Abstract
The evolution of the structure-related morphological characteristics and fractional composition of an “Al–2B” composite powder has been studied in the course of the synthesis by the mechanical alloying method. The internal stresses, specific interface areas, and statistical characteristics of fractional composition have been determined as depending on the duration of powdered mixture treatment. Mechanisms for development of the composition and structure of the composite powder are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The use of boron-containing fillers, in particular, Al–2B composite powders, which are produced by the self-propagating high-temperature synthesis (SHS compositions), is a promising way to develop new high-energy materials. The combustion heat per unit mass and volume of such composites are, respectively, no lower and substantially higher than those of hydrocarbon fuels. However, the use of Al–2B composite powders can have a marked effect only given appropriate kinetic parameters of ignition and combustion. These parameters essentially depend on the fractional composition and structure-related morphological characteristics of the powders. These characteristics include the size distribution, shape, and surface state of composite particles; the degree of the composition uniformity; and the specific surface area of the interfaces between the components.
The most simple and available method for the production of Al–2B composite powders is based on combined mechanical treatment of the components in cooled activating ball mills [1–3]. The characteristics of the final product are determined by the process regime, i.e., the intensity and duration of the treatment, intensity of cooling, composition of grinding fluid, particle sizes, and physicochemical properties of initial powders.
The qualitative scheme of the synthesis is universal for the processes of mechanical alloying used to produce dispersion-reinforced composite materials [3]. Combined mechanical activation is accompanied by the intense incorporation of solid submicron boron particles into the near-surface layers of relatively large and plastic aluminum particles and, then, composite particles. Two competing processes proceed simultaneously—namely, fracture and “cold welding” of composite particles. The alternation of these processes leads to homogenization of the particle composition: as the treatment duration increases, the distribution of the components in the particle bulk becomes increasingly uniform. In the long run, a composite powder is formed with a maximum possible specific interface area between the components. When mechanical activation is carried out in a protective grinding fluid, a surface free of oxide films is formed. Repeated mechanical action on a powdered mixture results in the formation of a highly defective atomic structure of composite particles, with this structure being far from equilibrium.
Although the mechanical synthesis of composite powders, i.e., mechanical alloying, has for a long time been used in the processes of powder metallurgy, it has begun to be employed for the creation of composite high-energy materials relatively recently. The synthesis of high-energy composites is distinguished by the necessity of determining treatment conditions that would exclude the formation of noticeable amounts of undesirable chemical transformation products and, at the same time, ensure required physicochemical properties and kinetic characteristics of the transformations of a final product, including its interaction with oxidizing environments.
It should be noted that the study of the effect of mechanical activation on the transformation kinetics of high-energy condensed systems is a rapidly developing field of the chemical physics of combustion and explosion. At present, numerous original works and reviews have been published devoted to studying the properties of mechanically activated SHS composites [4, 5] and pyrotechnic [6] and explosive [7, 8] composites. A number of general regularities have been determined, such as a dramatic decrease in the temperature of spontaneous ignition and an increase in the conversion. Hypothetic mechanisms of the influence of mechanical activation on the transformation kinetics and the relation of the characteristics of ignition and combustion to the supraatomic structure of materials are widely discussed [5, 9]. Nevertheless, we cannot consider the contemporary level of understanding these mechanisms to be satisfactory. The ideas of the “technology–structure–properties” dependence for mechanically activated high-energy materials are, predominantly, confined to general considerations and need to be experimentally substantiated.
Concerning the Al–2B SHS composite considered in this work, it is necessary, first of all, to determine the characteristics of the powdered mixtures as depending on the treatment conditions and to formulate a detailed quantitative scenario for the formation of a composite powder. The refinement of this process via finding out the quantitative characteristics of variations in the composition and structure of a composite powder is of not only an academic interest. The practical concept of the question consists in the substantiation of rational parameters of the synthesis technology. The technology must ensure the absence of noticeable amounts of aluminum borides and products of the interaction with grinding fluid components and a minimal content of free boron in the final product, as well as a necessary fractional composition of the powder. Therefore, a problem arises concerning the control over the characteristics of the product by means of fitting technological parameters. Below, we present the results of studying the influence of mechanical activation time on the structure, morphology, and fractional composition of Al–2B composite powders.
