Abstract
A combinational-topological analysis of the crystalline structure (Ba,Ca)46Li102 (R\(\bar {3}\)c, hR888, V = 30 978 Å3) are performed by computers (the TOPOS software package). The framework-forming the 143-atom icosahedral nanocluster ico-K143 with a diameter of 20 Å is established by the method of the complete decomposition of a 3D atomic lattice into cluster structures. ico-K143 nanoclusters of a symmetry of g = \(\bar {3}\) are shown to be three-layered: Li @12(Li12) @32(Li12Ba20) @98(Ba32Li66). The second shell corresponds to the Bergman cluster with 32, 90, and 60 peaks, edges, and faces, respectively. The third new type of icosahedral shell consisting of 98 atoms is characterized by 98, 288, and 192 peaks, edges, and faces, respectively. The chemical composition of the third shell is made up of Ba32Li66. In the third shell, 32 Ba atoms were located above all the 32 atoms of the Li12Ba20 shell. The symmetry and topological code of the self-assembly of 3D structures from iсo-K143 nanoclusters-precursors are simulated in the following form: primary chain → layer → framework. The iсo-K143 nanoclusters formed densely packed 2D layers 36 positioned with a shift along the c axis. The distance between centers of iсo-K143 clusters is determined by the translation vector value ahex = 19.913 Å. The large voids in the 3D framework are occupied by 12-atom Ba3Li9 clusters with the symmetry g = 32.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
At present, there are several types of two-layered icosahedral clusters known in the chemistry of intermetallics that differ by the number of atoms in the second shell: 1@12@32 (Bergman cluster [1]), 1@12@42 (Mackay cluster [2]), and 1@12@50 [1].
Intermetallics containing Bergman nanoclusters are formed in multiple binary and ternary systems. Up to 150 crystalline structures have been investigated [3]. The chemical composition and structure of binary intermetallics with Bergman nanoclusters-precursors were investigated in [1].
Ternary and binary intermetallics containing local regions in the forms of Bergman icosahedral clusters represent the most complex crystalline structures in terms of crystal chemistry. Table 1 demonstrates intermetallics with a unit cell volume exceeding 10 000 Å3 containing up to 1100 atoms [4–12].
The crystal structure of (Ba,Ca)46Li102 (Li33.3Ba13.1Ca3, [12]) exhibits the largest values of the unit cell volume V = 30 979 Å3 and the translation vector с = 90.213 Å (Table 1). The CN values of 15 (for one atom), 16 (for seven atoms), and 17 (for two atoms) were found for 10 crystallographically independent Ba atoms. In the crystal structure of (Ba,Ca)46Li102, the Ca atoms are statistically distributed along the crystallographic positions of 8 Ba atoms [12].
The crystal structure of (Ba,Ca)46Li102 was described in the form of bonded Li(Li4Ba8) icosahedra with Li(Li12) and Li(Li9Ba3) icosahedra located between them [12].
The present study carries out a geometrical and topological analysis of the crystal structure of the (Ba,Ca)46Li102 intermetallic system by the ToposPro software package [13]. The symmetry and topological code of the process of cluster self-assembly from the 143-atom nanocluster in the form of the primary chain \({\text{S}}_{3}^{1}\) → layer \({\text{S}}_{3}^{2}\) → framework \({\text{S}}_{3}^{3}\) was established.
This study represents a follow-up of [1, 2, 14–22] in the simulation of processes of self-the assembly of systems on the suprapolyhedral level and the geometric and topological analysis of crystal structures by advanced computer methods.
TECHNIQUES USED IN COMPUTER ANALYSIS
The geometric and topological analysis was carried out by the ToposPro [13] software package that allows performing a multipurpose automated investigation of the crystal structure built on the presentation of structures as “folded graphs” (quotient graphs). The data on the functional role of the atoms at the formation of the crystal structure were obtained by calculating the coordination sequences, i.e., the number sets {Nk}, where Nk is the number of atoms in the k-coordination sphere of a specific atom.
The obtained values of the coordination sequences of atoms in 3D-lattices are provided in Table 2, where the number of neighboring atoms in the adjacent region, i.e., in the first coordination sphere, are given in bold. All the atoms exhibited various sets of the coordination sequences {Nk}, so that they are all different topologically (and functionally).
