Abstract—
A combinatory topological analysis and simulation of the self-assembly of the crystal structure of Ca11Hg54–hP65 (a = b = 17.114 Å, c = 10.442 Å, hexagonal group P-6) are performed by computer methods (ToposPro software package). A total of 184 variants of the cluster representation of the 3D atomic lattice with a number of structural units of 3–7 are established. The polyhedral clusters K8 = 0@Ca2Hg6, which appeared to be hexagonal bipyramids, the polyhedral clusters K11 = 0@Ca3Hg8, and the polyhedral clusters with the central Hg atom K12 = Hg(Ca3Hg8) are determined. The centers of the Ca2Hg6, 0@Ca3Hg8 and Hg(Ca3Hg8) clusters occupied the highly symmetric positions 1c, 1b, and 1f with a –6 symmetry. The precursor clusters Ca2Hg6 represent templates on the surface of which atomic shells of 38 atoms are formed. The composition of the two-layered template cluster K46 is 0@8(Ca2Hg6)@38(Hg6 + CaHg6)2(Ca6Hg6). The symmetry and topological code for the self-assembly processes of the 3D structure from the nanoclusters-precursors K46 with participation of the polyhedral clusters 0@Ca3Hg8 and Hg(Ca3Hg8) are simulated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The crystal chemistry family of double Hg intermetallides includess about 200 compounds [1]. The formation of Hg intermetallides was established in 45 A–Hg systems. The largest number of crystallochemically different intermetallides (eleven) was found in the binary system Ca–Hg, with a wide range of changes in the Hg : A atomic ratio from 11 to 0.33 (Table 1, [2–6]).
The crystal structures of six Hg-intermetallides were included in the crystal chemistry families of the most common types of crystal structures, with several hundred specimen each. Three Hg intermetallides belonged to the crystal chemistry families with more than forty species (Table 1). The authors of [7, 8] simulated the cluster self-assembly of widely spread types of crystal structures established for Hg intermetallides.
The most crystallochemically complex family of intermetallides Ca11Hg54–hP65 included two more compounds: Sr11Hg54–hP65 [5] and Yb11Hg54–hP [9]. The crystal structure of the intermetallide Ca11Hg54–hP65 [5] with the spatial group P-6 (no. 174) and V =1492.7 Å3 was characterized by 18 crystallographically independent atoms with the Wyckoff sequence l6k3j4ihgfa. For Hg atoms, a wide spectrum of coordination number (CN) values was established: 11 (5 atoms), 12 (8 atoms), and 13 (1 atom). Ca atoms exhibited the CN of 14 (1 atom), 15 (2 atoms), and 16 (1 atom).
In the present study, we performed the geometric and topological analysis of the crystal structure of the intermetallide Ca11Hg54–hP65 by the ToposPro software package [10]. The symmetry and topological code for the processes of self-assembly of the 3D structure from precursor clusters K46 were simulated in the following form: primary chain → microlayer → microframework.
The present study represents a followup to the works [7, 8, 11–16] in the field of simulation of the processes of self-assembly of systems on the suprapolyhedral scale and the geometrical and topological analysis of crystal structures by advanced computer methods.
COMPUTER ANALYSIS METHODS
The geometrical and topological analysis was performed by means of the ToposPro software package [10], which made it possible to conduct a multipurpose study of the crystal structure automatically, using the representation of structures in the form of “folded graphs” (factor graphs). The data on the functional role of atoms in the formation of a crystalline structure were obtained by calculating coordination sequences, i.e., sets of numbers {Nk}, where Nk was the number of atoms in the kth coordination sphere of a given atom.
The obtained values of the coordination sequences of atoms in the 3D lattices are provided in Table 2, in which the number of neighboring atoms in the most proximate surrounding, i.e., in the first coordination sphere of the atom, was separated. All atoms were characterized by different sets of the coordination sequences {Nk}; therefore, all the atoms were topologically (and functionally) different.
The algorithm for automatic decomposition of a structure of any intermetallide, represented in the form of a folded graph, onto cluster units was based on the following principles: a structure was formed as a result of self-assembly from precursor clusters. Here, the precursor clusters formed a structure framework, voids in which were filled with spacer clusters (consisting of a small number of atoms), the precursor nanoclusters did not have shared internal atoms; however, they could have shared atoms on the surface, the precursor clusters occupied highly symmetric positions and the set of precursor nanoclusters and spacer clusters included all atoms of the structure.
