Abstract
The TOPOS software package is used for the combinatorial topological analysis and simulation of the self-assembly of the K52Na12Tl36In122-hP224 (spatial group P-3m1, a = b = 16.909, c = 28.483 Å, V = 7 052 Å3) crystalline structure. A total of 1649 variants of the cluster representation of a 3D atomic lattice with the number of structural units ranging from 4 to 10 are found. Two frame-forming three-layer icosahedral nanoclusters are detected: K132 and K116 with symmetry g = –3m and a diameter of 17 Å. The chemical composition of the shells of the three-layer 132-atom nanocluster K132 is 0@12(In6Tl6)@30(In6Na6K18)@90(In72Na12K6) and that of the 116-atom nanocluster K116 is (0@12(In6Tl6)@26(In12K14)@78(In36Tl20K12). The symmetrical and topological code of the self-assembly of the 3D Na12K52Tl36In122 structure from the precursor K132 and K116 nanoclusters is reconstructed in the following form: primary chain → layer → frame. The framework’s voids are occupied by spacer atoms K and Tl.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Crystallization of potassium intermetallic compounds was detected in binary systems K–M (with the participation of 15 chemical elements M), in 63 ternary systems K–M1–M2 (with the participation of 27 elements) and in 30 quaternary systems K–M1–M2–M3 (with the participation of 29 elements) [1, 2].
Twelve quaternary intermetallic compounds are formed in 11 systems K–Na–M1–M2 with the participation of atoms M1 and M2 = Au, Mg, Zn, Cd, Al, Ga, In, Sn, Sb, Bi) (Table 1, [3–14]). In the K–Na–Cd–Tl system, the formation of two compounds is detected; and in the other systems, of one compound (Table 1). With the participation of the large atoms K and Rb, and K and Cs in quaternary systems only 3 and 2 intermetallic compounds are formed [1, 2].
In [15] for the quaternary intermetallic compound K23Na8Cd12In48-hP91 (a = b =17.114, c = 10.442 Å, spatial group P6/mmm), a new type of polyhedral precursor cluster was detected: K8 = 0@8(Na2In6) and K8 = 0@K2In6 in the form of a hexagonal bipyramid. The Na2In6 clusters were templates on the surfaces of which atomic shells of 36 atoms were formed. The composition of the two-layer templated cluster is K44 = 0@8(Na2In6)@36(In6Cd6K6)2.
The crystalline structure of the most complex quaternary intermetallic compound K52Na12Tl36In122-hP224 in terms of crystalline chemistry [13] is characterized by the high values of the parameters of the hexagonal cell: a = b =16.909 Å, c = 28.483 Å, V = 7 052 Å3, spatial group P-3m1(164), and 32 crystallographic independent atoms with unique Wyckoff positions j8i20d3c. Two Na atoms have coordination numbers (CN) = 13 and 15; eight K atoms have CN = 12, 14, 15, and 16; sixteen In atoms have CN = 9, 10, 11, 12, and 16; and six Tl atoms have CN = 10, 11, 13, and 14.
This work using the ToposPro software package [16] performs geometrical and topological analysis of the crystalline structure of the intermetallic compound K52Na12Tl36In122-hP224. The symmetrical and topological code of the self-assembly of the crystalline structure of the intermetallic compound made of nanoclusters K132 and K116 is reconstructed in the following form: primary chain → microlayer → microframe.
This work is a continuation of the studies [15, 17–23] in the field of simulation of the self-organization of systems at the supra-polyhedral level and the geometrical and topological analysis of crystalline structures by advanced computer methods.
METHODS OF COMPUTER ANALYSIS
The geometrical and topological analysis was performed using the ToposPro software package [16], which allows us to perform a multipurpose automatic study of the crystalline structure based on the representation of structures in the form of convoluted graphs (factor graphs). The data on the functional role of atoms upon the formation of a crystalline structure were obtained by computing the coordination sequences, that is, the sets of numbers {Nk}, where Nk is the number of atoms in the kth coordination sphere of the given atom.
The obtained values of the coordination sequence of atoms in 3D lattices are summarized in Table 2, where the number of neighboring atoms in the nearest surrounding area is highlighted in bold, that is, in the first coordination sphere of the atom. All atoms are characterized by various sets of coordination sequences {Nk}.
The automatic decomposition algorithm of the structure of any intermetallic compound was described elsewhere and implemented using the ToposPro software package [16].
SELF-ASSEMBLY OF THE K52Na12Tl36In122-hP224 CRYSTALLINE STRUCTURE
The applied simulation method of the crystalline structure is based on the determination of the hierarchical sequence of its self-assembly in crystallographic space [15, 17]. At the first level of the system’s self-organization, the mechanism of the primary chain formation of nanoclusters of the zero level formed at the template stage of the system’s chemical evolution is determined, then the mechanism of self-assembly of the layer chain (the 2nd level), and then of the layer of the structure’s 3D frame (the 3rd level).