MATERIALS AND METHODS
Powders of ASD-1 aluminum and B-99V amorphous boron were used in the experiments. Mixed powders were treated in an AGO-2U laboratory water-cooled planetary-type activating mill at a mixture feed of 10 g, a ball feed of 100 g, a cylinder rotation rate of 1061.5 rpm, and a treatment duration of 3–21 min. The balls were made of ShKh-15 alloy and had a diameter of 6 mm. Hexane (reagent grade) was used as a grinding fluid. Aluminum and boron powders were mixed in a ratio of 55/45 wt % for 20 min in a vibrating mill without balls.
The structural and morphological studies were performed by electron microscopy, X-ray spectroscopic microanalysis, and X-ray diffractometry. A JSM-6460LA electron microscope equipped with an attachment for energy-dispersive X-ray spectroscopic microanalysis (EDAX) was used. The primary electron beam energy, sample current, and angle between a sample surface and the detector were 15 keV, 1 nA, and 40°, respectively. The volume concentrations of the elements were determined using the ZAF-correction program. Powder samples of two types were used, i.e., pellets nearly 1 mm thick prepared with a hand-power press and placers of particles on a conducting adhesive tape.
Special samples were prepared to determine the character of the distribution of boron particles in the bulk of composite particles and boron contents in them. Composite powders were mixed with a powdered polymer, and the mixture was pressed and cured at an elevated temperature. Then, the samples were polished, and the polished sections were etched by argon ion beam .
Diffractometric studies were performed with an EMPYREAN X-ray diffractometer (CuKα radiation) within an angle range of 5°–140°. Two wavelengths of 1.5406 and 1.5444 Å with an intensity ratio in the spectra of 2 : 1 were taken into account in the calculations. The full-profile refinement of the calculation results was performed by the Rietveld method for all diffraction patters.
The fractional composition of the powders was determined with a Microtrac S3500 high-resolution liquid-phase laser diffractometer. Loosely packed aggregates were eliminated by 3-min ultrasonic dispersing.
Before serial experiments, the morphology and the fractional and chemical compositions were determined for the purchased aluminum and boron powders and the same powders subjected to mechanical activation in hexane.
The particles of the ASD-1 aluminum powder had an almost spherical shape. The average size of the particles calculated from their relative-number distribution was 3.89 µm and their specific surface area was 0.33 m2/g, while particles with sizes of 2.4–18.0 µm composed 90% of the sample mass. The basic substance content was 99.5%. The characteristic thickness of the oxide layer on the particle surface was in a range of 12–15 nm.
The initial powder of B-99V amorphous boron contained isometric particles with irregular shapes. The number-average particle size was 0.56 µm, specific surface area calculated within the model of spherical particles was 1.19 m2/g, and the real specific surface area was no larger than 2.5 m2/g. The basic substance content was no less than 99%. Initial powder particles consisted of nanosized crystallites with a characteristic size of about 5 nm. They contained chlorine (nearly 0.07%) and trace amounts of metal impurities and crystalline boron hydroxide. Particles with sizes of 0.2–10 µm composed the majority of the sample mass, with large particles being represented by well-distinguishable aggregates.
EVOLUTION OF FRACTIONAL COMPOSITION AND MORPHOLOGY
Figure 1 shows the distribution of the relative volume occupied by powder particles over their sizes. As the treatment duration increases, the distribution, which is initially bimodal and determined by a large difference between the sizes of aluminum and boron particles, is transformed into a unimodal one. At the same time, the particles become substantially larger. The submicron fraction, which mainly contains free boron, i.e., that unincorporated into aluminum, and amounts to nearly 6% of the initial mixture, almost completely disappears at a treatment duration of more than 7 min. On the other hand, a significant amount of large particles that were absent in the initial mixture arise in the system.