The algorithm was implemented by the ToposPro software package [10].
SELF-ASSEMBLY OF THE CRYSTAL STRUCTURE OF Ca11Hg54–hP65
The method of simulation of the crystal structure that we used was based on determining the hierarchical sequence of its self-assembly in the crystallographic space [11]. At the first level of the system self-assembly, the mechanism of formation of a primary chain of the structure from 0-level nanoclusters formed at the template stage of the chemical evolution of the system is determined, then the mechanism of self-assembly from the layer chain (2nd level), and, thereafter, from the layer of three-dimensional framework (3rd level).
Crystallographic data. Parameters of the hexagonal cell: a = b = 13.389, c = 9.615 Å.
Space group P-6 (no. 174) with elements of the point symmetry: g = –6 (1a, 1b, 1c, 1d, 1e, 1f), 3 (2g, 2h, 2i), m (3j, 3k). The order of the group was 6.
Polyhedral clusters K8, K11 and K12. 184 variants of the cluster representation of the 3D atomic lattice with a number of structural units from 3 to 7 was established.
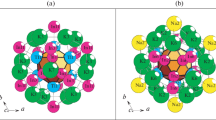
The polyhedral clusters K8 = 0@Ca2Hg6 which appeared to be hexagonal bipyramids, the polyhedral clusters K11 = 0@Ca3Hg8 and the polyhedral clusters with the central Hg atom K12 = Hg (Ca3Hg8) were determined (Fig. 1).
The centers of the clusters Ca2Hg6, 0@Ca3Hg8 and Hg(Ca3Hg8) occupied the most highly symmetric positions 1c, 1b, and 1f with a –6 symmetry.
Suprapolyhedral precursor clusters K46. The clusters Ca2Hg6 represented templates, on the surface of which atomic shells were formed of 38 atoms (Fig. 2). The composition of the two-layered cluster K46 was 0@8(Ca2Hg6)@38(Hg6 + CaHg6)2(Ca6Hg6).
Self-assembly of the crystalline structure. Primary chain. The formation of primary chains \(S_{3}^{1}\) occured upon binding of K46 clusters by triple Hg3 rings towards the direction [001]. The distance between the centers of the K46 in the primary chain determined the value of the translation vector c = 9.818 Å.
2D layer. The layer \(S_{3}^{2}\) was formed upon binding of the primary chains \(S_{3}^{1}\) towards the direction [100] (Fig. 3). A distance between the axes of the primary chains determined the value of the translation vector a = 13.602 Å. The voids in the framework were occupied by the polyhedral clusters 0@Ca3Hg8 and Hg(Ca3Hg8) and the spacer Hg(14) atoms (Fig. 3).
Self-assembly of the framework. The 3D framework structure \(S_{3}^{3}\) was formed upon binding of the 2D layers (with a shift) towards the direction [010] (Fig. 3). In the 3D framework, the distance between the equivalent 2D layers determined the value of the vector b = 13.602 Å.
CONCLUSIONS
The combinatory-topological analysis and simulation of cluster self-assembly of the Ca11Hg54–hP65 crystal structure have been performed.
The polyhedral clusters K8 = 0@Ca2Hg6, which had appeared to be hexagonal bipyramids, the polyhedral clusters K11 = 0@Ca3Hg8, and the polyhedral clusters with the central Hg atom K12 = Hg(Ca3Hg8) have been determined.
The clusters Ca2Hg6 represented templates, on the surface of which atomic shells of 38 atoms have been formed. The composition of the two-layered cluster K46 was 0@8(Ca2Hg6)@38(Hg6 + 6)2(Ca6Hg6).
The symmetry and topological code of the processes of self-assembly of the 3D structures from K46 precursor nanoclusters in the form primary chain → layer → framework have been simulated. The voids between the primary chains have been occupied by the polyhedral clusters 0@Ca3Hg8 and Hg(Ca3Hg8), as well as by the Hg spacer atoms.
REFERENCES
Villars, P. and Cenzual, K., Pearson’s Crystal Data-Crystal Structure Database for Inorganic Compounds (PCDIC), Materials Park, OH: ASM Int., 2010.
Bruzzone, G. and Merlo, F., The calcium-mercury system, J. Less-Common Met., 1973, vol. 32, pp. 237–241.