The algorithm of the automated decomposition of any intermetallic structure, represented as a folded graph, on the cluster units was based on the following principles. A structure is formed as a result of the self-assembly from clusters-precursors. Here, the clusters-precursors formed a framework of the structure, where voids were occupied by clusters-spacers (consisting of a small number of atoms). Nanoclusters-precursors did not have shared internal atoms, although shared surface atoms could be observed. The clusters-precursors occupied highly symmetric positions. A set of nanoclusters-precursors and clusters-spacers consisted of all the atoms of the structure. The algorithm was implemented in the ToposPro software package [13].
SELF-ASSEMBLY OF THE CRYSTAL STRUCTURE OF (Ba,Ca)46Li102
The applied method of the simulation of the crystalline structure was based on the determination of the hierarchic sequence of its self-assembly in the crystallographic dimension [14, 15]. At the first level of the system’s self-assembly, the mechanism was determined by the formation of the primary chain of the structure from nanoclusters of the 0-level, formed at the template stage of the system’s chemical evolution, then the mechanism of self-assembly from the chain of the level was initiated (2nd level) and, thereafter, from the layer a 3D framework of the structure (3rd level) was initiated.
Crystallographic data on(Ba,Ca)46Li102. The space group R\(\bar {3}\)c is characterized by elements with the point symmetry g = 32 (6a), g = –3 (6b), 3 (12c), –1 (18d), and 2 (18e).
Table 2 demonstrates the local surroundings of the Li and Ba atoms and the coordination sequence values in the 3D atomic network.
The number of variations of the decomposition of a 3D atomic lattice with of 3, 4, 5, 6, and 7 structural units equaled 3, 16, 53, 44, and 17 (total 133 variations), respectively.
The variant of describing the crystal structure of (Ba,Ca)46Li102 suggested in [12] implying isolation of the layer from the bonded Li(Li4Ba8) icosahedra with the Li(Li12) and Li(Li9Ba3) icosahedra located between them corresponds to the variation with the largest number of elementary structural units of 7 (Table 3). Upon the selection of such a type of intermetallics simulation, apart from the three investigated elementary polyhedral clusters, the functional role of another three isolated elementary polyhedral clusters (Ba1(1)(1@16), Li3(1)(1@12), and Ba4(1)(1@17)) and atoms-spacers (Li5(0)(1)) must be considered.
Quasis-pherical clusters the iсo-K143 of Li(Li12)(Li12Ba20)(Ba32Li66) composition, Ba3Li9 clusters-spacers, and Li atoms-spacers were established as the framework-forming nanoclusters (Table 4, Figs. 1, 2).
The iсo-K143 nanocluster appeared to be three-layered of composition 1@12@32@98 with the center in the position 6b (1/3, 2/3, 1/6) and the point symmetry g = –3.
The center of the Ba3Li9 cluster-spacer with the point symmetry g = 32 was located in the position 6a (2/3, 1/3, 0.583) (Fig. 1).
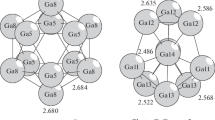
The second 32-atom icosahedral shell-deltohedron corresponded to the Bergman cluster with 32, 90, and 60 peaks, edges, and faces, respectively (Fig. 2). The second shell contained 20 Ba atoms located on 20 edges of an icosahedron and 12 Li atoms bonded to 12 Li atoms (peaks of the icosahedrons).
The third new type of 98-atom icosahedral shell-deltohedron exhibited of 98, 288, and 192 peaks, edges, and faces. The chemical composition of the shell is Ba32 Li66. In the third shell, all 32 Ba atoms were located above 32 atoms of the Berman shell (Fig. 3).
The basic 3D lattice for the framework-forming iсo-K143 clusters corresponded to the hexagonal densest packing of clusters with CN = 12 = 6 (in the layer) + 3 (above the layer) + 3 (under the layer).
