionally been challenging due to variable baseline glucose levels, which can lead to inaccuracies in measuring stress levels.
In 2015, Roberts et al. introduced the stress hyperglycemia ratio (SHR), a method that improves the accuracy of stress hyperglycemia assessments by adjusting blood glucose measurements based on baseline levels determined by HbA1c6. The SHR provides a more precise evaluation by taking into account individual variations in glucose metabolism. Typically, the SHR can range from values below 1.0 (indicating below-normal glucose metabolism) to higher values that indicate varying degrees of hyperglycemia, depending on the patient’s baseline HbA1c and acute glucose levels. For instance, an SHR value below 1.0 may suggest hypoglycemia or other metabolic abnormalities, a value around 1.0 suggests normal glucose metabolism, while higher values indicate significant stress-induced hyperglycemia. While prior research primarily focused on SHR’s predictive capacity for adverse outcomes in sepsis7 and coronary artery disease8, recent studies have expanded its value to predicting stroke recurrence, onset, and mortality in AIS patients9. Despite these advances, the reliability of SHR in AIS patients admitted to the intensive care unit (ICU), who often suffer complex pathophysiological profiles, remains uncertain.
Diabetes alters glucose homeostasis, potentially affecting the predictive accuracy of the SHR. Conversely, in non-diabetic patients, an elevated SHR observed upon admission may indicate significant stress-induced hyperglycemia, which could adversely affect prognosis, necessitating rigorous monitoring and intervention. Therefore, it is imperative to evaluate the prognostic value of SHR in patients with AIS separately based on their diabetic status.
In this study, we aimed to investigate the relationship between SHR and all-cause mortality in this vulnerable patient group. Additionally, we sought to determine whether SHR holds predictive value in AIS patients both with and without diabetes. Our findings have the potential to guide more effective clinical interventions and medical management strategies.
Materials and methods
Database introduction
The data for this study were derived from the Medical Information Mart for Intensive Care IV (MIMIC-IV, v2.2) database. MIMIC-IV (v2.2) is a comprehensive, population-based, critical care database that is publicly available10. It encompasses extensive data from over 60,000 ICU admissions, making it one of the most robust repositories of critical care information globally. This dataset includes not only demographic details, vital signs, laboratory findings, medication records, and clinical notes but also complex physiological waveforms and imaging reports, providing a holistic view of patient care. The corresponding author (Guangdong Wang, Record ID: 60106105) was authorized to use the MIMIC-IV database after completing the National Institutes of Health’s online education program.
Population selection criteria
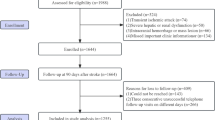
This study focused on patients diagnosed with AIS, as classified by the ICD-9 and ICD-10. Exclusion criteria were as follows: (1) patients under 18 years; (2) patients not on their first ICU admission; (3) ICU stays shorter than 24 h; and (4) absence of SHR values. After applying these criteria, 2029 patients who met the inclusion requirements were selected and subsequently categorized into three groups based on their SHR values (Fig. 1).
Data extraction and SHR calculation
Data extraction from the MIMIC-IV database was performed using PostgreSQL, covering a broad spectrum of clinical parameters. Collected demographic information included gender, age, and race. Recorded vital signs comprised heart rate, mean blood pressure (MBP), respiratory rate, and blood oxygen saturation (SpO2). Comorbidities such as hypertension, heart failure, arterial fibrillation, myocardial infarction, chronic pulmonary disease, diabetes and renal disease were documented. Laboratory parameters included hemoglobin, platelets, white blood cells (WBC), blood urea nitrogen (BUN), creatinine, sodium, potassium, international normalized ratio (INR), prothrombin time (PT), and activated partial thromboplastin time (APTT). Severity of disease was assessed using indices like the Charlson Index, Glasgow Coma Scale (GCS), Sequential Organ Failure Assessment (SOFA), Acute Physiology Score (APS III), Simplified Acute Physiology Score (SAPS II), and the National Institutes of Health Stroke Scale (NIHSS).Therapies documented included antiplatelet, anticoagulation, thrombolysis, mechanical thrombectomy. The SHR was calculated with the formula6: SHR = (admission blood glucose (mg/dL))/(28.7 × HbA1c(%) − 46.7).