Cenzual, K., Gelato, L.M., Penzo, M., and Parthe, E., Inorganic structure types with revised space groups. I, Acta Crystallogr., Sect. B: Struct. Sci., 1991, vol. 47, pp. 433–439.
Puselj, M. and Ban, Z., New ternary gamma-brass phases in the systems Ca–MIB(IIB)–Hg, Z. Naturforsch., B: Anorg. Chem., Org. Chem., 1980, vol. 35, pp. 1594–1595.
Tkachuk, A.V. and Mar, A., Alkaline-earth metal mercury intermetallics A(11–x)Hg(54+x) (A = Ca, Sr), Inorg. Chem., 2008, vol. 47, no. 4, pp. 1313–1318.
Puselj, M. and Ban, Z., Contribution to knowledge about the mercury–calcium system, Croat. Chem. Acta, 1978, vol. 51, no. 1, pp. 75–79.
Ilyushin, G.D., Modeling of self-organization processes in crystal-forming systems: Symmetry and topology code for the cluster self-assembly of crystal structures of intermetallic compounds, Russ. J. Inorg. Chem., 2017, vol. 62, no. 13, pp. 1730–1769.
Ilyushin, G.D., Modeling of the self-orinnization processes in crystal-forming systems. Tetrahedral metal clusters and the self-assembly of crystal structures of intermetallic compounds, Crystallogr. Rep., 2017, vol. 62, no. 5, pp. 670–683.
Tambornino, F. and Hoch, C., The simplest representative of a complex series: The Hg-rich amalgam Yb11Hg54, Z.Kristallogr., 2017, vol. 232, pp. 557–565.
Blatov, V.A., Shevchenko, A.P., and Proserpio, D.M., Applied topological analysis of crystal structures with the program package topospro, Cryst. Growth Des., 2014, vol. 14, no. 7, pp. 3576–3585. https://topospro.com/.
Ilyushin, G.D., Modelirovanie protsessov samoorganizatsii v kristalloobrazuyushchikh sistemakh (Modeling of Self-Organization Processes in Crystal-Forming Systems), Moscow: Editorial URSS, 2003.
Blatov, V.A., Ilyushin, G.D., and Proserpio, D.M., Nanocluster model of intermetallic compounds with giant unit cells: β,β'-Mg2Al3 polymorphs, Inorg. Chem., 2010, vol. 49, no. 4, pp. 1811–1818.
Shevchenko, V.Ya., Blatov, V.A., and Ilyushin, G.D., Symmetry and topology codes of cluster self-assembly for icosahedral structures of the NaZn13-cF112 and TRB66-cF1944 family, Glass Phys. Chem., 2015, vol. 41, no. 4, pp. 341–351.
Ilyushin, G.D., The crystal chemistry of lithium intermetallic compounds: A survey, Russ. J. Inorg. Chem., 2018, vol. 63, no. 14, pp. 1786–1799.
Shevchenko, V.Ya., Blatov, V.A., and Ilyushin, G.D., Modeling the processes of self-organization in crystal-forming systems: New two-layer clusters–precursors 0@(Na2Cd6)@(Na12Cd26) and 0@(Na3Cd6)@(Na6Cd35) for the self-assembly of the Na26Cd141–hP168 crystal structure, Glass Phys. Chem., 2019, vol. 45, no. 4, pp. 311–317.
Ilyushin, G.D., Symmetry and topology code of the cluster self-assembly of intermetallic compounds A[16]2 B[12]4 of the Friauf Families Mg2Cu4 and Mg2Zn4, Crystallogr. Rep., 2018, vol. 63, pp. 543–552.
Funding
This work was carried put with the support of the Russian Foundation for Basic Research (project no. 19-02-00636) and the Ministry of Science and Higher Education as a part of the State Order of the Russian Federal Research Center for Crystallography and Photonics.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by D. Marinin
Rights and permissions
About this article
Cite this article
Shevchenko, V.Y., Blatov, V.A. & Il’yushin, G.D. Cluster Self-Organization of Intermetallic Systems: New Two-Layer Cluster-Precursor K46 = 0 @8(Ca2Hg6)@38(Hg6 + CaHg6)2(Ca6Hg6) for Self-Assembly of the Crystal Structure of Ca11Hg54–hP65. Glass Phys Chem 46, 1–5 (2020). https://doi.org/10.1134/S1087659620010198
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659620010198