Crystallographic data. The spatial group P-3m1 (no. 164) is characterized by the elements with point symmetry: g = –3m (1a, 1b), 3m (2c, 2d), 2/m (3e, 3f), 2 (6g, 6h), and m (6i). The group’s order is 12.
Table 2 shows the local surrounding of K, Na, Tl, and In atoms and their coordination sequences in a 3D atomic lattice.
The number of variants of representing a 3D atomic lattice with the number of structural units ranging from 4 to 10 was 1649 (Table 3).
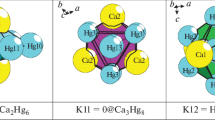
Two variants of different crystallographic icosahedral clusters ico-In6Tl6(0@12) were detected with the symmetry –3m, occupying the highly symmetric positions 4a and 4b.
Icosahedrons ico-In6Tl6(0@12) are templates on which three-layer clusters K132 and K116 are formed with the size of 17 Å (Figs. 1, 2).
The chemical composition of the shells of the nanocluster K132 is 0@12(In6Tl6)@30(In6Na6-K18)@-90(In72Na12K6). The chemical composition of the shells of the nanocluster K116 is 0@12(In6Tl6)@26(In12K14)@78(In36Tl20K12).
Self-assembly of crystalline structure. The nanoclusters K132 (Table 4) and K116 (Table 5) are the frame-forming clusters.
Layer. The \(S_{3}^{2}\) basic layer is formed of clusters K132 upon the binding of the primary chains with a shift (Fig. 3). The distance between the centers of clusters K132 in the primary chain and in the layer determines the values of the translation vectors a = b = 16.909 Å.
Packet. The layer of clusters K116 is formed on the surface of the layer of clusters K132 (Fig. 4). In the voids of the layer of clusters K116, spacer atoms K8 and Tl10 occupy positions 2d (1/3, 2/3, 0.174) and Tl10 (2/3, 1/3, 0.185) with symmetry 3m. The thickness of the two-layer packet corresponds to the absolute value of the translation vector c = 28.483 Å.
Self-assembly of shell. The 3D framework structure \(S_{3}^{3}\) is formed upon the binding of two-layer packets in the Z direction.
CONCLUSIONS
Two frame-forming nanoclusters have been detected using the decomposition of the 3D atomic lattice: K132 and K116 with symmetry g = –3m. The voids of the layer of K116 clusters are occupied by spacer atoms K and Tl.
The chemical composition of shells of the nanocluster K132 is 0@12(In6Tl6)@30(In6Na6K18)-@90(In72Na12K6) and that of the nanocluster K116 is 0@12(In6Tl6)@26(In12K14)@78(In36Tl20K12).
The symmetrical and topological code of the self-assembly of the 3D structure from the precursor nanoclusters K132 and K116 has been reconstructed in the following form: primary chain → layer → frame.
REFERENCES
Villars, P. and Cenzual, K., Pearson’s Crystal Data-Crystal Structure Database for Inorganic Compounds (PCDIC), Materials Park, OH: ASM Int., 2018.
Inorganic Crystal Structure Database (ICSD), Karlsruhe, Germany: Fachinformationszentrum; USA: Natl. Inst. Standard Technol.
Somer, M., Carrillo-Cabrera, W., Peters, E.M., Peters, K., and von Schnering, H.G., Crystal structure of sodium potassium antimonidetriantimonidoaluminate, Na3K6Sb(AlSb3), Z. Kristallogr., 1995, vol. 210, pp. 527–527.
Somer, M., Carrillo-Cabrera, W., Peters, E.M., Peters, K., and Vonschnering, H.G., Crystal structure of sodium potassium antimonidetriantimonidogallate, K6Na3Sb(GaSb3), Z. Kristallogr., 1995, vol. 210, pp. 143–143.
Kim, S.-J., Kraus, F., and Faessler, T.F., Na6ZnSn2, Na4.24K1.76ZnSn2, and Na20Zn8Sn11: Three intermetallic structures containing the linear {Sn–Zn–Sn}6-unit, J. Am. Chem. Soc., 2009, vol. 131, pp. 1469–1478.
Eisenmann, B. and Klein, J.D., Zintl-anionen [Sn2As6]10– und [Sn2Sb6]10– in alkaliverbindungen, Z. Kristallogr., 1991, vol. 196, pp. 213–229.
Tillard-Charbonnel, M.M., Belin, C.H.E., Manteghetti, A.P., and Flot, D.M., Heteroatomic centering of icosahedral clusters. Crystal and electronic structure of the K6(NaCd)2Tl12Cd compound containing the not-so-naked Tl12Cd12-polyanion, Inorg. Chem., 1996, vol. 35, pp. 2583–2589.
Dong, Z-C. and Corbett, J.D., Na14K6Tl18M (M = Mg, Zn, Cd, Hg) and Na13.5 Sm0.5K6Tl18Na: Novel octahedral and centered icosahedral cluster phases related to the Mg2Zn11-type structure, Angew. Chem., Int. Ed. Engl., 1996, vol. 36, pp. 1006–1008.