The kinetics of the particle enlargement is illustrated by the main statistical characteristics presented for the powder system in Figs. 2 and 3 as depending on the treatment time. These dependences show that, at an initial stage of mechanical activation, the average particle sizes calculated from the distributions of the relative volume, the surface areas and numbers of particles, and the width and median of the relative volume distribution rapidly increase at a treatment duration of 3–7 min. At the same time, the specific surface area of the powder calculated in terms of the spherical particle model dramatically decreases.
The process of the composite powder synthesis has a pronounced two-stage character. As its duration increases, the particle growth decelerates and the time scale of the fractional composition evolution increases drastically. At the second stage, variations in the main statistical parameters of the fractional composition appear to be nonmonotonic. The nonmonotonic dependence on the mechanical treatment time takes place for not only the average values, but also the standard deviations and medians of relative volume distributions, surface area, and number of particles in the composite powder.
The results of the morphological studies also indicate that the synthesis process has a two-stage character. At treatment durations of 3–7 min, particles with an anisotropic platelike shape compose a substantial fraction of the mixed powder. As the duration increases, the fraction of such particles dramatically decreases. At a treatment duration of 9 min and longer, the main volume of the powder system is represented by isometric particles with an acute-angled habit.
The evolution of the fractional composition and morphology of the particles of individual aluminum powder was studied to clarify the reasons for the two-stage character of the process. Figure 4 shows the electron micrographs, which illustrate the qualitative differences in the particle shapes and sizes of pure aluminum and the Al–2B system after 9-min treatment. Pure aluminum powder contains large platelike particles, while the particles of the composite powder are isometric and have manyfold smaller sizes. The differences in the particle shapes and sizes increase with the mechanical activation time.
EVOLUTION OF THE COMPOSITION AND STRUCTURE OF COMPOSITE PARTICLES
The X-ray spectroscopic microanalysis data for the pressed samples are presented in Fig. 5 as depending on the treatment duration.
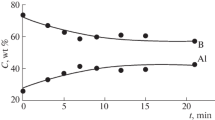
It should be clarified that the formal values of the relative contents of the components obtained for the pressed samples in no way correspond to the values averaged over the volume of the powder sample irrespective of the treatment time. The averaged values are characterized by the stoichiometric weight ratio of the components equal to 55% Al/45% B for all samples. As a matter of fact, the presented dependences reflect the kinetics of boron incorporation into aluminum and the homogenization of the composition of particles in the composite powder. At an initial stage of the treatment, the system contains a large amount of free boron, the powder of which possesses a low bulk density, occupies a large relative volume, and has a substantially lower X-ray absorption coefficient than does aluminum. As a result, the boron concentration averaged over the polished section surface area is markedly higher than the real one. As the treatment duration increases, the amount of free boron decreases, and its percentage in the composite particle bulk approaches the stoichiometric one.
The content of oxides in treated mixed powers was estimated as the atomic fraction of oxygen. The obtained values have appeared to be somewhat higher than those in the initial powders. This is explained by oxidation in the course of sample preparation for the analysis and is caused by the increased chemical activity of mechanically activated aluminum and boron. According to the X-ray diffraction data, oxide phases occur in the X-ray amorphous state. However, at a treatment duration of 5–7 min the diffraction patterns of the powders exhibit a small diffuse peak at angle 2θ = 33.05°, which corresponds to the phase of Fe2O3. The appearance of a small amount of this phase is associated with wear of the grinding bodies and activator cylinder walls.
In addition, the results of the diffraction studies have led to the conclusion that no noticeable amounts of crystalline aluminum boride, carbide, or nitride phases are present in the composite powders. Nevertheless, the possibility of the presence of trace amounts of these phases in the amorphized state cannot be excluded. It should also be noted that the narrow and low-intensity peak at 2θ = 28.10°, which was observed in the diffraction pattern of the initial mixture and corresponded to crystalline boron hydroxide, has completely disappeared already at the minimal treatment duration of 3 min.
Boron particles (both initial and incorporated into the composite powders) occur in the amorphous state. Reflections corresponding to crystalline boron are absent in the diffraction patterns of all powders. Dependences of the main characteristics of the microstructure of the aluminum component of the composite powders on the treatment duration are presented in Table 1. At an initial stage of the treatment, the coherent-scattering region (CSR) sizes and lattice constant value drastically decrease, while the second-order microdeformations, which characterize stresses in subgrains and mosaic blocks, increase. As the treatment duration increases, the variations in the microstructure parameters acquire an oscillating character. The correlation of the time dependence of the parameters remains preserved.