Handling missing data
Table S1 presents the missing data metrics for this study. For variables with fewer than 10 missing entries (e.g., MBP, respiratory rate, SPO2, GCS, hemoglobin, platelets, WBC), the mean or median values were used as substitutes. For variables such as INR, PT, and APTT, where missing data constituted less than 10% of the total, multiple imputation methods were employed to fill in the gaps.
Outcomes
The primary outcomes were 30-day and 90-day all-cause mortality, and the secondary outcomes were hospital mortality, length of hospital stay and ICU stay.
Statistical Analysis
For variables exhibiting a normal distribution, mean ± SD were presented and subjected to Analysis of Variance (ANOVA). Variables not conforming to normal distribution were assessed using the Mann–Whitney U test or Kruskal–Wallis test as appropriate. Categorical variables were expressed as counts and percentages and analyzed via the χ2 test or Fisher’s exact test when necessary. Kaplan–Meier survival analysis was employed to investigate the association between SHR and overall mortality, with differences evaluated using the log-rank test.
Univariate Cox regression analysis identified risk factors, which were then used in a multivariate Cox proportional hazards regression model to quantify the influence of SHR on overall mortality among patients with AIS. SHR was included as both a categorical variable (with the lowest level, T1, as the reference) and a continuous variable. The trend P value was also calculated. Three models were developed to analyze clinically significant factors, reporting hazard ratios (HR) and 95% confidence intervals (CIs). Additionally, we divided patients into two groups based on diabetes status and conducted the same multivariate analyses for each subgroup. Restricted cubic splines (RCS) explored potential nonlinear relationships between SHR and clinical outcomes.
A sensitivity analysis was conducted to explore the associations between the SHR and mortality outcomes in patients with AIS, considering the availability of NIHSS scores. Patients were divided into two groups: those with available NIHSS scores (n = 362) and those without (n = 1667). Additionally, subgroup analyses stratified by age, gender, hypertension, heart failure, arterial fibrillation, myocardial infarction, diabetes, antiplatelet and anticoagulation therapy assessed the consistency of SHR’s prognostic value for primary outcomes.
All statistical analyses were conducted using R software (version 4.4.1) and SPSS (version 26.0). A P value below 0.05 was deemed to indicate statistical significance.
Results
Patient Characteristics
The baseline characteristics of the 2029 AIS patients are detailed in Table 1.The cohort’s median age was 72 years (IQR: 61–82), with 996 males and 1033 females. The median SHR was 1.01 (IQR: 0.88–1.19). Patients were stratified into three tertiles based on SHR at ICU admission: Tertile 1 (T1: 0.31–0.92), Tertile 2 (T2: 0.92–1.12), Tertile 3 (T3: 1.12–4.37). Higher SHR tertiles were associated with older age, higher heart rate, respiratory rate, WBC, BUN, creatinine levels, and more frequent use of thrombolysis therapy. These patients also had higher rates of heart failure, myocardial infarction, diabetes, and renal disease, along with higher disease severity scores. Conversely, they had lower MBP, hemoglobin levels, platelet counts, sodium, potassium, APTT and use of antiplatelet therapy compared to lower tertiles. The comparison between T3 and T1–2 groups revealed similar trends (Table S2).
Table S3 contrasted the 90-day survivors with non-survivors. Non-survivors were older and predominantly female, with higher heart rate, respiratory rate, WBC, BUN, creatinine, INR, and PT, and were less likely to require antiplatelet and anticoagulation therapy. They also had a higher prevalence of heart failure, myocardial infarction and renal disease, with significantly higher disease severity scores. SHR levels were notably higher in non-survivors (1.1 vs. 1.0, P < 0.001), with SHR distribution by clinical outcomes depicted in Fig. S1c,d.