Asbrand, M., Eisenmann, B., and Engelhardt, H., Dimere und polymere Pnictidostannat(IV)-anionen-darstellung und kristallstrukturen von Na2K3[SnP3], Na2Cs3[SnP3] und Na2K3[SnBi3], Zeitschr. Naturforsch., B, 1998, vol. 53, pp. 405–410.
Flot, D.M., Tillard-Charbonnel, M., and Belin, C., Na8K23Cd12In48: A zintl phase containing icosahedral and triangular indium units and displaying a remarkable condensed metal fullarene stuffed with a tubular cluster. Synthesis, crystal, and electronic structures, J. Am. Chem. Soc., 1996, vol. 118, pp. 5229–5235.
Huang, D. and Corbett, J.D., Na9K16Tl18Cd3: A novel phase containing (Tl8Cd3)(–) and Tl5(7) clusters, Inorg. Chem., 1999, vol. 38, pp. 316–320.
Huang, D., Dong, Zh.-ch., and Corbett, J.D., Na12K38Tl48Au2: A metallic zintl phase with naked icosahedral fragments Tl7(7–) and Tl9(9–) plus Au(–), Inorg. Chem., 1998, vol. 37, pp. 5881–5886.
Flot, D.M., Tillard Charbonnel, M.M., and Belin, C., Crystal structure of sodium potassium thallide indide, Na6K26In42 – x(InyTl38 – y) (x = 1.08; y = 20.12), Z. Kristallogr. (New Struct.), 1998, vol. 213, pp. 225–226.
Flot, D., Vincent, L., Tillard-Charbonnel, M., and Belin, C., Crystal structure of sodium potassium cadmium gallium, Na21K14Cd17Ga84, Z. Kristallogr.(New Struct.), 1997, vol. 212, pp. 509–510.
Shevchenko, V.Ya., Blatov, V.A., and Ilyushin, G.D., Modeling self-organization processes in crystal-forming systems: New two-layer cluster–precursor K44 = 0@8(Na2In6)@36(In6Cd6K6)2 for the self-assembly of the K23Na8Cd12In48–hP91 crystal structure, Glass Phys. Chem., 2019, vol. 45, no. 6, pp. 405–411.
Blatov, V.A., Shevchenko, A.P., and Proserpio, D.M., Applied topological analysis of crystal structures with the program package ToposPro, Cryst. Growth Des., 2014, vol. 14, pp. 3576–3585.
Ilyushin, G.D., Modelirovanie protsessov samoorganizatsii v kristalloobrazuyushchikh sistemakh (Modeling of Self-Organization Processes in Crystal-Forming Systems), Moscow: Editorial URSS, 2003.
Ilyushin, G.D., Modeling of the self-organization processes in crystal-forming systems. tetrahedral metal clusters and the self-assembly of crystal structures of intermetallic compounds, Crystallogr. Rep., 2017, vol. 62, pp. 670–683.
Ilyushin, G.D., Symmetry and topology code of the cluster self-assembly of intermetallic compounds \({\text{A}}_{2}^{{[16]}}{\text{B}}_{4}^{{[12]}}\) of the friauf families Mg2Cu4 and Mg2Zn4, Crystallogr. Rep., 2018, vol. 63, pp. 543–552.
Blatov, V.A., Ilyushin, G.D., and Proserpio, D.M., New types of multishell nanoclusters with a Frank-Kasper polyhedral core in intermetallics, Inorg. Chem., 2011, vol. 50, pp. 5714–5724.
Ilyushin, G.D., Modeling of self-organization processes in crystal-forming systems: Symmetry and topology code for the cluster self-assembly of crystal structures of intermetallic compounds, Russ. J. Inorg. Chem., 2017, vol. 62, pp. 1730–1769.
Pankova, A.A., Akhmetshina, T.G., Blatov, V.A., and Proserpio, D.M., A collection of topological types of nanoclusters and its application to icosahedra-based intermetallics, Inorg. Chem., 2015, vol. 54, no. 13, pp. 6616–6630.
Ilyushin, G.D., Crystal chemistry of lithium intermetallic compounds: A survey, Russ. J. Inorg. Chem., 2018, vol. 63, no. 14, pp. 1786–1799.
Funding
This work was supported by the Ministry of Science and Higher Education as part of a state contract of the Crystallography and Photonics Center, Russian Academy of Sciences and the Russian Foundation for Basic Research (project no. 19-02-00636).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by I. Moshkin
Rights and permissions
About this article
Cite this article
Shevchenko, V.Y., Blatov, V.A. & Il’yushin, G.D. Cluster Self-Organization of Intermetallic Systems: Three-Layer Icosahedral Nanoclusters K132 = 0@12(In6Tl6)@30(In6Na6K18)@90(In72Na12K6) and K116 = 0@12(In6Tl6)@26(In12K14)@78(In36Tl20K12) for the Self-Assembly of the K52Na12Tl36In122-hP224 Crystalline Structure. Glass Phys Chem 46, 195–202 (2020). https://doi.org/10.1134/S1087659620030116
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659620030116