RESULTS AND DISCUSSION
The data obtained enable us to formulate a quantitative scheme for the development of the fractional composition, structure, and morphology of “Al–2B” composite powder. The kinetic of variations in the component and fractional compositions of the composite powder is governed by the ratio between three interdependent processes: boron incorporation into aluminum, fracture of particles, and their aggregation via the “cold welding” of small particles. At the initial stage, when the treatment duration is 3–7 min, the enlargement of particles prevails due to the incorporation of highly dispersed boron into aluminum and “cold welding.” The density of relative volume distribution over particle sizes shifts to the right (Fig. 1).
As the treatment time and the fraction of large particles increase, the particle enlargement process decelerates (Figs. 2, 3). The boron content in the bulk of composite particles and the main characteristics of the fractional composition are stabilized almost simultaneously. At the same time, the particles of the anisometric platelike shape completely disappear. The morphology of the composite powder particles suggests that their fracture has a quasi-brittle character. Comparison of the morphologies of the mechanically activated particles of pure aluminum and the composite powder leads us to conclude that the reason for this is the saturation of the composite particles with boron.
The growth of the boron content in the composite particles entails an increase in the yield stress, i.e., dispersion strengthening caused by an increase in the concentration of the barriers hindering the slip of dislocations. An approximate qualitative estimate may be made using expression [10]
which relates yield stress increment \(\Delta {{\sigma }_{{0.2}}}\) to aluminum shear modulus G, Burgers vector b, effective distance λ between boron particles, and particle size d. At fraction of boron particles α = 0.4 and characteristic particle size d = 0.5 µm, the calculation by relation (1) yields a Δσ0.2 value of about 100 MPa.
On the other hand, an increase in the boron content must lead to a decrease in the ultimate strength of the composite material because of a decrease in the strength of aluminum–boron adhesion contacts due to the presence of an oxide film on the boron particle surface and the adsorption of grinding fluid components at the interface. Ignoring adhesion, the ultimate strength may be estimated by the following relation [11]:

where α = \({{\left( {1 + \frac{\lambda }{d}} \right)}^{{ - 3}}}\) and β is the coefficient of the order of unity (for regular cubic packing of dispersed particles, β = 1.21). Typical values of the ultimate strength and yield stress for aluminum having a characteristic block size on the order of 0.1 µm and containing small amounts of iron, silicon, and magnesium lie in the ranges of σm = 170–220 MPa and σ0.2 = 80–120 MPa [12]. The calculations by relations (1) and (2) show that the thresholds of the strength and plasticity approach each other at a relative volume concentration of boron lying in a range of 0.20–0.25. As the boron content rises, the yield stress becomes higher than the ultimate strength and the fracture acquires a quasi-brittle character. The intensity of particle fracture increases, and, at a boron content in aluminum particles close to stoichiometric, the processes of fracture and “cold welding” are equilibrated, and the fractional composition is stabilized. Relatively with each other small oscillations of the average statistical characteristics of the system seem to be associated with the dependence of the yield stress and ultimate strength of aluminum on the parameters of the block structure.
The evolution of the block structure of the aluminum component of the composite powder is governed by the competition of grain fragmentation and dynamic recrystallization. The dramatic decrease in the CSR sizes at the initial stage of the treatment is related to the development of strong compressive stresses in the particle bulk, with these stresses manifesting themselves as a decrease in the lattice constant. The maximum compressive stresses are realized at a mechanical activation time of 7 min and the minimal size of the CSR. The estimation of the maximal stress under the assumption that the material occurs in the state of a uniform compression gives a value of about 240 MPa. At the stage of nonmonotonic variations in the structure parameters at treatment durations of 9–21 min, the stresses vary within a range of 50–100 MPa, thereby corresponding to specific energies of elastic deformation of 12.5–25.0 J/g.