Clinical outcomes across SHR tertiles
The study’s overall 30-day and 90-day mortality rates were 19.9% and 25.7%, respectively (Table 1). The T3 group experienced notably longer hospital stays (9.82 days vs. 7.59 days, P < 0.001) and ICU stays (4.10 days vs. 3.00 days, P < 0.001), along with a higher hospital mortality rate (22.9% vs. 11.4%, P < 0.001), compared to the T1-2 group (Table S2). Mortality rates across SHR tertiles showed that higher tertiles faced significantly increased 30-day and 90-day mortality rates (Fig. S1a,b). In addition, SHR is positively correlated with disease severity scores such as GCS, SOFA, APS III and SAPS II (Fig. S2). Kaplan–Meier curves (Fig. 2) confirmed that higher SHR significantly increased mortality risk (log-rank P < 0.001).
Association between SHR and primary outcomes
Cox proportional hazards analysis, adjusting for confounders from Table S4 (P < 0.05), indicated that patients in the second and third SHR tertiles had a significantly increased mortality risk at 30 and 90 days compared to the first tertile (Table 2). The trend of HR values across SHR tertiles is shown in Fig. 3a,b. RCS regression models confirmed that mortality risk increased nonlinearly with rising SHR levels (Fig. 3c,d). Multivariate logistic regression analysis highlighted significant predictors of hospital mortality, including age, SPO2, heart failure, SOFA, WBC, BUN, SHR, antiplatelet therapy and anticoagulation therapy (Table S5).
HR (95% CI) for all-cause mortality according to SHR tertiles after adjusted for age, gender, hypertension, heart failure, myocardial infarction, renal disease, antiplatelet therapy, anticoagulation therapy, MBP, SPO2, GCS, SOFA, hemoglobin, WBC, BUN, creatinine, INR and PT. The T1 is the reference. (a) HR (95% CI) for 30d-mortality according to SHR tertiles. (b) HR (95% CI) for 90d-mortality according to SHR tertiles. RCS curve of SHR with all-cause mortality. (c) RCS curve for 30-day mortality. (d) RCS curve for 90-day mortality.
In patients with diabetes, the highest SHR tertile significantly increased the risk of 30-day and 90-day mortality compared to the lowest tertile, a pattern also seen in non-diabetic patients (Table 3).
Sensitivity analysis
The sensitivity analysis demonstrated that an elevated SHR is significantly associated with increased 30-day and 90-day mortality in patients with AIS, and this association is significant both when adjusted and not adjusted for NIHSS scores (Table S6).
Subgroup analysis
Subgroup analysis across various demographics and medical conditions (Fig. 4) consistently demonstrated that higher SHR levels were associated with an increased risk of mortality across age, gender, hypertension, heart failure, arterial fibrillation, myocardial infarction, diabetes, and antiplatelet therapy subgroups (all P for interaction > 0.05). However, in the anticoagulation therapy subgroup, there was a significant interaction between SHR and 90-day mortality (P for interaction = 0.022).
Discussion
Our results indicate that the SHR serves as a potent predictor of mortality in patients with AIS. The significant association between elevated SHR levels and increased mortality, particularly noted in the higher tertiles, highlights its role in identifying patients at elevated risk of adverse outcomes. This association persists across various subgroups, illustrating SHR’s robustness as a clinical marker. Importantly, while diabetes status appears to modulate the strength of this relationship, the influence of SHR on mortality remains significant even among diabetic patients. This underscores the potential of SHR to enhance risk stratification and guide more tailored therapeutic strategies, regardless of underlying diabetes, thereby optimizing ICU management and potentially improving survival outcomes.