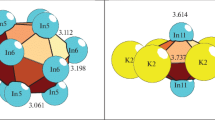
Self-assembly of the(Ba,Ca)46Li102crystalline structure. Primary chain. The self-assembly of the primary chains from the iсo-K143 cluster proceeded along the X axis (Fig. 4). Here, the localization of two atoms-spacers (Li2) occurred between iсo-K143 clusters.
The distance between the centers of iсo-K143 clusters corresponds to the translation vector value a = 19.913 Å.
Self-assembly of the layer. The formation of the \(S_{3}^{2}\) layer proceeded upon the complementary bonding of the clusters from the adjacent primary chains with a shift (Fig. 5). At this stage of self-assembly, localization occurs in the voids of the Ba3Li9 cluster-spacer.
The distance between the centers of iсo-K143 clusters from the adjacent primary chains corresponded to the translation vector value b = 19.913 Å.
Self-assembly of the framework. The structure of the framework of \(S_{3}^{3}\) is formed upon the bonding of two basic layers with a shift. The distance between layers along the Z axis determines the translation vector value c/6 = 90.213 Å/6.
CONCLUSIONS
The framework-forming 143-atom icosahedral nanocluster with a diameter of 20 Å has been established via the method of the complete decomposition of the 3D atomic lattice into cluster structures. ico-K143 nanoclusters have been determined as three-layered Li @12(Li12) @32(Li12Ba20) @98(Ba32Li66) with a symmetry of g = \(\bar {3}\).
The symmetrical and topological code of the self-assembly processes of 3D structures from iсo-K143 nanoclusters-precursors in the primary chain → layer → framework form has been reconstructed. iсo-K143 nanoclusters form densely packed 2D layers 36 located with a shift along the c axis.
The 12-atom Ba3Li9 clusters-spacers with a symmetry of g = 32 that occupied voids in the 3D framework structure and Li atoms-spacers that participated in the bonding of iсo-K143 upon the formation of the primary chain and layer have been identified.
REFERENCES
Akhmetshina, T.G. and Blatov, V.A., A Bergman, Bergman-based and 63-atom nanoclusters in intermetallics, Struct. Chem., 2016, vol. 27, pp. 1685–1692.
Akhmetshina, T.G. and Blatov, V.A., A fascinating building unit: Mackay cluster in intermetallics, Struct. Chem., 2017, vol. 28, pp. 133–140.
Villars P., Cenzual K., Pearson’s Crystal Data-Crystal Structure Database for Inorganic Compounds (PCDIC), Materials Park, OH: ASM International, 2010.
Todorov, E. and Sevov, S.C., Synthesis, characterization, electronic structure, and bonding ofheteroatomic deltahedral clusters: Na49Cd58.5Sn37.5, A network structure containing the first empty icosahedron without a group 13 element and the largest closo-deltahedron, J. Am. Chem. Soc., 1997, vol. 119, pp. 2869–2876.
Li, B. and Corbett, J.D., Electronic stabilization effects: Three new K–In–T (T = Mg, Au, Zn) network compounds, Inorg. Chem., 2006, vol. 45, pp. 8958–8964.
Leblanc, M., Le Bail, A., and Audier, M., Crystalline phases related to the icosahedral Al–Li–Cu phase: A single crystal X-ray diffraction study of the tetragonal τ-Al56(Cu,Zn)11Li33 phase, Phys. B (Amsterdam, Neth.), 1991, vol. 173, pp. 329–355.
Charbonnel, M. and Belin, C., Synthesis and crystal structure of the new nonstoichiometric phase Li3N-a5Ga19.56, Nouv. J. Chim., 1984, vol. 8, pp. 595–599.
Kreiner, G., Towards realistic quasiperiodic st-ructures: Modelling, synthesis and structure of (Ga,Zn)175–δMg97+δ—A large 3/2-2/1-2/1 Fibonacci approximant, J. Alloys Compd., 2002, vol. 338, pp. 261–273.
Tillard-Charbonnel, M., Belin, C., and Chouaibi, N., Crystal structure of sodium gold gallium, Na128Au81Ga275, Z. Kristallogr., 1993, vol. 206, pp. 310–312.