Defects in the bulk of Al crystallites, as well as interfaces and intercrystallite boundaries also contribute to the excess free energy of composite powder particles. Commonly, the contribution from the defects in the bulk of nanosized crystallites may be ignored, because the high stresses squeeze mobile defects into intercrystallite regions during the formation of the block structure. In the case of Al–2B composite powders, this is confirmed by relatively low values of the Debye–Waller factor, which characterizes the root-mean-square shift of aluminum crystal lattice atoms from the equilibrium positions.
Under ignorance of adhesion, the maximal values of the specific interfacial energy in composite particles may be estimated using the sum of aluminum and boron surface energies. At an average boron particle size of 0.5 µm, which agrees with the data of electron microscopy, this results in a value of about 5 J/g at a treatment duration of more than 7 min. At a low content of free boron in the system, the contribution from the interfacial energy must be almost constant.
The obtained experimental data do not yield a direct assessment of the free energy of intercrystallite interfaces. However, the surface stresses (mechanical surface tension) may be evaluated [13]. Assume that the aluminum matrix has a regular cellular structure, each cell of which contains a crystallite, the size of which is equal to the CSR size, surrounded with a thin layer of a grain-boundary phase. It follows from the mechanical equilibrium condition that the normal stresses at a cell boundary are equal to zero, while the stresses in the plane of the boundary are continuous. Within the framework of this model scheme, the pressure in a crystallite and the surface stress are related by the Laplace equation. The corresponding assessments yield the maximum value of the surface stresses of 2.3 N/m upon a 7-min treatment, values lying in a range of 1.3–1.8 N/m at treatment for 9–15 min, and 0.8 N/m at a treatment time of 21 min, with the described time dependence being nonmonotonic. The high level of the surface stresses at intermediate treatment times is mainly due to the nonequilibrium state of the boundaries. The stress relaxation in the course of time is associated with a decrease in the degree of the nonequilibrium during the dynamic recrystallization.
On the other hand, the area of the intercrystallite boundaries reaches a maximal value of 30 m2/g at treatment time t = 7 min, acquires a minimum value of 14 m2/g at t = 12 min, and increases again to 28 m2/g at t = 21 min. Thus, the first stage is ended by the formation of ultimately ground and strongly nonequilibrium block structure of the aluminum component. Then, the area of the interfaces and the surface stresses vary nonmonotonically. At the maximum duration of treatment, the grinding of the structure is accompanied by a decrease in the surface stresses, which may be explained by an increase in the fraction of the low-energy small-angle boundaries in the course of the secondary fragmentation of grains.
It should be noted that the values of the free energy of large-angle boundaries of grains in aluminum polycrystals commonly lie in a range of 0.5–0.7 J/m2 [14], so that the contribution of the boundaries to the total excess energy of composite particles does not exceed 20 J/g. At the same time, the fraction of atoms in the grain-boundary phase is rather large: at a boundary width of 1 nm a relative free volume of 6%, which is characteristic of large-angle grain boundaries in aluminum polycrystals [15], it amounts to several percent.
The above-presented estimates cannot pretend to an exact quantitative description of the evolution of the mechanical properties and structure of the composite particles. They only illustrate the causes and interrelation of the processes that result in the formation of the internal structure of particles and the fractional composition of a composite powder. Concerning the energy characteristics, it should be noted that the total excess energy of a powder is two orders of magnitude lower than the heat of the reaction between aluminum and boron and cannot play a significant role in the energy balance of the transformation. However, the value of the excess energy of the defective structure is a sign of an increase in the reactivity and a change in the thermophysical characteristics of the aluminum component. In particular, determination of the melting temperature of aluminum contained in the composite powder has shown it to be reduced by more than 100 K.
CONCLUSIONS
The method of mechanical alloying has been employed to synthesize SHS composite powders with an Al–2B composition. The structure-related morphological characteristics and fractional composition of the mixed powder have been studied as depending on treatment duration. It has been found that the synthesis process has a pronounced two-stage character. At the first stage, solid highly dispersed boron particles are intensely incorporated into aluminum, the particles are enlarged, and the block structure of the aluminum component becomes finer. Then, the main statistical parameters of the fractional composition, the parameters of the block structure, and the relevant excess energy of the composite powder vary nonmonotonically.