AIS remains a significant cause of mortality and disability worldwide11. Despite advances in secondary prevention strategies, many patients continue to experience adverse outcomes. One of the critical challenges in treating AIS is the limited time window available for effective vascular recanalization, which benefits only a subset of patients1. Consequently, addressing modifiable risk factors associated with AIS is crucial. Among these factors, stress hyperglycemia is noteworthy. One such critical factor is stress hyperglycemia, which manifests as an elevation in blood glucose levels above baseline during acute events such as stroke or injury12. Approximately half of AIS patients experience stress hyperglycemia13; however, its definition varies across studies, which typically measure it using random or fasting blood glucose levels at admission. This condition, occurring in both diabetic and non-diabetic individuals, is associated with the incidence and severity of stroke and serves as a prognostic indicator of unfavorable outcomes14,15. The underlying mechanisms involve fluctuations in blood glucose levels that may accelerate reactive oxygen species production in mitochondria, leading to vascular endothelial apoptosis16,17. This exacerbates cerebral perfusion impairment, intensifies ischemic brain damage, and worsens neurological deficits. Critical illness triggers increased production of counterregulatory hormones such as cortisol and catecholamines, which raise glucose levels by antagonizing insulin18. Concurrently, systemic inflammation releases cytokines like TNF-α and IL-6 that impair insulin signaling, thereby exacerbating hyperglycemia19. Insulin resistance also typically increases during critical illnesses, and in some cases, pancreatic dysfunction may further impair insulin secretion20. Additionally, ICU nutritional strategies, particularly the administration of parenteral glucose, can intensify these effects if caloric inputs exceed metabolic demands or insulin capacity21.
However, due to the lack of reliable indicators for evaluating stress-induced hyperglycemia, the research results of stress-induced hyperglycemia in acute stroke are inconsistent. To address these issues, the SHR was introduced by Roberts et al. in 20156. Unlike stress-induced blood glucose, SHR measures immediate fluctuations and is adjusted for the average estimated blood glucose levels from the patient’s first three months, thus reflecting the relative changes during stress compared to baseline levels. This correction makes SHR a more reliable indicator for assessing the intensity of stress-induced hyperglycemia, enhancing its clinical relevance. Zhu et al.15 found that the risk of stroke recurrence in patients with AIS increases with higher SHR; in addition, SHR is also associated with the short-term prognosis of cerebral anterior circulation vascular occlusion after thrombectomy treatment22, which provides a reference for the efficacy evaluation of mechanical thrombectomy for stroke; Li et al.23 showed that SHR increases the risk of hemorrhagic transformation in AIS, and increases the degree of neurological deficit in AIS. Li and Yuan et al.23,24 showed that SHR, independent of diabetes status, was linked to a higher probability of severe neurological deficit and mortality within a year in the AIS. Our study corroborates these findings, underscoring SHR as an independent predictor of short-term outcomes in AIS patients. A higher SHR is indicative of an increased prognostic risk, reinforcing its value as a crucial clinical metric in the management of AIS.
Severe patients exhibiting acute stress reactions are predisposed to develop stress hyperglycemia, complicating the management of blood sugar levels. Extensive research, particularly in ICU settings, has traditionally linked stress hyperglycemia with adverse clinical outcomes25,26. However, recent investigations challenge this association, suggesting a more complex relationship. For example, a retrospective study found that sepsis patients with stress-induced hyperglycemia exhibited lower ICU mortality compared to those with normoglycemia27. Bellomo et al. propose that stress-induced hyperglycemia and the accompanying insulin resistance are evolutionary adaptations, activated under stress to supply critical energy to the immune system and brain28. This perspective suggests that mild to moderate stress hyperglycemia (7.8–12 mmol/L) may confer a protective effect, diverging from the detrimental impacts traditionally associated with diabetic hyperglycemia. Consequently, stringent glucose control might not only lack benefit but could also precipitate risks such as hypoglycemia. The 2009 AACE/ADA consensus has shifted to recommend less restrictive glucose targets of 7.8–10 mmol/L for critically ill patients, advising against lowering blood glucose below 6.1 mmol/L29.
In our findings, monitoring the SHR can play a role in reducing the all-cause for AIS patients admitted to ICU. In ICU setting, managing stress hyperglycemia involves adopting a balanced approach to glycemic control. Current strategies include maintaining blood glucose levels within a specific target range using insulin therapy, with a preference for intravenous administration due to its adjustability30. Additionally, recent studies have suggested that GLP-1 receptor agonists (GLP-1 RAs) could offer advantages over traditional insulin therapy for managing stress hyperglycemia in AIS. GLP-1 RAs not only help regulate blood glucose but also provide neuroprotective benefits, which are crucial for AIS patients. Despite these promising findings, more clinical research is needed to fully understand the benefits of GLP-1 RAs and establish comprehensive treatment protocols31. Nutritional management, emphasizing enteral over parenteral nutrition, plays a crucial role in mitigating hyperglycemia by carefully controlling carbohydrate intake to meet but not exceed energy requirements32. Continuous glucose monitoring is emerging as a tool to enhance real-time glucose management, although its efficacy in the ICU setting continues to be evaluated33.