Smetana, V., Babizhetskyy, V., Vajenine, G., and Simon, A., Neue Li26-cluster in Li13Na29Ba19, Z. Anorg. Allg. Chem., 2006, vol. 632, pp. 2115–211.
Smetana, V., Vajenine, G.V., Kienle, L., Duppel, V., and Simon, A., Intermetallic and metal-rich phases in the system Li–Ba–In–N, J. Solid State Chem., 2010, vol. 183, no. 8, pp. 1767–1775.
Smetana, V., Babizhetskyy, V., Hoch, C., and Simon, A., Icosahedral Li clusters in the structures of Li33.3Ba13.1Ca3 and Li18.9Na8.3Ba15.3, J. Solid State Chem., 2007, vol. 180, pp. 3302–3309.
Blatov, V.A., Shevchenko, A.P., and Proserpio, D.M., Applied topological analysis of crystal structures with the program package ToposPro, Cryst. Growth Des., 2014, vol. 14, no. 7, pp. 3576–3585. http://topospro.com/.
Ilyushin, G.D., Modelirovanie protsessov samoorganizatsii v kristalloobrazuyushchikh sistemakh (Modeling of Self-Organization Processes in Crystal Forming Systems), Moscow: Editorial URSS, 2003.
Ilyushin, G.D., Theory of cluster self-organization of crystal-forming systems. geometrical-topological modeling of nanocluster precursors with a hierarchical structure, Struct. Chem., 2012, vol. 20, no. 6, pp. 975–1043.
Pankova, A.A., Blatov, V.A., Ilyushin, G.D., and Proserpio, D.M., γ-Brass polyhedral core in intermetallics: The nanocluster model, Inorg. Chem., 2013, vol. 52, no. 22, pp. 13094–13107.
Shevchenko, V.Ya., Blatov, V.A., and Ilyushin, G.D., Symmetry and topology codes of cluster self-assembly for icosahedral structures of the NaZn13-cF112 and TRB66-cF1944 family, Glass Phys. Chem., 2015, vol. 41, no. 4, pp. 341–351.
Shevchenko, V.Ya., Blatov, V.A., and Ilyushin, G.D., Modeling of self-organization processes in crystal-forming systems: Symmetry and topological codes of cluster self-assembly of a 2D layered icosahedral structure of Sc18B238 (Pbam, oP514), Glass Phys. Chem., 2016, vol. 42, no. 3, pp. 221–229.
Shevchenko, V.Ya., Blatov, V.A., and Ilyushin, G.D., The symmetric and topological code of the cluster self-assembly of the crystal structure of ε-Mg23Al30 of K63 nanoclusters, Glass Phys. Chem., 2017, vol. 43, no. 6, pp. 512–520.
Blatov, V.A., Ilyushin, G.D., and Proserpio, D.M., Nanocluster model of intermetallic compounds with giant unit cells: β,β'-Mg2Al3 polymorphs, Inorg. Chem., 2010, vol. 49, no. 4, pp. 1811–1818.
Shevchenko, V.Ya., Blatov, V.A., and Ilyushin, G.D., Intermetallic compounds of the NaCd2 family perceived as assemblies of nanoclusters, Struct. Chem., 2009, vol. 20, no. 6, pp. 975–982.
Blatov, V.A. and Ilyushin, G.D., New method for computer analysis of complex intermetallic compounds and nanocluster model of the samson phase Cd3Cu4, Crystallogr. Rep., 2010, vol. 55, no. 7, pp. 1100–1105.
ACKNOWLEDGMENTS
This study was supported by the Russian Foundation for Basic Research (project no. 16-12-00105) and the Federal Agency for Scientific Organizations (agreement no. 007-GZ/ChZ3363/26).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by D. Marinin
Rights and permissions
About this article
Cite this article
Shevchenko, V.Y., Blatov, V.A. & Ilyushin, G.D. Cluster Self-Organization of Intermetallic Systems: New 143-Atom Icosahedral Nanocluster-Precursor and the Self-Assembly of a Crystalline Framework (Ba,Ca)46Li102 (R\(\bar {3}\)c, hR888). Glass Phys Chem 44, 503–510 (2018). https://doi.org/10.1134/S1087659618060172
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659618060172