The fractional composition results from the balance established between the rates of fragmentation and “cold welding.” The rate ratio between these processes is governed by the degree of particle saturation with boron and, to a lower extent, parameters of the block structure. The evolution of the block structure is, in turn, determined by the competition of grain fragmentation and dynamic recrystallization, the rates of which also depend on the content of boron and the size of crystallites. At the initial stage, a decrease in the CSR size is associated with the generation of strong compressive stresses in the bulk of crystallites. At the second stage the stresses relax; however, they remain rather strong. Evaluation of the excess energy has indicated that the reactivity of the composite powder can be substantially increased and the melting temperature of the aluminum matrix can be decreased.
REFERENCES
Gilman, P.S. and Benjamin, J.S., Annu. Rev. Mater. Sci., 1983, vol. 13, p. 279.
Gilman, P.S. and Nix, W.D., Metall. Trans. A, 1983, vol. 12, p. 813.
Kuz'mich, Yu.V., Kolesnikova, I.G., Serba, V.I., and Freidin, B.M., Mekhanicheskoe legirovanie (Mechanical Alloying), Moscow: Nauka, 2005.
Bernard, F. and Gaffet, E., Int. J. SHS, 2001, vol. 10, p. 109.
Rogachev, A.S. and Mukas’yan, A.S., Gorenie dlya sinteza materialov: vvedenie v strukturnuyu makrokinetiku (Combustion for Synthesis of Materials: Introduction to Structural Macrokinetics), Moscow: Fizmatlit, 2013.
Reddy, B.S.B. and Das, S., J. Mater. Sci., 2007, vol. 42, p. 9366.
Dolgoborodov, A.Yu., Makhov, M.N., Kolbanev, I.V., Streletskii, A.N., and Fortov, V.E., JETP Lett., 2005, vol. 81, p. 311.
Dolgoborodov, A.Yu., Makhov, M.N., Streletskii, A.N., Kolbanev, I.V., Gogulya, M.F., and Fortov, V.E., Khim. Fiz., 2004, vol. 33, no. 9, p. 85.
Rogachev, A.S., Kochetov, N.A., Kurbatkina, V.V., Levashov, B.A., Grimchuk, P.S., Rabinovich, O.S., Sachkova, V.N., and Bernar, F., Fiz. Goreniya Vzryva, 2006, vol. 42, no. 4, p. 61.
Shtremel’, M.A., Prochnost’ splavov (Strength of Alloys), Moscow: MISIS, 1999.
Bazhenov, S.L., Berlin, A.A., Kul’kov, A.A., and Oshmyan, V.G., Polimernye kompozitsionnye materialy. Prochnost’ i tekhnologiya (Polymer Composites. Strength and Technology), Dolgoprudnyi: Izd. Dom Intellekt, 2010.
Fizicheskie velichiny. Spravochnik (Physical Quantities: A Handbook), Grigor’ev, I.S. and Meilikhov, E.Z., Eds., Moscow: Energoatomizdat, 1991.
Rusanov, A.I., Termodinamicheskie osnovy mekhano-khimii (Thermodynamic Fundamentals of Mechanochemistry), St. Petersburg: Nauka, 2006.
Hasson, G. and Gou, C., Scr. Met., 1971, vol. 5, p. 889.
Chuvil’deev, V.N., Neravnovesnye granitsy zeren v metallakh. Teoriya i prilozheniya (Nonequilibrium Grain Boundaries in Metals. Theory and Applications), Moscow: Fizmatlit, 2004.
ACKNOWLEDGMENTS
We are grateful to A.D. Aliev and A.A. Shiryaev for carrying out electron microscopic and diffractometric studies with the equipment of the Center for Collective Use of Physical Methods of Investigation, Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by A. Kirilin
Rights and permissions
About this article
Cite this article
Malkin, A.I., Klyuev, V.A., Ryazantseva, A.A. et al. The Development of the Structure, Morphology, and Fractional Composition of “Al−2B” Composite Powders in the Course of Mechanical Activation. Colloid J 81, 703–710 (2019). https://doi.org/10.1134/S1061933X19060115
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X19060115