Our subgroup analysis observed a significant interaction between SHR and 90-day mortality in the anticoagulation therapy subgroup (P for interaction = 0.022). In this subgroup, elevated SHR was significantly associated with an increased risk of death (HR 1.79, 95% CI 1.40–2.29), whereas in the no therapy group, high SHR did not significantly affect mortality risk (HR 0.61, 95% CI 0.24–1.55). Several mechanisms might explain these differential impacts. Anticoagulants like warfarin can affect liver function and interfere with vitamin K pathways, potentially altering glucose metabolism and exacerbating the adverse effects of hyperglycemia34. Elevated SHR in this context could reflect a dysregulated metabolic state that contributes to higher mortality. Additionally, AIS triggers inflammatory and coagulation pathways. In patients receiving anticoagulation therapy, the interaction between these pathways and high glucose levels might be more detrimental. Hyperglycemia enhances platelet activation and aggregation35, which, when combined with anticoagulation, could lead to unstable coagulation dynamics, increasing the risk of hemorrhagic complications or recurrent thrombotic events. These findings emphasize the importance of stringent glucose monitoring and control in patients on anticoagulation therapy to reduce the increased risk of mortality in AIS care.
Our study offers a comprehensive examination of the impact of the SHR on patient outcomes in AIS, analyzing a robust cohort of 2029 patients and ensuring a broad representation across diverse demographics. However, several potential limitations should be considered: Firstly, although we selected AIS patients admitted to the ICU, our study lacks specific data reflecting the severity of AIS, such as the NIHSS scores and detailed imaging information. These metrics are critical for accurately assessing stroke severity and may significantly influence patient outcomes. The absence of these data points limits our ability to fully adjust for stroke severity in our analyses. Secondly, we did not collect information on whether patients were on glucose-lowering medications or insulin prior to ICU admission. Pre-existing glycemic management could have a significant impact on SHR levels and subsequent patient outcomes. Thirdly, our study focused on the impact of baseline SHR on patient prognosis. While baseline SHR provides valuable initial insights, a dynamic assessment of SHR over the course of ICU stay could offer more comprehensive information on the relationship between glycemic control and patient outcomes. Continuous monitoring of SHR might reveal temporal patterns and fluctuations that are critical for understanding and managing stress-induced hyperglycemia in AIS patients.
In conclusion, our findings extend the utility of the SHR to patients with AIS who required ICU admission, demonstrating its potential as a valuable tool for stratifying risk of all-cause mortality within this patient population. Routine monitoring of the SHR may enhance clinical decision-making and management of AIS. Nevertheless, to substantiate these results, multicenter, prospective studies are imperative for thorough validation.
Data availability
The data that support the findings of this study are available from MIMIC-IV. Access to the database can be obtained through PhysioNet at https://physionet.org/content/mimiciv/2.2/.
References
Feske, S. K. Ischemic stroke. Am. J. Med. 134, 1457–1464. https://doi.org/10.1016/j.amjmed.2021.07.027 (2021).
Lawrence, E. S. et al. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke 32, 1279–1284. https://doi.org/10.1161/01.str.32.6.1279 (2001).
Li, L. et al. Trends and predictors of myocardial infarction or vascular death after ischaemic stroke or TIA in China, 2007–2018: Insights from China National Stroke Registries. Stroke Vasc. Neurol. 6, 214–221. https://doi.org/10.1136/svn-2020-000503 (2021).
Woo, E., Ma, J. T., Robinson, J. D. & Yu, Y. L. Hyperglycemia is a stress response in acute stroke. Stroke 19, 1359–1364. https://doi.org/10.1161/01.str.19.11.1359 (1988).
Hashmat, A., Ya, J., Kadir, R., Alwjwaj, M. & Bayraktutan, U. Hyperglycaemia perturbs blood–brain barrier integrity through its effects on endothelial cell characteristics and function. Tissue Barriers https://doi.org/10.1080/21688370.2024.2350821 (2024).
Roberts, G. W. et al. Relative hyperglycemia, a marker of critical illness: Introducing the stress hyperglycemia ratio. J. Clin. Endocrinol. Metab. 100, 4490–4497. https://doi.org/10.1210/jc.2015-2660 (2015).
Li, L. et al. Association of stress hyperglycemia ratio and mortality in patients with sepsis: Results from 13,199 patients. Infection https://doi.org/10.1007/s15010-024-02264-3 (2024).
Zhang, Y. et al. Effects of the stress hyperglycemia ratio on long-term mortality in patients with triple-vessel disease and acute coronary syndrome. Cardiovasc. Diabetol. 23, 143. https://doi.org/10.1186/s12933-024-02220-3 (2024).
Pan, H., Xiong, Y., Huang, Y., Zhao, J. & Wan, H. Association between stress hyperglycemia ratio with short-term and long-term mortality in critically ill patients with ischemic stroke. Acta Diabetol. https://doi.org/10.1007/s00592-024-02259-4 (2024).
Johnson, A. E. W. et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci. Data 10, 1. https://doi.org/10.1038/s41597-022-01899-x (2023).
Li, J., Zhong, Q., Yuan, S. & Zhu, F. Global burden of stroke attributable to high systolic blood pressure in 204 countries and territories, 1990–2019. Front. Cardiovasc. Med. 11, 1339910. https://doi.org/10.3389/fcvm.2024.1339910 (2024).
Dungan, K. M., Braithwaite, S. S. & Preiser, J. C. Stress hyperglycaemia. Lancet 373, 1798–1807. https://doi.org/10.1016/s0140-6736(09)60553-5 (2009).
Capes, S. E., Hunt, D., Malmberg, K., Pathak, P. & Gerstein, H. C. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: A systematic overview. Stroke 32, 2426–2432. https://doi.org/10.1161/hs1001.096194 (2001).
Chen, G. et al. Admission random blood glucose, fasting blood glucose, stress hyperglycemia ratio, and functional outcomes in patients with acute Ischemic stroke treated with intravenous thrombolysis. Front. Aging Neurosci. 14, 782282. https://doi.org/10.3389/fnagi.2022.782282 (2022).
Zhu, B. et al. Stress hyperglycemia and outcome of non-diabetic patients after acute ischemic stroke. Front. Neurol. 10, 1003. https://doi.org/10.3389/fneur.2019.01003 (2019).
Nair, S. S., Sylaja, P. N., Sreedharan, S. E. & Sarma, S. Maintenance of normoglycemia may improve outcome in acute ischemic stroke. Ann. Indian Acad. Neurol. 20, 122–126. https://doi.org/10.4103/0972-2327.194301 (2017).
MacDougall, N. J. & Muir, K. W. Hyperglycaemia and infarct size in animal models of middle cerebral artery occlusion: Systematic review and meta-analysis. J. Cereb. Blood Flow Metab. 31, 807–818. https://doi.org/10.1038/jcbfm.2010.210 (2011).
Finfer, S. et al. Hypoglycemia and risk of death in critically ill patients. N. Engl. J. Med. 367, 1108–1118. https://doi.org/10.1056/NEJMoa1204942 (2012).
Pickup, J. C. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 27, 813–823. https://doi.org/10.2337/diacare.27.3.813 (2004).
Gunst, J. & Verbruggen, S. C. Insulin resistance in critical illness: Consequences for nutrition therapy and glucose management. Curr. Opin. Crit. Care 29, 286–292. https://doi.org/10.1097/mcc.0000000000001055 (2023).
Blackburn, G. L., Wollner, S. & Bistrian, B. R. Nutrition support in the intensive care unit: An evolving science. Arch. Surg. 145, 533–538. https://doi.org/10.1001/archsurg.2010.97 (2010).
Chen, X. et al. High stress hyperglycemia ratio predicts poor outcome after mechanical thrombectomy for Ischemic stroke. J. Stroke Cerebrovasc. Dis. 28, 1668–1673. https://doi.org/10.1016/j.jstrokecerebrovasdis.2019.02.022 (2019).
Li, J. et al. Effect of stress hyperglycemia on neurological deficit and mortality in the acute Ischemic stroke people with and without diabetes. Front. Neurol. 11, 576895. https://doi.org/10.3389/fneur.2020.576895 (2020).
Yuan, C. et al. The stress hyperglycemia ratio is associated with hemorrhagic transformation in patients with acute ischemic stroke. Clin. Interv. Aging 16, 431–442. https://doi.org/10.2147/cia.S280808 (2021).
Lee, T. F. et al. Relative hyperglycemia is an independent determinant of in-hospital mortality in patients with critical illness. Crit. Care Med. 48, e115–e122. https://doi.org/10.1097/ccm.0000000000004133 (2020).
Krinsley, J. S. et al. The interaction of acute and chronic glycemia on the relationship of hyperglycemia, hypoglycemia, and glucose variability to mortality in the critically ill. Crit. Care Med. 48, 1744–1751. https://doi.org/10.1097/ccm.0000000000004599 (2020).
Wernly, B., Lichtenauer, M., Hoppe, U. C. & Jung, C. Hyperglycemia in septic patients: An essential stress survival response in all, a robust marker for risk stratification in some, to be messed with in none. J. Thorac. Dis. 8, E621-624. https://doi.org/10.21037/jtd.2016.05.24 (2016).
Marik, P. E. & Bellomo, R. Stress hyperglycemia: An essential survival response! Crit. Care 17, 305. https://doi.org/10.1186/cc12514 (2013).
Moghissi, E. S. et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 32, 1119–1131. https://doi.org/10.2337/dc09-9029 (2009).
Davies, M. J. et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 45(2753–2786), 2022. https://doi.org/10.2337/dci22-0034 (2022).
Marlet, I. R., Ölmestig, J. N. E., Vilsbøll, T., Rungby, J. & Kruuse, C. Neuroprotective mechanisms of glucagon-like peptide-1-based therapies in ischaemic stroke: A systematic review based on pre-clinical studies. Basic Clin. Pharmacol. Toxicol. 122, 559–569. https://doi.org/10.1111/bcpt.12974 (2018).
McClave, S. A. et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J. Parenter. Enteral Nutr. 40, 159–211. https://doi.org/10.1177/0148607115621863 (2016).
Faulds, E. R., Dungan, K. M. & McNett, M. Implementation of continuous glucose monitoring in critical care: A scoping review. Curr. Diab. Rep. 23, 69–87. https://doi.org/10.1007/s11892-023-01503-5 (2023).
Yamagishi, S. I. Concerns about clinical efficacy and safety of warfarin in diabetic patients with atrial fibrillation. Cardiovasc. Diabetol. 18, 12. https://doi.org/10.1186/s12933-019-0818-0 (2019).
Undas, A., Wiek, I., Stêpien, E., Zmudka, K. & Tracz, W. Hyperglycemia is associated with enhanced thrombin formation, platelet activation, and fibrin clot resistance to lysis in patients with acute coronary syndrome. Diabetes Care 31, 1590–1595. https://doi.org/10.2337/dc08-0282 (2008).
Funding
No specific fund supports the current analysis.
Author information
Authors and Affiliations
Contributions
Y.Z. conceived the study, performed the data analysis, and drafed the manuscript. X.Y., T.L. and W.J. participated in manuscript revision. G.W. collected the data, drew the figure and revised manuscript. All authors participated in the interpretation of the fndings and approved the fnal version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was an analysis of a third-party anonymized publicly available database with pre-existing institutional review board (IRB) approval. The Institutional review boards at the Beth Israel Deaconess Medical Center (protocol 2001-P-001699/14) and Massachusetts Institute of Technology (protocol 0403000206) have approved the data collection and the use of MIMIC-IV for research purposes and granted waiver of informed consent.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Yin, X., Liu, T. et al. Association between the stress hyperglycemia ratio and mortality in patients with acute ischemic stroke. Sci Rep 14, 20962 (2024). https://doi.org/10.1038/s41598-024-71778-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71778-5
- Springer Nature Limited